ABSTRACT
This study describes an efficient and reusable process for ethanol production from medium containing whey powder, using alginate immobilized ethanologenic E. coli strains either expressing (TS3) or not expressing (FBR5) Vitreoscilla hemoglobin. Reuseabilities of the FBR5 and TS3 strains were investigated regarding their ethanol production capacities over the course of 15 successive 96-h batch fermentations. The ethanol production was fairly stable over the entire duration of the experiment, with strain TS3 maintaining a substantial advantage over strain FBR5. Storage of both strains in 2 different solutions for up to 60 d resulted in only a modest loss of ethanol production, with strain TS3 consistently outperforming strain FBR5 by a substantial amount. Strains stored for 15 or 30 d maintained their abilities to produce ethanol without dimunition over the course of 8 successive batch fermentations; again strain TS3 maintained a substantial advantage over strain FBR5 throughout the entire experiment. Thus, immobilization is a useful strategy to maintain the advantage in ethanol productivity afforded by expression of Vitreoscilla hemoglobin over long periods of time and large numbers of repeated batch fermentations, including, as in this case, using media with food processing wastes as the carbon source.
Introduction
In recent years, there has been great concern regarding environmental pollution due to the use of fossil fuels. The increase of world population and high oil prices have also increased efforts to efficiently develop production of renewable energy sources;Citation1,2 these include biofuels. Among the biofuels, bioethanol is most commonly used. Most bioethanol is produced from fermentation of sugar from maize starch or sugarcane, but it can also be produced from non-food sources such as lignocellulose or different wastes.Citation3 There is an increasing interest in using food processing wastes in this role.
Cheese whey is a dairy industry waste produced in high amounts, the disposal of which results in environmental pollution due to its high organic content. Whey production is about 180 to 190 × 106 tons per year world wide,Citation4 whereas only about half of this is reused in the food industry.Citation5,6 Ethanol is produced from whey in countries such as New Zealand, the United States, and Denmark.Citation5,7 Ethanol production from whey cannot, however, be an economical process due to the low lactose content of whey, which results in low levels of ethanol production.Citation8 High concentrations of lactose, however, can be provided by using whey powder to achieve high concentrations of ethanol. Previous studies have shown that whey and whey powder can be used for ethanol production by lactose fermenting microorganisms such as Kluyveromyces lactis, Kluyveromyces marxianus and Candida pseudotropicalis.Citation7,9-13
There is a great need for effective, low cost, and reusable methodologies for bioethanol production that can be applied at an industrial scale.Citation14,15 One such methodology, immobilization of microorganims, has been studied extensively in recent years.Citation16-18 It has been shown that the immobilization of ethanologenic microorganisms offers many advantages over planktonic cell cultures. These include easy separation of product from the growth medium, elimination of the need for a fresh inoculum for each batch, reuse of microorganisms for several cycles, enhanced productivity, lowered contamination risks, and enhanced stability of the ethanologenic strains.Citation19-20 Immobilized yeast cells have been used in continuous fermentation systems,Citation1 and carriers such as alginate, chitosan or cellulose have been extensively investigated.Citation17
Immobilization of microorganisms presents challenges, including carrier gel degradation, limited mass transfer and difussion of nutrients, diffuculties in estimating the effects of immobilization on the metabolism or physiology of the microorganisms, and cell leakage.Citation21 To overcome these diffuculties, parameters such as cell concentration or carrier type have been extensively investigated.
Although, as mentioned above, ethanol production using immobilized cells has been investigated in recent years,Citation1,22 there are few studies of ethanol production by immobilized microorganisms using whey as the main substrate.Citation7,8,18 Efficient ethanol production is important from an economic viewpoint. For example, ethanologenic Escherichia coli strain FBR5 has advantages over yeast as a fermenting organism, since it is able to use various sugars to produce ethanol as the only end product from fermentation.Citation23-25
Bacterial hemoglobin (Vitreoscilla hemoglobin, VHb) is an effective tool for engineering of microorganisms for enhancement of growth and productivity.Citation26,28 Enhancement of ethanol production was achieved by expressing VHb in strain FBR5 (producing strain TS3).Citation29,30 Strains FBR5 and TS3 have been compared for their ethanol production efficiencies using different carbon sources including pure sugars and maize stover,Citation29,30 potato processing waste water,Citation31 sugar beet molasses, whey and whey powder,Citation32 and maize and potato processing waste.Citation33 Recently immobilization of strain TS3 was found to add an additional advantage to that afforded by VHb expression.Citation18
The purpose of the work reported here was to expand upon our previous workCitation18 and develop an efficient, reusable process for producing ethanol from whey powder containing medium using alginate immobilized strain TS3. It was found that the immobilized cells could be reused or stored for fairly extended times and retain both their ethanol producing ability and the increase in ethanol production coincident with VHb expression. The details of this work are described below.
