ABSTRACT
In this study, a Ganoderma lucidum polysaccharide GLP-1–1 was isolated from a culture broth with Mw of 22014 Da. Monosaccharide contained glucose, mannose, and galactose with mole percentages of 92.33%, 7.55%, and 0.22%, respectively. Moreover, FTIR and methylation analysis were conducted to characterize the structural properties of GLP-1–1. The results of antioxidant activity analysis showed that GLP-1–1 had a great DPPH and ABTS radical scavenging activity. Meanwhile, GLP-1–1 also exhibited anti-tumor activity to A431 and MDA-MB-231 cells, and inhibitory rates were dose-dependent. During culturing with GLP-1–1, the G1/G0 cell percentage of A431 cells was increased from 48.64% to 84.52%, and the G1/G0 cell percentage of MDA-MB-231 cells was increased from 57.14% to 73.48%. Therefore, the anti-tumor activity of GLP-1–1 may be caused by inducing the G1/G0 arrest of tumor cells.
Introduction
Ganoderma lucidum is a valuable medicinal mushroom and has been used in China and other Asian countries for over 2000 y for improving health and longevity. The extracts from the sporophore and mycelium of G. lucidum contain polysaccharides, proteoglycans, triterpenoids, and other active compounds, which have been used to treat many diseases.Citation1 Among these active compounds, G. lucidum polysaccharide is one of the most studied biologic compounds and has several bioactivities, including antioxidant,Citation2,3 anti-tumor,Citation4,5 and immunomodulatory activities.Citation6,7
Recent researchers focused on the extraction of G. lucidum polysaccharide from sporophores by various methods, and extraction conditions were optimized to enhance the polysaccharide production.Citation3,8 The extracted polysaccharide was further used to detect the bioactivities. However, the polysaccharide and other biologic compounds in G. lucidum sporophores were easily influenced by cultivation conditions and their place of origin. Submerged fermentation was commonly used to produce bioactive products in industry. Recently, Peng et al.Citation9,10 reported the monosaccharide composition of G. lucidum polysaccharide was influenced by culture conditions and carbon sources. Therefore, the development of fermentation technology and further understanding of polysaccharide synthesis process may be helpful for the mass production of biologic G. lucidum polysaccharide in submerged fermentation.
In this study, G. lucidum obtained by submerged fermentation were purified, and the structural properties of isolated polysaccharide were investigated. Moreover, the antioxidant and anti-tumor activities of the isolated polysaccharide were also studied. Meanwhile, the possible reason behind polysaccharide anti-tumor activity was analyzed by detecting the cell cycle of used tumor cells.
Results and discussion
Isolation and purification of G. lucidum EPS
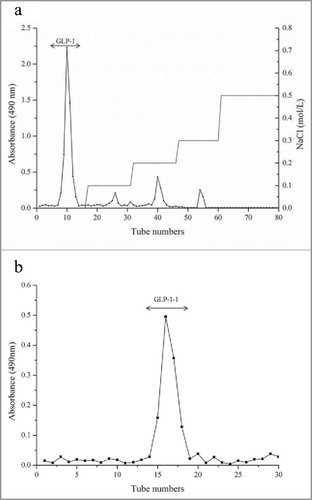
The obtained crude G. lucidum EPS was purified by a DEAE Fast Flow column, and eluted by distilled water and various concentrations of NaCl solutions. The EPS (GLP-1) eluted by distilled water was the dominant polysaccharide, after comparing with other EPS eluted by different concentrations of NaCl (). GLP-1 was further purified by a Sephadex G-75 gel filtration column, and a sharp peak (GLP-1–1) was obtained by eluting with distilled water (). In this study, GLP-1–1 was obtained and was used for further structure and biologic activity analysis.
Molecular weight and monosaccharide composition of G. lucidum polysaccharide
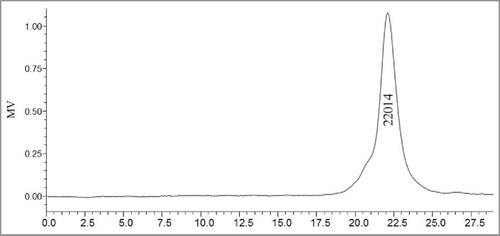
Dextrans and GLP-1–1 were determined by HPGFC, and a sharp peak of GLP-1–1 was shown in , indicating a homogenous polysaccharide component after purification. Various dextrans with different molecular weights were used as standard to calculate the equation. According to the equation, the molecular weight of GLP-1–1 was calculated by the retention time and was 22014 Da (). The monosaccharide composition of GLP-1–1 was analyzed by gas-liquid chromatography. The results showed that the Glc was the main monosaccharide in GLP-1–1, and mole percentage of Glc exceeded 90% (). Peng et al.Citation9,10 reported that carbon sources and culture conditions in submerged fermentation, all influenced the mole percentage of monosaccharides in G. lucidum EPS. In this study, the carbon source for the G. lucidum culture was glucose, and thus the dominant monosaccharide of the main polysaccharide, GLP-1–1, was Glc, which exceeded 90%.
