?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Glucose concentration is closely related to the metabolic activity of cells and it is the most important substance as the energy source of a living body which plays an important role in the human body. This paper proposes an optical method that can measure the concentration of glucose. The change in glucose concentration was observed by using CIE diagram, and wavelength and purity values were detected. Also, even small changes in glucose concentration can be evaluated through mathematical modeling. This system is simple, economical, and capable of quantifying optical signals with numerical values for glucose sensing. This method can be applicable to the clinical field that examines diabetes mellitus or metabolic syndrome.
Introduction
Glucose plays an important role of gratifying human sensation, producing energy and balancing our body in the non-nutritional aspect.Citation1 Also, the research findings showing that glucose helps to improve cognitive ability and memory were published and the effect was authenticated in all ages.Citation2 Intake of glucose has a good effect on word memory, story memory, shortening of reaction time, calculation ability improvement etc. In particular, some studies showed that the memory of old people with Alzheimer disease and those with Down syndrome has been improved and alcohol metabolism is also known to be influenced by glucose.Citation3-5 However, even if glucose has a positive impact such as recognition, memory improvement, too excessive intake may cause a hypoglycemia symptom.Citation6
Currently, glucose concentration is measured by using enzyme electrode, liquid chromatograph, glucose concentration analyzer, and electrical method etc.Citation7-12 However, enzyme electrode has a problem in accuracy of measured values and safety of electrode. In addition, it is difficult to use liquid chromatograph or glucose concentration analyzer in laboratory or industry because it requires a lot of incidental equipment in addition to the analyzer.Citation13-15 Refractometer can be used as glucose concentration analyzer and it contains expensive optical elements such as prism, analyzer, and polarizer. However, this technique is not suitable to collect data continuously because it requires calibration with distilled water after every 15 measurements. Furthermore, prism is sensitive to the change of temperature and humidity which can cause measuring errors. The principle of the method for detecting glucose concentration by the electrical method is that glucose is oxidized by glucose oxidase. However, this method has a problem in credibility because it measures the current amount in the correlation not direct concentration of glucose so there is a problem of regarding it as the accurate concentration of glucose.
This study is to measure the degree of discoloration of the sample to quantitatively analyze the concentration of glucose and present a method that can detect optical characteristics of the sample when color reaction occurs.Citation16-19 The discoloration characteristics depending on the concentration change of glucose were observed by using the indicator of Benedict's solution. As a method using small photodiode and color coordinates, the method presented in this study can determine the amount and color of the sample contained in the substance when color reaction occurs and estimate the concentration of glucose. It is also possible to detect wavelength and purity of solution by converting RGB values into color coordinate values. With the system proposed in this study, non-experts can detect the state change of a substance by measuring discoloration information. It also has the advantage that even a very small amount of sample can be measured quickly and easily without loss of the sample by measuring the state of a substance with the optical method. Since this can be applied to urinalysis, it will be applicable to the clinical field that examines diabetes mellitus or metabolic syndrome.
Material and methods
CIE diagram
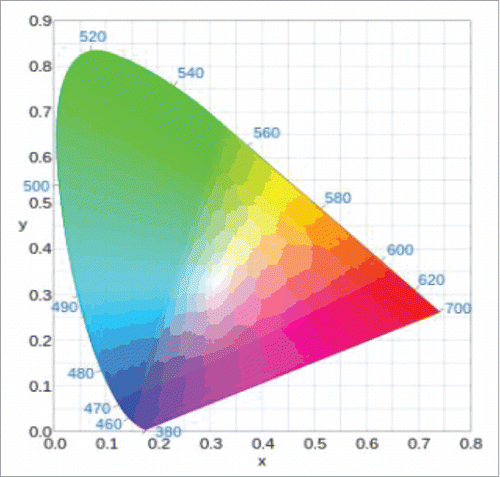
CIE 1931 color space is one of color space defined mathematically based on research about human color recognition. Human eyes recognize colors by combining RGB 3 colors and therefore, the distribution map of all visible lights becomes a 3-dimensional shape.
is the chromaticity distribution table of CIE 1931 color space drawn by using x and y variables. The outer curve-shaped boundary corresponds to monochromatic light and wavelength of each monochromatic light is marked with a nanometer. All colors in the visible light can be expressed as positive X, Y, and Z values. If marking any 2 points in this chromaticity distribution table, all colors that can be expressed by mixing 2 colors are present on a straight line connecting the 2 points. Also, all colors that can be shown by mixing 3 colors are present in a triangle consisting of 3 points.