Materials and methods
Strains
Ethanologenic VHb expressing E. coli strain TS3Citation33 had been generated from pLOI297-bearing strain FBR5.Citation25 LB plates supplemented with 8 % xylose were used for maintanance of these strains. Antibiotics (100 μg/mL ampicillin for strain FBR5, and 100 μg/mL ampicillin and 50 μg/mL streptomycin for TS3) were added to the growth media.Citation29
Growth medium
Cheese whey powder (CWP, containing 75 % total sugar/dry basis) was obtained from Bahcivan Gıda (Kırklareli, Turkey). Whey powder solution (128 g of whey powder suspended in 400 mL of distilled water) was autoclaved at 121°C for 15 min. The solution was cooled to room temperature and centrifuged at 15,000 xg for 10 min. The supernatant is “whey powder solution” (adapted from Ozmihci and Kargi).Citation9-12
Whey powder medium (WPM, pH 7.0) was prepared by combining 400 ml of whey powder solution, 100 mL of sterile yeast extract solution (5 g/L), 100 mL of CaCl2 (50 g/L),Citation34 and sterile distilled water to give 1 L final volume.
Immobilization method
The immobilization procedure was performed as described previously.Citation18 Briefly 6 % (w/v) alginate solution was prepared using sodium alginate powder (Sigma Aldrich, United Kingdom), sterilized by exposure to UV light for 30 min and diluted with sterilized distilled water.
One or 2 colonies of each strain were inoculated into growth medium and incubated at 37 °C at 180 rpm overnight. The bacterial cell suspension was prepared by centrifugation of each bacterial culture at 4000 xg for 15 min at 4 °C followed by resuspension of each pellet with 0.9 % NaCl (to give 107 cfu/mL). Following this 6 % (w/v) sodium alginate solution was mixed with each cell suspension in a 1:1 volume ratio. This mixture was dripped into 250 mL of sterile 0.1 M CaCl2 using a syringe (3P 21G 0.80×38 mm) and stirred continously at room temperature for 30 min.
To stabilize the texture of the beads, the beads were treated with 0.05 M sterile CaCl2 solution to harden for 1 h.Citation35 The mean diameter of the resulting Ca-alginate gel beads was approximately 3.0 mm. After washing the beads with sterile NaCl (0.9 %) they were stored in 2 different solutions as described below. The beads were rinsed with sterile distilled water before being used in fermentation experiments.
The numbers of viable cells in beads were determined by immersing the beads in 1 mL of phosphate buffer (1 M, pH 7.0) followed by disruption by vigorous mixing on a shaker for 1 min. Viable cell numbers were determined by plating serial dilutions on nutrient agar plates incubated at 37 °C for 24 h.Citation36 Cell leakage from the alginate beads was measured by making serial dilutions from the bead formation solution after 30 min (0.05 M CaCl2) and from both storage solutions after 12 h or at 15 d intervals during the 60 d storage at 4 °C, followed by plating on nutrient agar and incubation at 37 °C for 24 h.
Batch and repeated batch fermentations
Precultures and shake flask cultures for free and immobilized cells were performed as described previously.Citation18 Briefly, overnight cultures were prepared by inoculating one colony from the nutrient agar plates into 5 mL of WPM containing appropriate antibiotics followed by incubation at 37 °C at 180 rpm.
Immobilized cell batch cultures
Immobilized cell batch cultures were prepared by adding 40 beads containing immobilized cells (containing a total of 1 mL of bacterial preculture of OD600nm = 0.8) into 400 mL of medium in 500 mL flasks.Citation18 Each culture was capped with a rubber stopper pierced with a 22 gauge needle for CO2 exhaustion and incubated at 37°C and 180 rpm for 96 h. The ethanol, residual sugar and VHb levels were determined after 96 h of incubation. For VHb measurements, approximately 50 ml culture samples were centrifuged at 4000 xg for 10 min at 4 °C; the supernatants were used for ethanol and residual sugar measurements, while the pellets were used for VHb determinations.
Repeated batch cultures with non-stored immobilized cells
Repeated batch cultures were conducted smilarly to batch cultures. After each batch lasting 96 h, the beads were withdrawn, washed twice with sterile distilled water and resuspended in fresh medium to start a new batch fermentation. Including the initial batch runCitation18 a total of 15 repeated batch fermentations (over a 60 d period) were performed. Ethanol measurements were determined at the end of each 96 h incubation.
Repeated batch cultures with stored immobilized cells
Beads were stored at 4 °C for 60 d in 2 different solutions. Storage solution SS1 contained glucose (0.2 %) and yeast extract (0.2 %) (as described by Ghorbani et alCitation37). Storage solution SS2 contained only CaCl2 (2 %).Citation35 Beads stored in either storage solution were transferred to fresh medium for culturing at 15 d intervals after washing twice with sterile distilled water.
Analytical measurements
Initial and residual lactose contents and ethanol levels in fermentation medium were determined using an HPLC (Shimadzu 10A, Shimadzu, Colombia, MD) equipped with an autosampler (SIL-20AC), an exchange column and a refractive index detector (RID-10A). The samples were filtered through a 22 µm filter before injection. Sample volumes were adjusted to 20 µl and injected into a NH2 column (Interstil NH2 column, 5 µm, 4.6×250 mm, GL Sciences Inc., Shinjuku, Tokyo, Japan) operated at 25 °C and 0.5 mL/min with a mobile phase including acetonitrile (60 %) and ultra distilled water (40 %). The ethanol and sugar levels were determined by using standard curves correlating peak areas with concentrations of standard solutions. VHb expression levels were determined as described previously by dithionite treated-minus untreated difference spectra (Δε435–405 = 34 M−1 cm−1) of cell lysatesCitation38 and normalized to g wet weight of cells. Cell mass was also measured as OD at 600 nm of cell cultures using WPM as a blank and diluting cultures with WPM as necessary to keep measured ODs below 0.6; these measurements were used in the calculations of EtOH production per cell mass.