Table 1. Monosaccharide composition of different fractions of G. lucidum EPS
FTIR and methylation analysis
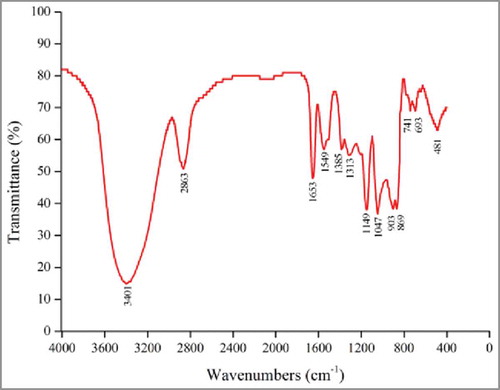
The organic groups in GLP-1–1 were analyzed by FTIR spectroscopy, and the results are shown in . In a previous study, Ross et al.Citation14 summarized the functional groups and absorptions of polysaccharides from several other studies. The spectra of GLP-1–1, shown in , indicate the possible functional groups. The absorption peak of around 3401 cm−1 can be attributed to -OH stretching in sugar residues.Citation15 Peaks at 2863 cm−1 may represent the stretching vibration of C-H. The absorbances at around 1653 cm−1 and 1385 cm−1, were associated with the carboxyl group, and a stretching vibration of C = O.Citation16,15 GLP-1–1 also showed the following absorption peaks: 1313 cm−1 could be attributed to carboxylic acid (C-O stretch); 1149 cm−1 corresponded to C-O-C and the -OH bending vibration in the pyran structure; 1047 cm−1 represented the bending vibration of hydroxyl groups; 903 cm−1 and 869 cm−1 may be associated with the α or β configurations of glycosidic linkages.
Different methylated sugars of G. lucidum EPS were identified by retention times and mass spectra in GC-MS, as shown in . The result showed that the detected methylated sugars contained mannose and glucose, and no galactose was detected in GC-MS. Mannose had only one linkage pattern of Man (1- and was the terminal sugar in GLP-1–1. The most abundant sugar in GLP-1–1 was glucose and had 6 different linkages. The result indicated that glucose was the backbone and branching residue sugar in GLP-1–1.
Table 2. Methylation analysis of GLP-1–1
Antioxidant activity
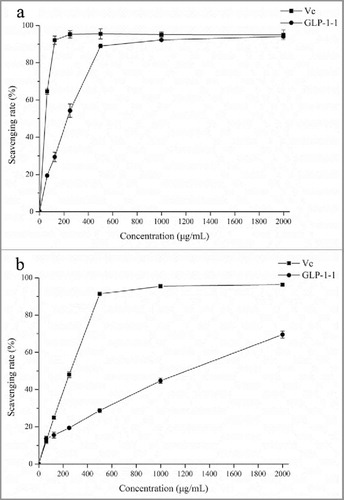
As the biologic macromolecules, polysaccharides from different resources had several activities, and antioxidant activity of polysaccharides was always detected.Citation16-18 In this study, DPPH and ABTS radical scavenging activity and reducing power analysis were investigated to evaluate G. lucidum EPS. In radical scavenging activity assay, the activity of GLP-1–1 all achieved the highest activity of 89.02% (DPPH) and 69.53% (ABTS), and maintained a steady level while increasing to 2000 μg/mL ( and ). Wu et al.Citation16 isolated 3 polysaccharides from purple sweet potato, and all 3 polysaccharides exhibited different DPPH and ABTS scavenging activity. As the dominant polysaccharide, GLP-1–1 had a great antioxidant activity, and was the main active component in G. lucidum polysaccharide.