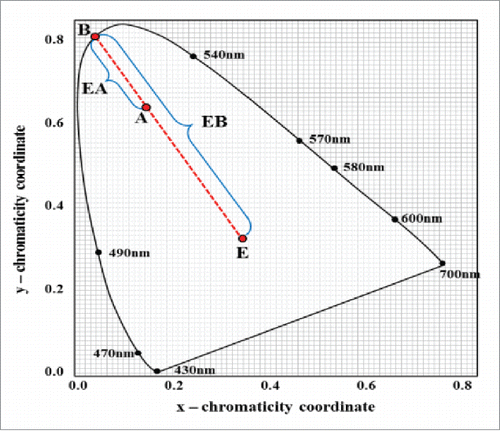
describes the CIE chromaticity diagram, established by the International Commission on Illumination. R, G, and B values can be measured with optical detector and these values are used to calculate coordinate values of color in CIE color space. The wavelength and purity of a certain color can be obtained by measuring the distance from coordinate value to spectrum line. The point B is situated at spectrum line and the purity of any point on the boundary (spectrum line) is 100%. The point E indicates the white color point and the purity value can be expressed as the ratio of EA/EB.Citation20
The R, G, and B values can be converted to X, Y, and Z values by equation (Equation1(1)
(1) ). Then, chromaticity coordinates x and y can be determined from the X, Y, and Z values by equation (Equation2
(2)
(2) ). The Y value indicates the degree of the luminance or brightness of a certain color in CIE coordinates. The chromaticity of a color can be expressed by x and y parameters which are dependent on 3 tristimulus values X, Y, and Z.Citation21
(1)
(1)
(2)
(2)
Color sensor and color reaction
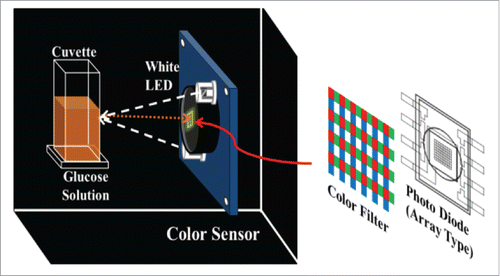
shows the schematic of experimental setup. A sensor used to obtain RGB values is a color sensor composed of photodiodes and optical elements (an optical sensor TCS3200 (TAOS, USA)). This is a sensor which converts an optical signal into a digital value. The sensor has several photodiodes arranged in an array type and combines the incident light through each color filter and then prints it out to a digital value between 0 and 255. Red, Green, and Blue optical filters are included inside the sensor and the photodiodes detect the intensity of the light passing through the optical filter and convert it into a digital signal. Light generated from White LED is reflected in the sample and incident on the photodiode.
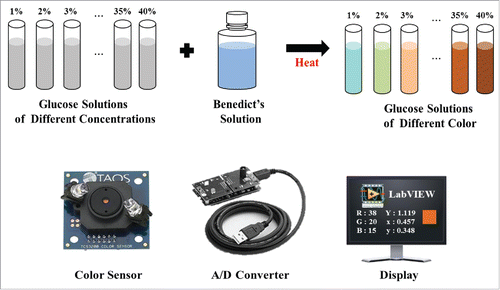
shows the procedure of detecting glucose concentration. In order to observe the color change according to the concentration of glucose, glucose solutions of different concentrations (1% ∼40%) of 10 ml and Benedict's solution 2 ml were mixed and heated for a certain period of time (2 minutes) and then the results of color change were obtained by using a color sensor. Glucose solution by each concentration was prepared by mixing distilled water with 40% glucose solution. The light received in the sensor was stored as a value from 0 to 255 through A/D conversion and the stored values were calculated by equations (Equation1 and 2) to obtain color coordinate values.
Results and discussion
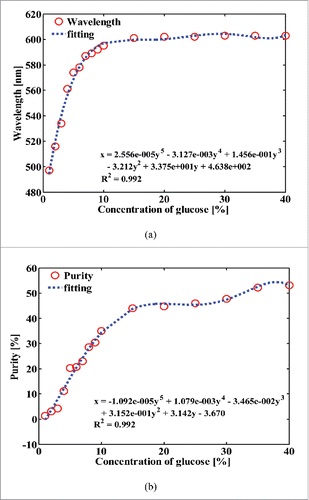
According to the change of glucose concentration, the obtained RGB values by the color sensor through A/D conversion and the variations of the calculated wavelength and purity values are shown in . It was not easy to distinguish the change of color reaction with naked eyes. However, if examining color reaction results according to the concentration of glucose numerically by using the color sensor, it can be observed that RGB values change according to the concentration of glucose, and wavelength and purity changes can be calculated with RGB values. Most show a tendency that as the concentration increases, wavelength and purity increase as well. Wavelength tends to be constant in some sections but purity values tend to increase gradually as the concentration increases. Therefore, the change of glucose concentration can be inferred with purity values.