The fermentation efficiency (%) was determined according to Fernandes et al.Citation39 by dividing ethanol produced (g/L) by the theoretical maximum yield and multiplying by 100 (53.83 g of ethanol is the maximum theoretical yield that can be achieved from 100 g of lactose). The sugar conversion efficiency (%) was obtained by dividing ethanol production (g/L) by the initial total biomass (whey powder) concentration in the starting medium (102.56 g/L) and multipling by 100.Citation40
Statistical analysis
Student's t tests were performed using Excel 2016. The P values were calculated with one-tailed t tests, corresponding to the hypothesis that VHb would enhance ethanol production for TS3 compared with strain FBR5, which lacks VHb. Results were considered significant for P values less than or equal to 0.05.
Results
In previous work, we immobilized ethanologenic E. coli FBR5 and its VHb expressing derivative, TS3, and investigated the potential advantages of combining immobilized systems and VHb technology for ethanol production from cheese whey powder.Citation18 Using whey powder containing fermentation medium containing 8 % lactose and 0.5 % yeast extract in shake flask cultures, ethanol production by free cells was 2.6 % and 3.1 % for FBR5 and TS3, respectively, and by immobilized cells was 3.0 % and 3.5 % for FBR5 and TS3, respectively. Ethanol production reached 3.9 % for immobilized strain TS3 grown in a fermenter. From an industial point of view, then, an extension of this approach using immobilized VHb expressing strains in repeated batch fermentations could be a useful approach for efficient ethanol production. This methodology might also be useful with any food waste as an inexpensive carbon source in batch and continuous fermentations. In the work reported here, we have investigated that possibility.
Performance of repeated batch cultures over a period of 14 successive transfers
Immobilized FBR5 and TS3 strains that had been grown through one fermentation cycleCitation18 were transferred and reused for 14 successive additional 96 h batch fermentation runs. For all 14 runs strain TS3 had a substantial advantage in ethanol production over strain FBR5 (17 % to 119 % depending on the cycle number; ; Table S1). This was most pronounced in the final 3 runs, in which the ethanol production by strain FBR5 steadily decreased, while that of strain TS3 reamained fairly constant over the entire 14 cycles. The highest ethanol production (4.4 %, v/v) was achieved by strain TS3 in run 8. In these experiments both the ethanol fermentation and conversion efficiencies were also found to be higher with strain TS3 than strain FBR5 (ranging from 7 to 95 % greater for the fermentation efficiency and 17 to 115 % greater for the conversion efficiency over the course of the 14 cycles) ( and S1). Both of these paramenters were fairly constant for both strains throughout the course of the 14 cycles, except for a dropoff for strain FBR5 in the last 3 cycles (coincident with its decrease in ethanol production). Ethanol production per unit of biomass was also greater for immobilized TS3 than for immobilized FBR5 for all 14 cycles, the advantage ranging from 5 % to 93 % ( and S1). The VHb expression levels, which ranged from 7 to 16 nmol/g, were fairly consistent across all cycles, but quite low compared with those measured in previous studies with free TS3 cells,Citation31-33 and fairly comparable to those seen with immobilized TS3 cells used for only one cycle of growth.Citation18 () It is possible that immobilization may lower VHb expression or inhibit its extraction from cells in some as yet unknown way.
Figure 1. Ethanol production (v/v, %) by immobilized E. coli strains FBR5 and TS3 in WPM reused for 14 successive fermentation cycles following an initial (single) fermentation culture. Values are averages of 2 individual experiments; error bars indicate standard deviations (n-1). T-tests showed that the FBR5 and TS3 values were different, with P values between 0.002 and 0.05, for all batches except number 3 (P value of 0.07)
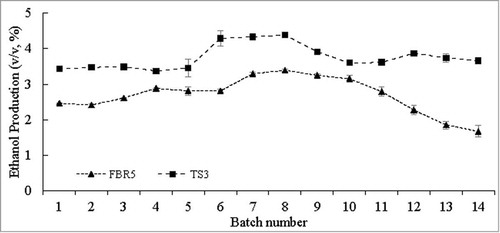
Table 1. Conversion efficiencies (%), fermentation efficiencies (%), ethanol production per unit of biomass (EtOH/OD), and VHb (nmol/g) expression (strain TS3 only) of immobilized E. coli strains FBR5 and TS3 grown in WPM for 14 successive fermentation cycles following an initial (single) fermentation culture. Values are averages of 2 individual experiments (standard deviations (n-1) are in parentheses). T-tests showed that the FBR5 and TS3 values were different, with P values between 0.001 and 0.05, for all comparisons except Fermentation Efficiency for batches number 5 and 10, and EtOH/OD for batches 5, 7, 8, and 11 (P values between 0.06 and 0.19)
Performance of immobilized cells stored for up to 60 d
Immobilized strain TS3 cells maintained their advantage at a nearly constant level over immobilized strain FBR5 after storage for 15, 30, 45 or 60 d (); the magnitude of the advantage was similar to that of unstored cells (). Ethanol production was nearly constant for both strains when stored in SS1 for up to 60 d, but storage in SS2 led to a modest lowering of ethanol levels for both strains that continued from day 15 through day 60. The data on sugar conversion efficiencies and ethanol fermentation efficiencies closely paralleled those of ethanol production ( and S2). This was true also of ethanol production per unit of biomass ( and S2). In any case, the immobilized cells and the advantage coincident with VHb expression were both very stable with regard to ethanol production. The VHb levels for strain TS3 () were very similar to those for unstored cells () and fairly constant throughout the full 60 d of storage, the one possible exception being a lower level at day 60, especially for storage solution SS1 ().