Anti-tumor activity
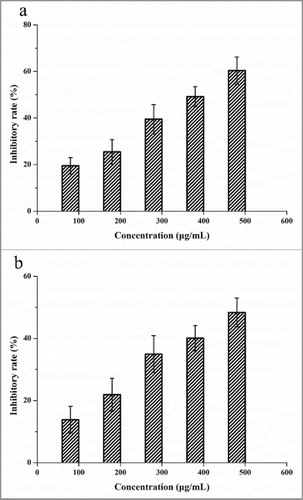
Anti-tumor activity of G. lucidum polysaccharide against several tumor cell lines in vivo and in vitro was reported by many studies.Citation2,5,13 In this study, the anti-tumor activity analysis of G. lucidum EPS was performed by detecting the inhibitory rate of A431 and MDA-MB-231 cells (). The anti-tumor activity of GLP-1–1 against A431 and MDA-MB-231 cells was dose-dependent, and the inhibitory rate increased in a concentration range of 100 – 500 µg/mL. The highest inhibitory rates of GLP-1–1 to A431 and MDA-MB-231 were 60.38% and 48.34%, respectively. This result indicated GLP-1–1 was the main active component of G. lucidum polysaccharides. Similarly, anti-tumor activity against 3 hepatocarcinoma cell lines (HepG2, BEL-7402, Huh-7) of the G. lucidum polysaccharide was reported.Citation4 Moreover, the G. lucidum polysaccharides also exhibited great anti-tumor activity in the gastric rat model.Citation2 Combined with the results of this study, G. lucidum polysaccharides showed the potential to develop as an effective drug for cancer treatment.
Cell cycle analysis of A431 and MDA-MB-231 cells
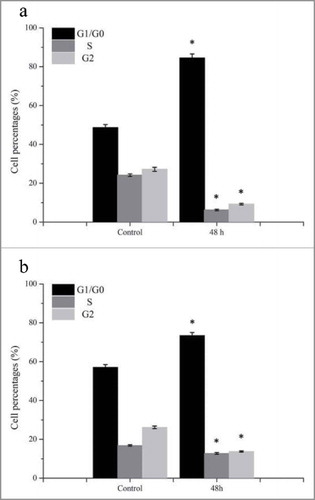
In vivo experiments, G. lucidum polysaccharides suppressed the growth and induced the apoptosis of tumor cells, by enhancing an immune response.Citation2 Moreover, T and B lymphocyte proliferation was also enhanced by G. lucidum polysaccharides.Citation7 Zhang et al.Citation13 reported that polysaccharides only exhibited anti-tumor activity as the existence of functional p53 protein in various human carcinoma cell lines. In this study, the percentage of cells in the G1/G0 phase was increased after incubating with 2000 μg/mL GLP-1–1 for 48 h (). Comparing with control, G1/G0 cell percentage of A431 raised approximately 73.77%, and 28.6% for MDA-MB-231. This result suggested that the inhibited growth of tumor cells may be caused by the arrest of the G1/G0 phase of A431 and MDA-MB-231 cells. Similarly, the G1 phase arrest of HepG2 induced by isolated G. lucidum polysaccharide was also reported, and caused the apoptosis of HepG2 cellsCitation4 Moreover, Wang et al.Citation19 regarded the cell cycle arrest as an extrinsic apoptotic pathway in the Boschniakia rossica polysaccharide study. Therefore, anti-tumor activity of GLP-1–1 in this study may be achieved by inducing a G1/G0 phase arrest.
Materials and methods
G. lucidum culture and EPS preparation
The fungal mycelium of G. lucidum was preserved by our laboratory and maintained in potato dextrose agar (PDA) at 4°C. The seed and fermentation medium of G. lucidum were composed of: 20 g/L glucose, 5 g/L peptone, 10 g/L wheat bran, 3 g/L KH2PO4, and 2 g/L MgSO4·7H2O (pH 6.6). In submerged fermentation, mycelia squares from PDA slants were inoculated to the seed medium and cultured at 30°C and 150 rpm for 2 weeks. The seed culture medium containing G. lucidum mycelia was inoculated to 150 mL fermentation medium and then cultured at 30°C and 150 rpm for 7 d.
The culture broth from the G. lucidum fermentation medium was obtained by centrifuging to remove mycelia, and crude EPS was collected by precipitating with 3 volumes of 95% (v/v) ethanol overnight. The obtained crude EPS was dissolved in distilled water, and insoluble compound in solution was removed by centrifuging. Crude EPS in solution was concentrated by lyophilization for further research.
Purification of crude G. lucidum EPS
The deproteinated EPS was purified by DEAE fast flow column, and eluted by distilled water and 0.5 mol/L NaCl solution. Different fractions were collected with 5 ml per tube, and the major fractions were desalted. Then, different fractions were concentrated by lyophilization for further purification in Sephadex G75 column. After loading EPS, different fractions were eluted by distilled water, and collected fractions were lyophilized for further research. All fractions collected during purification were detected by phenol-sulfuric acid method at 490 nm.
