ABSTRACT
Worldwide, millions of patients are affected annually by healthcare-associated infection (HCAI), impacting up to 80,000 patients in European Hospitals on any given day. This represents not only public health risk, but also an economic burden.
Complementing routine hand hygiene practices, cleaning and disinfection, antimicrobial coatings hold promise based, in essence, on the application of materials and chemicals with persistent bactericidal or –static properties onto surfaces or in textiles used in healthcare environments.
The focus of considerable commercial investment and academic research energies, such antimicrobial coating-based approaches are widely believed to have potential in reduction of microbial numbers on surfaces in clinical settings. This belief exists despite definitive evidence as to their efficacy and is based somewhat on positive studies involving, for example, copper, silver or gold ions, titanium or organosilane, albeit under laboratory conditions. The literature describes successful delay and/or prevention of recontamination following conventional cleaning and disinfection by problematic microbes such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin resistant enterococci (VRE), among others. However, there is a scarcity of studies assessing antimicrobial surfaces other than copper in the clinical environment, and a complete lack of published data regarding the successful implementation of these materials on clinically significant outcomes (including HCAI).
Through its Cooperation in Science and Technology program (COST), the European Commission has funded a 4-year initiative to establish a network of stakeholders involved in development, regulation and use of novel anti-microbial coatings for prevention of HCAI. The network (AMiCI) comprises participants of more than 60 universities, research institutes and companies across 29 European countries and, to-date, represents the most comprehensive consortium targeting use of these emergent technologies in healthcare settings.
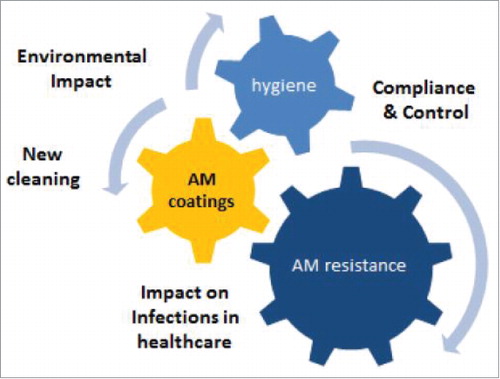
More specifically, the network will prioritise coordinated research on the effects (both positive and negative) of antimicrobial coatings in healthcare sectors; know-how regarding availability and mechanisms of action of (nano)-coatings; possible adverse effects of such materials (e.g., potential emergence of microbial resistance or emission of toxic agents into the environment); standardised performance assessments for antimicrobial coatings; identification and dissemination of best practices by hospitals, other clinical facilities, regulators and manufacturers.
Introduction
Healthcare-associated infections (HCAI), also termed nosocomial infections, are complications of healthcare that result in elevated patient morbidity and mortality.Citation1 HCAI increase healthcare costs for patients, hospitals and insurers due to extended hospitalization and associated care. There is also a psychological burden placed on patients, their carers and families, in addition to opportunity costs subsequent to patients' and their carers' inability to work, attend school, etc., while unanticipated reduction of hospital capacity impacts the efficiency of healthcare.Citation2,3 It has been estimated that, in the US alone, HCAI affects approximately 2 million patients annually of whom approximately 90,000 die. This is associated with an annual cost estimated to range from US$ 28 billion to 45 billion.Citation4 Similarly, in the European Union, the European Center for Disease Prevention and Control (ECDC) advises that approximately 4.1 million acute care patients acquire a HCAI annually, with 37,000 deaths attributed directly to HCAI.Citation5
There is a dichotomy in awareness of HCAI, with much media and public focus on Gram-positive Staphylococcus aureus-associated outbreaks, while the reality is that Gram-negative species currently represent the greater risk. More specifically, monitoring of outbreak incidence and individual cases has shown that while, for instance, the prevalence of meticillin-resistant Staphylococcus aureus (MRSA) is stabilizing and even decreasing in some European countries, other HCAI are increasing (e.g., Escherichia coli and Klebsiella pneumoniae).Citation5 Such monitoring, including pan-European surveillance, has been expanded to encompass long-term care facilities (LTCF) in addition to hospitals.Citation6 Further to that, more comprehensive data are emerging across Europe, such as in Ireland where a recent national median HCAI prevalence of 4.2% in long-term care facilities was reported albeit not consistently.Citation7,8
Incidence of HCAI, however, are generally considered preventable.Citation9 Although hand hygiene is widely regarded as the most effective preventative measure for health care workers, there is a recognized need for novel methods in addition to appropriate cleaning, use of disinfectants and antibiotics.Citation10,11 One such approach involves antimicrobial (nano)-coatings (AMC),Citation12 in which integrated active ingredients are responsible for the elimination of microorganisms that come into contact with treated surfaces. There has been an emphasis on bactericidal applications for use in healthcare settings, and a developing confidence in the potential for such coatings albeit based somewhat on studies involving, for example, silver or gold ions, titanium or organosilane performed under laboratory conditions.Citation13 However, this confidence is bolstered by reports describing successful delay and/or prevention of recontamination following conventional cleaning and disinfection by problematic microbes such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin resistant enterococci (VRE), among others.Citation14-Citation17