Figure 2. Ethanol production (v/v, %) by immobilized E. coli strains FBR5 and TS3 grown in a single fermentation in WPM following storage in either SS1 (a) or SS2 (b) solutions for either 15, 30, 45, or 60 d. Values are averages of 2 individual experiments; error bars indicate standard deviations (n-1). T-tests showed that the FBR5 and TS3 values were different, with P values between 0.01 and 0.05, for all storage conditions except 45 d in SS1 (P value of 0.06)
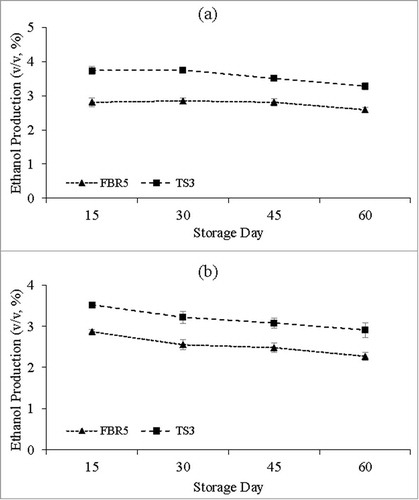
Table 2. Conversion efficiencies (%), fermentation efficiencies (%), ethanol production per unit of biomass (EtOH/OD), and VHb (nmol/g) expression (strain TS3 only) of immobilized E. coli strains FBR5 and TS3 reused once (growth in WPM) after storage in SS1 or SS2 solutions for either 15, 30, 45, or 60 d. Values are averages of 2 individual experiments (standard deviations (n-1) are in parentheses). T-tests showed that the FBR5 and TS3 values were different, with P values between 0.01 and 0.05, for all comparisons except Conversion Efficiency for SS1 storage for 45 d, Fermentation Efficiency for SS1 storage for 60 d, and, EtOH/OD for SS1 storage for 30 and 45 d and SS2 storage for 45 d (P values between 0.06 and 0.10)
Performance of stored immobilized cells used in repeated batch cultures over a period of 8 successive transfers
When the strains were stored in SS1 or SS2 solutions for 15 and 30 d and then used for 8 successive repeated batch fermentations, results similar to those seen above were obtained. In all cases strain TS3 outperformed strain FBR5 in ethanol production, conversion efficiency, fermentation efficiency, and ethanol produced per cell mass (; and S3). In all cases with both strains the levels of all 4 parameters were generally very stable through all 8 transfers. There was little difference in the performance of either strain stored in SS1 for either 15 or 30 d, but there was a decrease in ethanol production from 15 to 30 d of storage in SS2 for both strains. Although there was some variability in VHb levels in strain TS3, these did not show a discenible pattern, and the VHb levels were generally in line with those in the experments described above ().
Figure 3. Ethanol production (v/v, %) of immobilized E. coli strains FBR5 and TS3 for 8 successive fermentation cycles following storage in either SS1 (a, 15 d; c, 30 d) or SS2 (b, 15 d; d, 30 d) solutions; growth was in WPM. Values are averages of 2 individual experiments; error bars indicate standard deviations (n-1). T-tests showed that the FBR5 and TS3 values were different for all batches, with P values between 0.001 and 0.041
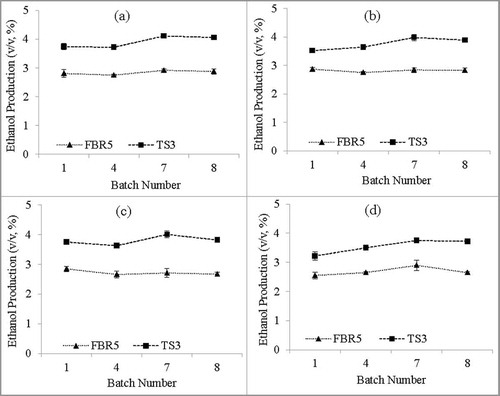
Table 3. Conversion efficiencies (%), fermentation efficiencies (%), ethanol production per unit of biomass (EtOH/OD), and VHb (nmol/g) expression (strain TS3 only) of immobilized E. coli strains FBR5 and TS3. Growth was in WPM for 8 successive transfers following storage in SS1 or SS2 solutions for either 15 or 30 d. Values are averages of 2 individual experiments (standard deviations (n-1) are in parentheses). T-tests showed that the FBR5 and TS3 values were different, with P values between 0.001 and 0.05, for all comparisons except Fermentation Efficiency for 30 d storage, batch number 4, and EtOH/OD for 30 d storage, batch number 4 (P values between 0.06 and 0.07)
Discussion
Robustness of enhancement of ethanol production by VHb-expression combined with immobilization
It is of interest that regardless of the number of successive transfers or the length of storage, all 4 of the parameters of ethanol production (ethanol production, ethanol conversion efficiency, fermentation efficiency, and ethanol production per unit of biomass) remained generally very constant for both strains. In addition, the absolute levels of ethanol in the fermentation broth achieved with the immobilized, VHb-expressing strain (about 4 %) exceeds the level needed for cost-effective recovery.Citation5,9 Also of note are our findings that for a wide variety of storage and repeated fermentation conditions, the advantage afforded by VHb expression regarding ethanol production, which has been seen in several studies using medium made with various wastes,Citation18,31-33 was substantial and very stable. Thus, as dicussed in more detail below, the approach described here may be of use in extending the use of VHb expressing ethanologenic E. coli to large-scale, practical applications using various low cost carbon sources derived from waste materials.