Table 1. The changes of RGB values, wavelength, and purity due to glucose concentration.
is the result graph and equation of modeling wavelength and purity values shown in . is the results of modeling wavelength values detected through color coordinates and is the results of modeling purity detected in color coordinates. Wavelength and purity values are in the monotonically increasing region according to the change of glucose concentration so slight concentration differences can be also predicted through inverse modeling. In order to detect the concentration of glucose, we devised a method that can convert wavelength or purity into the concentration of glucose. Therefore, as shown in equation (Equation3(3)
(3) ), we used a modeling technique that can see the X variable, the concentration of glucose if detecting the Y variables, wavelength and purity values.
Here, A0 ∼ An mean coefficient values of the inversely converted data obtained by modeling results, X means the concentration of glucose and Y means wavelength or purity value. By using this modeling equation, the concentration of glucose not measured can be estimated with wavelength and purity values obtained by converting RGB values. Wavelength values of do not show notable differences in the range of more than 15% concentration. However, in , it can be found that purity changes clearly according to the concentration. That is, as the concentration increases, purity values tend to increase gradually so the values of the glucose concentration can be detected by inversely modeling purity values.
Also, coefficients of determination(R2), a measure of showing the goodness of fit of the regression equation obtained through modeling and original data obtained through experimental results were found to be very high, (a) R2 = 0.992, p < 0.01, (b) R2 = 0.992, p < 0.01, respectively, indicating that it is statistically significant.
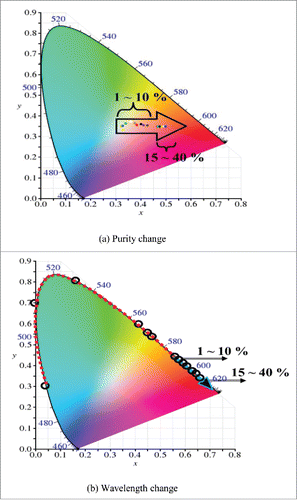
shows the changes of wavelength and purity values by glucose concentration. It is the one schematized by substituting Red, Green, and Blue values that were measured by the sensor into equations (Equation1 and 2), and marking the coordinates (x and y values) in CIE diagram. It can be seen that color coordinates move by the concentration change of glucose. Unlike the way of predicting the concentration change incorrectly by observing the color change with naked eye like the conventional method, the method proposed in this study was to mark the concentration change of glucose easily by observing and imaging it through the color coordinate change. Visualized data has the advantages of being able to understand the data easily and show the characteristics of the data itself fast and obviously. This is more effective than the memory of the linguistic form and the effect of the memory can be significantly improved because it helps users to easily understand the information provided and make them remember the visual form. If looking at , it can be found that as the concentration of glucose increases, color coordinates move from center (white) to spectrum line (outer border in the CIE diagram). These are results consistent with the trend that as the concentration of glucose increases, sediment is also increased and purity of the color is increased. If adding Benedict's reagent in the glucose solution and then heating it, the solution changes gradually from blue to dark orange. That is, colors change in the order of blue-green-yellow-dark orange according to the concentration of glucose. This trend appears evident in . As the concentration of glucose increases, wavelength values in spectrum line increase as well.
Conclusion
This paper proposed a method of measuring the concentration of glucose by using optical devices and indicator, and showing it numerically. In particular, we observed color coordinate movement according to the concentration of glucose by using CIE color coordinate, and obtained wavelength and purity data. The proposed optical method can calculate concentrations through inverse conversion modeling of these data. This study is differentiated from existing studies. First, data were digitized by using the principle of color coordinates and color sensor, not the method of observing the degree of color reaction by using a spectrometer or colorimetric method as in previous studies. This shows the possibility that can replace the expensive equipment as well as subjective methods used in the past and makes it possible to determine simply and accurately in a short time. Second, this study is not limited to Benedict's solution and can be applied to all fields using the indicator showing color changes such as urinalysis and iodo-starch reaction etc. By being applied to metabolic syndrome diagnosis, the diagnosis of diabetes mellitus, water analysis, and serum cholesterol test, this will be not only helpful in clinical research but can be applied to environmental engineering, pharmaceutical and cosmetics fields.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Boeckxstaens GE, Horowitz M, Bermingham H, Holloway RH. Physiological variations in blood glucose concentration affect oesophageal motility and sensation in normal subjects. Neurogastroenterol Motil 1997; 9(4):239-46; PMID:9430792; http://dx.doi.org/10.1046/j.1365-2982.1997.d01-64.x
- Korol DL, Gold PE. Glucose, memory, and aging. Am J Clin Nutr 1998; 67(4):764S-771S; PMID:9537626
- Landin K, Blennow K, Wallin A, Gottfries CG. Low blood pressure and blood glucose levels in Alzheimer's disease. Evidence for a hypometabolic disorder? J Intern Med 1993; 233(4):357-63; PMID:8463769; http://dx.doi.org/10.1111/j.1365-2796.1993.tb00684.x
- Manning CA, Honn VJ, Stone WS, Jane JS, Gold PE. Glucose effects on cognition in adults with Down's syndrome. Neuropsychology 1998; 12(3):479; PMID:9674002; http://dx.doi.org/10.1037/0894-4105.12.3.479
- Carpenter TM, Lee RC. The effect of glucose on the metabolism of ethyl alcohol in man. J Pharmacol Exp Therap 1937; 60(3):264-285.