Molecular weight determination
The molecular weight of G. lucidum polysaccharide was detected by high-performance gel filtration chromatography (HPGFC) (Waters 600) with Ultrahydrogel™ Linear (7.8 × 300 mm). The column was eluted by 0.1 M NaNO3 at a flow rate of 0.9 mL/min. Various dextrans with different molecular weights were used as standards, and the molecular weight of GLP-1–1 was calculated according to the equation.
Monosaccharide composition
Twenty mg crude G. lucidum EPS was hydrolyzed by 2 mL of 1 mol/L H2SO4 at 100°C for 2 h and then neutralized with BaCO3. The obtained monosaccharide was concentrated by lyophilization and reacted with 20 mg hydroxylamine hydrochloride, 0.5 mLpyridine, and 0.5 mL acetic anhydride. The type and content of monosaccharide was detected by gas-liquid chromatography and inositol was used as internal standard.
Fourier transformation infrared (FT-IR) spectroscopy analysis
Crude G. lucidum EPS was grounded with KBr and pressed into a pellet. The obtained pellets were used for FTIR analysis (Nicolet Nexus 470), and FTIR spectra of GLP-1–1 were shown in the range of 400 to 4000 cm−1.
Methylation
G. lucdium polysaccharide in this study was methylated according to Ciucanu method reported by Needs and SwlvendranCitation11 previously. The methylated sample were further hydrolyzed, reduced, and peracetylated, and then analyzed by Shimadzu GCMSQP2010 system equipped with a capillary column as described by Wang et al.Citation12
Antioxidant activity analysis in vitro
The DPPH and ABTS radical scavenging activity was performed according to the method reported previously.Citation13 Vitamin C was used as the positive control in this experiment.
Anti-tumor activity in vitro
Two human tumor cell lines, epidermoid carcinoma A431 and breast carcinoma MDA-MB-231, were used to analyze the anti-tumor activity of G. lucidum EPS. Tumor cells were cultured by Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (Gibco), 100 μg/mL streptomycin, and 100 U/mL penicillin. Cells (1 × 104 cells/well) were seeded in a 96-well microplate and cultured at 37°C, in 5% CO2, for 24 h. After by medium with EPS for 48 h, MTT method was performed to detect the inhibitory rate of tumor cells, comparing with cells cultured without EPS.
Cell cycle analysis
A431 and MDA-MB-231 cells were cultured with 2000 μg/mL GLP-1–1 for 48h in a 6-well microplate, and culture conditions were the same as described above. Two tumor cells incubating without EPS were used as controls. Cells were collected and fixed with 70% ethanol in PBS. Then, cells were suspended by 0.5 mL PBS containing propidium iodide (50 mg/mL) and DNase-free RNase (100 mg/mL). The treated cells were analyzed by FACSCalibur (Becton Dickinson), and percentages of cells in different phases were calculated by obtained data.
Statistical analysis
Data were expressed as mean ± SD, and analyzed using the software program GraphPad Prism 5.0. Differences with p < 0.05 were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by the ‘863’ Program (No. 2013AA102203–06), the National Natural Science Foundation of China (No. 31271839 and 31571823), the National Key Research and Development Project from the Ministry of Science and Technology (2016YFD0400504), and the Fundamental Research Funds for the Central Universities of China (No. JUSRP11509).
References
- Ma HT, Hsieh JF, Chen ST. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015; 114:109-113; PMID:25790910; http://dx.doi.org/https://doi.org/10.1016/j.phytochem.2015.02.017
- Pan K, Jiang Q, Liu G, Miao X, Zhong D. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Biol Macromol 2013; 55:301-306; PMID:23370161; http://dx.doi.org/https://doi.org/10.1016/j.ijbiomac.2013.01.022
- Kan YJ, Chen T, Wu Y, Wu J, Wu J. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int J Biol Macromol 2015; 72:151-157; PMID:25149043; http://dx.doi.org/https://doi.org/10.1016/j.ijbiomac.2014.07.056
- Liu YJ, Shen J, Xia YM, Zhang J, Park HS. The polysaccharides from Ganoderma lucidum: Are they always inhibitors on human hepatocarcinoma cells? Carbohydrate Polymers 2012; 90(3):1210-1215; PMID:22939333; http://dx.doi.org/https://doi.org/10.1016/j.carbpol.2012.06.043