Medium composition
Here efficient ethanol production was achieved using medium containing only whey powder and yeast extract (as compared with an even richer medium). It has been reported that nitrogen sources such as yeast extract or peptone enhance ethanol production in yeast.Citation41,42 These nitrogen sources also enhance sugar utilization for increased ethanol yield.Citation42-43 Yeast extract also supplies cofactors such as biotin and riboflavin for enhancement of ethanol yield.Citation43 Nevertheless, the use of yeast extract adds significant expense to the medium used in our study, and further work might focus on finding lower cost substitutes that still provide the same benefits.
Immobilization of cells
Immobilization of organisms such as the yeast S. cerevisiaeCitation44-45 or bacteria like Zymomonas mobilisCitation46 and E. coli on different support materials for ethanol production from molassesCitation47 or whey powderCitation18 have been reported. The main advantages of immobilized cells compared with free cells for ethanol fermentation are in providing increased yield and viability for successive, repeated fermentations without the need to prepare fresh inocula each time reviewed by Kourkoutas et al.Citation16 Higher ethanol yields of immobilized versus free cells have been reportedCitation44,46 for yeast, and for ethanologenic E. coli.Citation18 Sar et al.Citation18 also demonstrated that the increase in ethanol production coincident with expression of VHb that occurs in media made with a wide variety of waste materials is maintained in immobilized cells, a result which was repeated in the work reported here.
Repeated batch fermentations and immobilized cell storage
The positive results we have seen in our system are similar to those seen by others using immobilized cells. Using S. cerevisiae immobilized on sweet sorgum stalks and using sweet sorgum juice as a carbon source, it was shown that immobilized yeast could be used for at least 8 successive batches with no reduction in ethanol production efficiencies.Citation48 Rattanapan et al.Citation49 immobilized S. cerevisiae on a thin-shell cocoon, and performed a 5 cycle repeated batch fermentation with enhanced stability and ethanol productivity. Santos and CruzCitation50 reported that ethanol production by Zymomonas mobilis immobilized on alginate for 12 24-h fermentation cycles increased between the fourth and the eighth cycles, while Ercan et al.Citation51 showed that for alginate immobilized S. cerevisiae the fermentation time was decreased and the immobilized cells were found to be reusable for 5 cycles in carob pod extract containing fermentation medium.
Regarding long-term storage, a previous study adapted S. cerevisiae SL 100 cells to a high (60 %) sucrose concentration during storage which resulted in higher ethanol levels compared with cells stored in water. The cells retained their metabolic activities and viabilities for more than a year when they were stored in water at 4 °C.Citation52 In the work reported here, neither long-term storage nor repeated batch fermentations appeared to greatly change VHb levels in strain TS3. As mentioned above, these levels are much lower than those reported for planktonic TS3 cells grown under similar conditions (90–323 nmol/g),Citation31-33 but similar to those previously reported for immobilized TS3 (6–63 nmol/g).Citation18 As mentioned above, the reasons for the low VHb levels in immobilized cells are not known, but even those low levels are not too different from the induced levels in native Vitreoscilla,Citation53 and so it is not surprising that they have a sizeable effect.
In our experiments, during the repetitious batch fermentations, no visible distruption of the alginate beads containing the cells was observed. It is possible that the inclusion of CaCl2 in our medium protected the beads from swelling.Citation54 The immobilization carriers are thought to protect the cells by providing stabilization of cell membranes and increased cell permeability Beg et al.Citation55 preventing the cells from entering the liquid medium while still allowing penetration of substrates. It is also possible that immobilized cells may not grow or grow slowly and become stable having minimized cellular activities,Citation56 and then regenerate with more vigorous growth at the beginning of each cycle upon exposure to fresh medium.
Conclusions
The work reported here extends out findings indicating that the combination of alginate immobilization and VHb expression for ethanologenic E. coli can be a useful strategy for bioethanol production using food processing wastes as a carbon source. Specifically, ethanol production levels and efficiencies as well as the advantage afforded by VHb expression could be maintained over extended periods of reuse of immobilized cells, storage of cells, or a combination of storage and reuse. These findings may be of use in large scale bioethanol production, particularly using food processing wastes, lignocellulosic wastes, or other inexpensive carbon sources.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KBIE_Supplemental_Materials_1303024.zip
Download Zip (34.8 KB)Acknowledgments
We would like to thank the Gebze Technical University (2016-A-13), Turkey and the Illinois Institute of Technology.