More broadly, however, chemical strategies and technologies for antimicrobial coatings have been described that utilize active eluting agents (e.g., ions or nanoparticles of silver, copper, zinc, or antibiotics, chloride, phytochemicals, iodine, ammonium etc.), immobilized molecules that become active upon contact (e.g., quaternary ammonium polymers or peptides), or light-activated molecules (e.g., TiO2 or photosensitizers).Citation18-Citation20 A 2016 comprehensive systematic review reported a paucity of studies evaluating non-copper antimicrobial surfaces in clinical environments, and a total lack of peer-reviewed data relating to successful implementation of materials other than copper on clinically relevant outcomes (including HCAI).Citation21 However, since that review was written, Molling et al. have reported a dominance of nanosilver in nanoparticle-based coatings, and associated adequate in situ performance.Citation13 Furthermore, copper touch surfaces in Finnish facilities such as hospital patient rooms and kindergartens lowered total bacterial counts and reduced occurrence of Staphylococcus aureus when compared with non-copper touch surfaces.Citation22 The application of copper and/or its alloys was also reported to reduce bacterial numbers on often touched surfaces in intensive care units.Citation23 A positive effect of AMC on reduction of HAIs was also shown by von Dessauer et al. whereby HAI rates of 10.6 versus 13.0 per 1,000 patient days were observed for copper- and non-copper-“exposed’ patients, respectively, albeit that the difference was not statistically significant.Citation24 In an alternate approach, recognizing that bacterial adhesion can be critical in successful host colonization or in the external environment,Citation25 the antimicrobial effects of coatings with controlled nanotopography are being developed which aim to repel bacterial adhesion based on physical properties.Citation26,27
But, prudence is needed. The introduction of (nano)-coatings with novel active components (e.g., nanosilver), some of which many be affected by varying end-user cleaning methods, may cause emission of bioactive agents into the environment and thereby facilitate potential exposure of humans, livestock and microorganisms to low concentrations of these. Indeed, the biocidal products are intrinsically toxic, i.e. showharmful effects toward different types of cells/organisms and, thus, not only affect the target organisms (such as pathogenic bacteria) but can be harmful also to humans and also to all types of environmental organisms.Citation28,29 As described in the One Health Initiative (http://www.onehealthinitiative.com), these agents (e.g., AgNP, Ag+, CuNP, and TiO2) may have potential for impact on organisms living in water and soil compartments, specifically. In addition, the slow infusion of active ingredients may induce antimicrobial resistances (AMR) that differ from antibiotic-driven mechanisms.Citation30 Indeed, several genes encoding enzyme synthesis responsible for resistance to metallic and other compounds used in AMCs have been identified within hospital environments with some described as plasmid-borne or readily transferable.Citation31-Citation34
The widespread introduction of such coatings clearly needs to be subject to expert risk-benefit analyses and is subject to significant scrutiny at the regulatory level; a lack of clear evidence of benefits and a poor understanding of risks may result in the loss of potentially valuable intervention strategies remaining available to the healthcare sector should such material not be approved by regulatory bodies.
Cost actions
COST Actions are a flexible, fast, effective and efficient networking instrument for researchers, engineers and scholars to cooperate and coordinate nationally funded research activities. COST Actions allow European researchers to jointly develop their own ideas in any science and technology field (http://www.cost.eu/COST_Actions). More specifically, COST Actions are bottom-up science and technology networks, open to researchers and stakeholders with a duration of 4 y. They utilize a range of networking tools, such as workshops, conferences, training schools, short-term scientific missions (STSMs), and dissemination activities.
AMiCI cost action (CA15114)
The network (http://www.cost.eu/COST_Actions/ca/CA15114) represents collaboration of stakeholders from different countries and disciplines, including third level institutes and universities, antimicrobial coating producers and processors, and organizations responsible for compliance with international standards. This network complements the closely related COST Action iPROMEDAI (http://www.cost.eu/COST_Actions/tdp/TD1305) the activities of which concentrate on timed presentation and localized delivery of antimicrobial compounds in medical devices, such as catheters, to reduce the incidence of device-associated infections due to biofilm formation.