- Zheng S, Jia Y, Zhao J, Wei Q, Liu Y. Ganoderma lucidum polysaccharides eradicates the blocking effect of fibrinogen on NK cytotoxicity against melanoma cells. Oncol Letters 2012; 3(3):613-616; PMID:22740961
- Chang CJ, Chen YY, Lu CC, Lin CS, Martel J, Tsai SH, Ko YF, Huang TT, Ojcius DM, Young JD, et al. Ganoderma lucidum stimulates NK cell cytotoxicity by inducing NKG2D/NCR activation and secretion of perforin and granulysin. Innate Immunity 2014; 20(3):301-311; PMID:23803412; http://dx.doi.org/https://doi.org/10.1177/1753425913491789
- Zhou HB, Liu G, Huang F, Wu X, Yang H. Improved production, purification and bioactivity of a polysaccharide from submerged cultured Ganoderma lucidum. Arch Pharm Res 2014; 37(12):1530-1537; PMID:24737396; http://dx.doi.org/https://doi.org/10.1007/s12272-014-0391-8
- Zhu X, Chen X, Xie J, Wang P, Su W. Mechanochemical-assisted extraction and antioxidant activity of polysaccharides from Ganoderma lucidum spores. Int J Food Sci Technol 2012; 47(5):927-932; http://dx.doi.org/https://doi.org/10.1111/j.1365-2621.2011.02923.x
- Peng L, Qiao S, Xu Z, Guan F, Ding Z, Gu Z, Zhang L, Shi G. Effects of culture conditions on monosaccharide composition of Ganoderma lucidum exopolysaccharide and on activities of related enzymes. Carbohydrate Polymers 2015; 133:104-109; PMID:26344261; http://dx.doi.org/https://doi.org/10.1016/j.carbpol.2015.07.014
- Peng L, Li J, Liu Y, Xu Z, Wu JY, Ding Z, Gu Z, Zhang L, Shi G. Effects of mixed carbon sources on galactose and mannose content of exopolysaccharides and related enzyme activities in Ganoderma lucidum. Rsc Adv 2016; 6(45):39284-39291; http://dx.doi.org/https://doi.org/10.1039/C6RA04798J
- Needs PW, Selvendran RR. Avoiding oxidative degradation during sodium hydroxide/methyl iodide-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohydrate Res 1993; 245(1):1-10; http://dx.doi.org/https://doi.org/10.1016/0008-6215(93)80055-J
- Wang Z, Liu Y, Sun Y, Mou Q, Wang B, Zhang Y, Huang L. Structural characterization of LbGpl from the fruits of Lycium barbarum L. Food Chem 2014; 159:137-142; PMID:24767036; http://dx.doi.org/https://doi.org/10.1016/j.foodchem.2014.02.171
- Zhang J, Chen JM, Wang XX, Xia YM, Cui SW, Li J, Ding ZY. Inhibitor or promoter? The performance of polysaccharides from Ganoderma lucidum on human tumor cells with different p53 statuses. Food Function 2016; 7(4):1872-1875; PMID:26999513; http://dx.doi.org/https://doi.org/10.1039/C5FO01628B
- Ross K, Siow Y, Dan B, Isaak C, Fukumoto L, Godfrey, D. Characterization of water extractable crude polysaccharides from cherry, raspberry, and ginseng berry fruits: chemical composition and bioactivity. Int J Food Properties 2015; 18(3):670-689; http://dx.doi.org/https://doi.org/10.1080/10942912.2013.837066
- Zhao L, Dong Y, Chen G, Hu Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydrate Polymers 2010; 80(3):783-789; http://dx.doi.org/https://doi.org/10.1016/j.carbpol.2009.12.029
- Wu QY, Qu H, Jia J, Kuang C, Wen Y, Yan H, Gui Z. Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydrate Polymers 2015; 132:31-40; PMID:26256321; http://dx.doi.org/https://doi.org/10.1016/j.carbpol.2015.06.045
- Zhang SS, Liu X, Yan L, Zhang Q, Zhu J, Huang N, Wang Z. Chemical compositions and antioxidant activities of polysaccharides from the sporophores and cultured products of Armillaria mellea. Molecules 2015; 20(4):5680-5697; PMID:25838171; http://dx.doi.org/https://doi.org/10.3390/molecules20045680
- Zhang TT, Lu CL, Jiang JG, Wang M, Wang DM, Zhu W. Bioactivities and extraction optimization of crude polysaccharides from the fruits and leaves of Rubus chingii Hu. Carbohydrate Polymers 2015; 130:307-315; PMID:26076631; http://dx.doi.org/https://doi.org/10.1016/j.carbpol.2015.05.012
- Wang ZH, Lu C, Wu C, Xu M, Kou X, Kong D, Jing G. Polysaccharide of Boschniakia rossica induces apoptosis on laryngeal carcinoma Hep2 cells. Gene 2014; 536(1):203-206; PMID:24334128; http://dx.doi.org/https://doi.org/10.1016/j.gene.2013.11.090