References
- Bangrak P, Limtong S, Phisalaphong M. Continuous ethanol production using immobilized yeast cells entrapped in loofa-reinforced alginate carriers. Braz J Microbiol 2011; 42:676-684; PMID:24031679; http://dx.doi.org/https://doi.org/10.1590/S1517-83822011000200032
- Ward OP, Singh A. Microbial Biotechnology in Agriculture and Aquaculture. Science Publishers New Hampshire 2005.
- Cardona CA, Sánchez ÓJ. Fuel ethanol production: Process design trends and integration opportunities. Bioresour Technol 2007; 98:2415-2457; PMID:17336061; http://dx.doi.org/https://doi.org/10.1016/j.biortech.2007.01.002
- Baldasso C, Barros TC, Tessaro IC. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011; 278:381-386; http://dx.doi.org/https://doi.org/10.1016/j.desal.2011.05.055
- Siso MIG. The biotechnological utilization of cheese whey: A review. Bioresour Technol 1996; 57:1-11; http://dx.doi.org/https://doi.org/10.1016/0960-8524(96)00036-3
- Ganzle MG, Haase G, Jelen P. Lactose: Crystallization, hydrolysis and value-added derivatives. Int Dairy J 2008; 18:685-694; http://dx.doi.org/https://doi.org/10.1016/j.idairyj.2008.03.003
- Das B, Sarkar S, Maiti S, Bhattacharjee S. Studies on production of ethanol from cheese whey using Kluyveromyces marxianus. Mater Today: Proceed 2016; 3:3253-3257; http://dx.doi.org/https://doi.org/10.1016/j.matpr.2016.10.006
- Guimarães PM, Teixeira JA, Domingues L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv 2010; 28:375-384; PMID:20153415; http://dx.doi.org/https://doi.org/10.1016/j.biotechadv.2010.02.002
- Ozmihci S, Kargi F. Effects of feed sugar concentration on continuous ethanol fermentation of cheese whey powder solution (CWP). Enzyme Microb Technol 2007a; 41:876-880; http://dx.doi.org/https://doi.org/10.1016/j.enzmictec.2007.07.015
- Ozmihci S, Kargi F. Ethanol fermentation of cheese whey powder solution by repeated fed-batch operation. Enzyme Microb Technol 2007b; 41:169-174; http://dx.doi.org/https://doi.org/10.1016/j.enzmictec.2006.12.016
- Ozmihci S, Kargi F. Comparison of yeast strains for batch ethanol fermentation of cheese-whey powder (CWP) solution. Lett Appl Microbiol 2007c; 44:602-606; http://dx.doi.org/https://doi.org/10.1111/j.1472-765X.2007.02132.x
- Ozmihci S. Kargi F Kinetics of batch ethanol fermentation of cheese-whey powder (CWP) solution as function of substrate and yeast concentrations. Bioresour Technol 2007d; 98:2978-2984; http://dx.doi.org/https://doi.org/10.1016/j.biortech.2006.10.005
- Ozmihci S, Kargi F. Ethanol production from cheese whey powder solution in a packed column bioreactor at different hydraulic residence times. Biochem Eng J 2008; 42:180-5; http://dx.doi.org/https://doi.org/10.1016/j.bej.2008.06.017
- Lin Y, Tanaka S. Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 2006; 69:627-642; PMID:16331454; http://dx.doi.org/https://doi.org/10.1007/s00253-005-0229-x
- Fasahati P, Woo HC, Liu JJ. Industrial-scale bioethanol production from brown algae: Effects of pretreatment processes on plant economics. Appl Energy 2015; 139:175-187; http://dx.doi.org/https://doi.org/10.1016/j.apenergy.2014.11.032
- Kourkoutas Y, Bekatorou A, Banat IM, Koutinas AA. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol 2004; 21:377-397; http://dx.doi.org/https://doi.org/10.1016/j.fm.2003.10.005
- Ivanova V, Petrova P, Hristov J. Application in the ethanol fermentation of immobilized yeast cells in matrix of alginate/magnetic nanoparticles, on chitosan-magnetite microparticles and cellulose-coated magnetic nanoparticles. Int Rev Chem Eng 2011; 3:289-299.