- Seo HC. The relationship between Sugar intake and emotional function of adolescent. J Br Educat 2013; 11:75-97.
- Kim JH, Lee KY, Im KT. Automatic measurement of cell and glucose concentration. Korean J Biotechnol Bioeng 1990; 5(1):37-42.
- Ahn WS, Kim J. T. Blood glucose measurement principles of non-invasive blood glucose meter: focused on the detection methods of blood glucose. J Biomed Eng Res 2012; 33:114-127; http://dx.doi.org/10.9718/JBER.2012.33.3.114
- Tian Y, Liu Y, Wang W, Zhang X, Peng W. CuO nanoparticles on sulfur-doped graphene for nonenzymatic glucose sensing. Electrochim Acta 2015; 156:244-251; http://dx.doi.org/10.1016/j.electacta.2015.01.016
- Zhou C, Yu X, Ma H, Liu S, Qin X, Yagoub A-G, Owusu J. Examining of athermal effects in microwave-induced glucose/glycine reaction and degradation of polysaccharide from Porphyra yezoensis. Carbohydr Polym 2013; 97:38-44; PMID:23769514; http://dx.doi.org/10.1016/j.carbpol.2013.04.033
- Chen CC, Lin CL, Chen LC. Functionalized carbon nanomaterial supported palladium nano-catalysts for electrocatalytic glucose oxidation reaction. Electrochim Acta 2015; 152:408-416; http://dx.doi.org/10.1016/j.electacta.2014.11.116
- Liang AH, Huang YJ, Jiang ZL. A rapid and sensitive immunoresonance scattering spectral assay for microalbumin. Clin Chim Acta 2007; 383:73-77; PMID:17532311; http://dx.doi.org/10.1016/j.cca.2007.04.020
- Jin Q, Shan L, Yue J, Wang X. Spectrophotometric determination of total serotonin derivatives in the safflower seeds with Ehrlich's reagent and the underlying color reaction mechanism. Food Chem 2008; 108:779-783; PMID:26059161; http://dx.doi.org/10.1016/j.foodchem.2007.11.022
- Jiang Z, Wang S, Liang A, Zhong F. A highly sensitive resonance scattering spectral assay for IgG using Fehling reagent–glucose–immunonanogold reaction. Talanta 2009; 77:1191-1196; PMID:19064111; http://dx.doi.org/10.1016/j.talanta.2008.08.017
- Song DB. Measurement of glucose concentration using a μFIA biosensor. J Korean Society Agri Machin 2003; 28(3):465-468.
- Iuliis AD, Arrigoni G, Andersson L, Zambenedetti P, Burlina A, James P, Arslan P, Vianello F. Oxidative metabolism of dopamine: A colour reaction from human midbrain analysed by mass spectrometry. Biochim Biophys Acta 2008; 1784:1687-1693; PMID:18675943; http://dx.doi.org/10.1016/j.bbapap.2008.07.002
- Horiguchi T, Koshiba Y, Ueda Y, Origuchi C, Tsutsui K. Reversible coloring/decoloring reaction of leuco dye controlled by long-chain molecule. Thin Solid Films 2008; 516:2591-2594; http://dx.doi.org/10.1016/j.tsf.2007.04.085
- Kinashi K, Horiguchi T, Tsutsui K, Ishida K, Ueda Y. Reversible multi-coloring reaction of spironaphtooxazine controlled by long-chain molecule. J Photochem Photobiol A 2010; 213:189-193; http://dx.doi.org/10.1016/j.jphotochem.2010.05.024
- Jiang Z, Huang Y, Liang A, Pan H, Liu Q. Resonance scattering detection of trace microalbumin using immunonanogold probe as the catalyst of Fehling reagent–glucose reaction. Biosens Bioelectron 2009; 24:1674-1678; PMID:18835769; http://dx.doi.org/10.1016/j.bios.2008.08.031
- Zhang L, Kong H, Chin CT, Wang T, Chen S. Cytoplasm segmentation on cervical cell images using graph cut-based approach. Bio-Med Mater Eng 2014; 24(1):1125-1131.
- Schanda J, Danyi M. Correlated Color-Temperature Calculations in the CIE 1976 Chromaticity Diagram. Color Research & Application 1977. 2(4):161–163; http://onlinelibrary.wiley.com/doi/10.1002/col.5080020403/pdf.