- Sar T, Stark BC, Akbas MY. Effective ethanol production from whey powder through immobilized E. coli expressing Vitreoscilla hemoglobin. Bioengineered 2016; 31:1-11; PMID:27579556; http://dx.doi.org/https://doi.org/10.1080/21655979.2016.1218581
- Kiyohara PK, Lima UA, Santos HS, Santos PS. Comparative study between yeasts immobilized on alumina beads and on membranes prepared by two routes. Braz J Microbiol 2003; 34:129-137; http://dx.doi.org/https://doi.org/10.1590/S1517-83822003000200008
- Najafpour G, Younesi H, Ismail KSK. Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour Technol 2004; 92(3):251-260; PMID:14766158; http://dx.doi.org/https://doi.org/10.1016/j.biortech.2003.09.009
- Phisalaphong M, Budiraharjo R, Bangrak P, Mongkolkajit J, Limtong S. Alginate-loofa as carrier matrix for ethanol production. J Biosci Bioeng 2007; 104:214-217; PMID:17964486; http://dx.doi.org/https://doi.org/10.1263/jbb.104.214
- Milessi TS, Antunes FA, Chandel AK, da Silva SS. Hemicellulosic ethanol production by immobilized cells of Scheffersomyces stipitis: Effect of cell concentration and stirring. Bioengineered 2015; 6:26-32; PMID:25488725; http://dx.doi.org/https://doi.org/10.4161/21655979.2014.983403
- Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF. Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 1987; 53:2420-2425; PMID:3322191
- Ingram LO, Conway T. Expression of different levels of ethanologenic enzymes from Zymomonas mobilis in recombinant strains of Escherichia coli. Appl Environ Microbiol 1988; 54:397-404; PMID:16347553
- Dien BS, Nichols NN, O'bryan PJ, Bothast RJ. Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl Microbiol Biotechnol 2000; 84–86:181-196.
- Stark BC, Dikshit KL, Pagilla KR. Recent advances in understanding the structure, function, and biotechnological usefulness of the hemoglobin from the bacterium Vitreoscilla. Biotechnol Lett 2011; 33:1705-1714; PMID:21603987; http://dx.doi.org/https://doi.org/10.1007/s10529-011-0621-9
- Stark BC, Dikshit KL, Pagilla KR. The biochemistry of Vitreoscilla hemoglobin. Computat Struct Biotechnol 2012; J 3:1-8.
- Stark BC, Pagilla KR, Dikshit KL. Recent applications of Vitreoscilla hemoglobin technology in bioproduct synthesis and bioremediation. Appl Microbiol Biotechnol 2015; 99:1627-1636; PMID:25575886; http://dx.doi.org/https://doi.org/10.1007/s00253-014-6350-y
- Sanny T, Arnaldos M, Kunkel SA, Pagilla KR, Stark BC. Engineering of ethanolic E. coli with the Vitreoscilla hemoglobin gene enhances ethanol production from both glucose and xylose. Appl Microbiol Biotechnol 2010; 88:1103-1112; PMID:20717665; http://dx.doi.org/https://doi.org/10.1007/s00253-010-2817-7
- Arnaldos M, Kunkel SA, Wang J, Pagilla KR, Stark BC. Vitreoscilla hemoglobin enhances ethanol production by Escherichia coli in a variety of growth media. Biomass Bioenerg 2012; 37:1-8; http://dx.doi.org/https://doi.org/10.1016/j.biombioe.2011.12.048
- Abanoz K, Stark BC, Akbas MY. Enhancement of ethanol production from potato-processing wastewater by engineering Escherichia coli using Vitreoscilla haemoglobin. Lett Appl Microbiol 2012; 55:436-443; PMID:22994421; http://dx.doi.org/https://doi.org/10.1111/lam.12000
- Akbas MY, Sar T, Ozcelik B. Improved ethanol production from cheese whey, whey powder, and sugar beet molasses by “Vitreoscilla hemoglobin expressing” Escherichia coli. Biosci Biotech Biochem 2014; 78:687-694; http://dx.doi.org/https://doi.org/10.1080/09168451.2014.896734
- Sumer F, Stark BC, Akbas MY. Efficient ethanol production from potato and corn processing industry waste using E. coli engineered to express Vitreoscilla haemoglobin. Environ Technol 2015; 36:2319-2327; PMID:25766084; http://dx.doi.org/https://doi.org/10.1080/09593330.2015.1026846
- Chang HN, Seong GH, Yoo IK, Park JK, Seo JH. Microencapsulation of recombinant Saccharomyces cerevisiae cells with invertase activity in liquid‐core alginate capsules. Biotechnol Bioeng 1996; 51:157-162; PMID:18624324; http://dx.doi.org/https://doi.org/10.1002/(SICI)1097-0290(19960720)51:2%3c157::AID-BIT4%3e3.0.CO;2-I
- Duarte JC, Rodrigues JAR, Moran PJS, Valença GP, Nunhez JR. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 2013; 3:2-8; PMID:23289832; http://dx.doi.org/https://doi.org/10.1186/2191-0855-3-31
- Chibata I, Tosa T, Satı T. Immobilized aspartase-containing microbial cells: preparation and enzymatic properties. Appl Microbiol 1974; 27:878-885; PMID:4208512
- Ghorbani F., Younesi H., Sari A.E., Najafpour G. Cane molasses fermentation for continuous ethanol production in an immobilized cells reactor by Saccharomyces cerevisiae. Renew Energy 2011; 36:503-509; http://dx.doi.org/https://doi.org/10.1016/j.renene.2010.07.016
- Liu CY, Webster DA. Spectral characterictics and interconversions of the reduced oxidized and oxygenated forms of purfied cytochrome o. J Biol Chem 1974; 240:4261-6.
- Fernandes MC, Ferro MD, Paulino AF, Mendes JA, Gravitis J, Evtuguin DV, Xavier AM. Enzymatic saccharification and bioethanol production from Cynara cardunculus pretreated by steam explosion. Bioresour Technol 2015; 186:309-315; PMID:25836040; http://dx.doi.org/https://doi.org/10.1016/j.biortech.2015.03.037
- Duangmanee T, Padmasiri SI, Simmons JJ, Raskin L, Sung S. Hydrogen production by anaerobic microbial communities exposed to repeated heat treatments. Water Environ Res 2007; 79:975-983; PMID:17910366; http://dx.doi.org/https://doi.org/10.2175/106143007X175762
- Pérez-Carrillo E, Cortés-Callejas ML, Sabillón-Galeas LE, Montalvo-Villarreal JL, Canizo JR, Moreno-Zepeda MG, Serna-Saldivar SO. Detrimental effect of increasing sugar concentrations on ethanol production from maize or decorticated sorghum mashes fermented with Saccharomyces cerevisiae or Zymomonas mobilis. Biotechnol Lett 2011; 33:301-307; PMID:20972698; http://dx.doi.org/https://doi.org/10.1007/s10529-010-0448-9
- Harde SM, Bankar SB, Ojamo H, Granström T, Singhal RS, Survase SA. Continuous lignocellulosic ethanol production using Coleus forskohlii root hydrolysate. Fuel 2014; 126:77-84; http://dx.doi.org/https://doi.org/10.1016/j.fuel.2014.02.046
- Ortiz‐Muñiz B, Carvajal-Zarrabal O, Torrestiana-Sanchez B. Aguilar-Uscanga MG Kinetic study on ethanol production using Saccharomyces cerevisiae ITV-01 yeast isolated from sugar cane molasses. J Chem Technol Biotechnol 2010; 85:1361-1367; http://dx.doi.org/https://doi.org/10.1002/jctb.2441
- Razmovski R, Vucurovic V. Ethanol production from sugar beet molasses by S. cerevisiae entrapped in an alginate-maize stem ground tissue matrix. Enzyme Microb Technol 2011; 48:378-385; PMID:22112953; http://dx.doi.org/https://doi.org/10.1016/j.enzmictec.2010.12.015
- Sembiring KC, Mulyani H, Fitria AI, Dahnum D, Sudiyani Y. Rice flour and white glutinous rice flour for use on immobilization of yeast cell in ethanol production. Energy Procedia 2014; 32:99-104; http://dx.doi.org/https://doi.org/10.1016/j.egypro.2013.05.013
- Behera S, Kar S, Mohanty RC, Ray RC. Comparative study of bio-ethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomyces cerevisiae cells immobilized in agar agar and Ca-alginate matrices. Appl Energy 2010; 87:96-100; http://dx.doi.org/https://doi.org/10.1016/j.apenergy.2009.05.030
- Panesar PS, Marwaha SS, Raj R. Screening of Zymomonas mobilis strain for ethanol production from molasses Indian. J Microbiol 2001; 41:187-198.
- Ariyajaroenwong P, Laopaiboon P, Jaisil P. Laopaiboon L Repeated-batch ethanol production from sweet sorghum juice by Saccharomyces cerevisiae immobilized on sweet sorghum stalks. Energies 2012; 5:1215-1228; http://dx.doi.org/https://doi.org/10.3390/en5041215
- Rattanapan A, Limtong S, Phisalaphong M. Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl Energy 2011; 88:4400-4404; http://dx.doi.org/https://doi.org/10.1016/j.apenergy.2011.05.020
- Santos VAQ, Cruz CHG. Ethanol and levan production by sequential bath using Zymomonas mobilis immobilized on alginate and chitosan beads. Acta Sci-Technol 2016; 38:263-271; http://dx.doi.org/https://doi.org/10.4025/actascitechnol.v38i3.27646
- Ercan Y, Irfan T, Mustafa K. Optimization of ethanol production from carob pod extract using immobilized Saccharomyces cerevisiae cells in a stirred tank bioreactor. Bioresour Technol 2013; 135:365-371; PMID:23010212; http://dx.doi.org/https://doi.org/10.1016/j.biortech.2012.09.006
- Melzoch K, Rychtera M, Hábová V. Effect of immobilization upon the properties and behaviour of Saccharomyces cerevisiae cells. J Biotechnol 1994; 32(1):59-65; http://dx.doi.org/https://doi.org/10.1016/0168-1656(94)90120-1
- Dikshit KL, Webster DA. Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 1988; 70:377-386; PMID:2850971; http://dx.doi.org/https://doi.org/10.1016/0378-1119(88)90209-0
- Cheong SH, Park HK, Kim BS, Chang HN. Microencapsulation of yeast cells in the calcium alginate membrane. Biotechnol Tech 1993; 7:879-884; http://dx.doi.org/https://doi.org/10.1007/BF00156366
- Beg QK, Bhushan B, Kapoor M, Andoldal GS. Prodution and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11-3. J Ind Microbiol Biotechnol 2000; 24:396-402; http://dx.doi.org/https://doi.org/10.1038/sj.jim.7000010
- Lú Chau T, Guillan A, Roca E, Nunez MJ. Lema JM Enhancement of plasmid stability and enzymatic expression by immobilising recombinant Saccharomyces cerevisiae. Biotechnol Lett 2000; 22:1247-1250; http://dx.doi.org/https://doi.org/10.1023/A:1005669618337