AMiCI focuses specifically on generating new capabilities related to:
systematic, international coordinated research on the effects (both positive and negative) of antimicrobial coatings in healthcare or other sectors;
know-how regarding the availability and use of different materials, mechanisms of action of (nano)-coatings and the desired use in different applications, procedures and products;
information about the possible adverse effects of such materials, e.g., the potential induction of new resistance mechanisms in bacteria or emission of toxic agents into the environment;
standard performance assessments for antimicrobial coatings, applicable in laboratory settings and, thereby, direct comparison of different coatings from different producers;
standard performance assessments to determine functionality of coatings in a(n) (extreme) test condition, field tests or benchmark methods to assess the efficacy in field conditions;
communication and/or publication of best practices by hospitals, other clinical facilities, regulators or product suppliers;
development of safe by design concepts allowing the identification of risks and uncertainties during innovation projects and enabling their seamless integration into the innovation process. In the context of AMCs, safe by design is intended to include the active antimicrobial ingredient (and other constituents of the coatings) whether integrated into a finished coating product or eluted from it such that its potential intended, unintended and/or unforeseen effects can be monitored, understood and mitigated, where possible and appropriate.
In doing so, AMiCI recognizes the critical importance of compliance with the European Biocidal Products Regulation (BPR, Regulation (EU) 528/2012), which concerns the placing on the market and use of biocidal products, which are used to protect humans, animals, materials or articles against harmful organisms, like pests or bacteria, by the action of the active substances contained in the biocidal product ().
The program
The AMiCI work has been designed to allow focus across 4 y of planned activities on 5 specific, interconnected themes.
Theme one: Antimicrobial materials that are safe by design
When developing antimicrobial coatings, insight regarding the site of use is crucial. The nature of the surface (e.g., clothing, movable door components, static surfaces, etc) must be considered. For example, the same antimicrobial coating if applied inappropriately of differing surfaces of differing characteristics may lead to a release of biocides due to incomplete chemical binding or the influence of UV radiation that is required to activate the relevant bioactive antimicrobial property. Therefore, this theme targets the generation of enhanced understanding of antimicrobial mechanisms and how these may be impacted through construction of physical infrastructure, application to surfaces, production processes etc such that available coatings can be adapted or new coatings developed to meet European Biocidal Product requirements (BPR).
Theme two: Performance assessment (laboratory – field – benchmark)
Current and new AMCs will enter the European market only if their efficacy is proven in appropriate field-testing, and when they comply with the BPR. Testing AMCs in a healthcare environment has ethical and practical challenges, and it is expected that the site of intervention (textiles, cleaning, surfaces) will have considerable influence on the overall ecosystem of the care environment. Therefore, it is important to develop laboratory, field and benchmark tests that can help predict the effectiveness of coating components as part of the cleaning processes used in healthcare. In addition, it is necessary to predict the overall efficiency of such interventions on bacterial contamination in healthcare facilities. This is considered critical as, although the efficacy and efficiency may be predicted and even demonstrated, the perceptions of healthcare professionals may continue to favor disinfection before adoption of new preventative technologies.
Further, it is anticipated that the continued implementation of the BPR (and other regulations elsewhere in the world) will result in significant reduction of available coatings in the market (access to global markets is often an important factor when a company considers the development of new coatings and systems). This combination of events signals the reasonably urgent need for guidelines by which healthcare facilities, as well as manufacturers, can produce and assess antimicrobial products. Part of such guidelines ought to be relatively simple, quick and reliable tests that can evaluate such coatings, their application and durability. AMiCI participants in this theme will work toward the design of suitable tests.
Theme three: Adverse effects / risk – benefit analyses
Introduction of antimicrobial (nano)coatings into widespread use has the potential to cause emission into the environment of bioactive components such as nanosilver, copper and/or zinc. It is, therefore, essential that the impact, following exposure of these active agents, on humans, livestock and other animals, and microorganisms be assessed. Given the context of their use, potential induction of antimicrobial resistance is of particular importance. However, comprehensive toxicological studies are also needed to allow risk - benefit analyses. AMiCI will attempt to generate the analytical data required to advise regulatory agencies, in addition to working with producers of antimicrobial coatings on the development of safe-by-design products.
Theme four: The new cleaning
For decades, cleaning and hygiene practices in healthcare have been conservative, with little variation in detergent and disinfectant technology or use. Innovation is now required due to both organic pollution risks and emergence of multidrug-resistant microbes. The introduction of antimicrobial coatings may represent a step-change. However, along with these products will come new practices, new environmental monitoring and training requirements. AMiCI will compare efficacy of cleaning practices in the context of new coating technologies across varying sites, and will generate guidelines appropriate for healthcare facilities. In doing so, due consideration will be paid to the fact that while evidence points to near patient surfaces having inadequate cleaning, mechanical cleaning processes in addition to chemical interactions may reduce the efficacy of AMCs to sub-inhibitory levels and result in generation of resistance, such as has been demonstrated with liquid disinfectants.Citation35,36 The possibility of over-reliance on AMCs and the potential compromising of adequate resourcing and appropriate cleaning procedures will also be explored.
Theme five: Communication and dissemination
Antimicrobial coatings are a relatively new technological solution in the battle against healthcare acquired infections. At present, potential users have little knowledge of their advantages, and associated challenges. AMiCI will coordinate communication of credible information regarding these innovations in understandable and accessible formats through social media, websites, conferences, trade fairs, patient and professional fora, and scientific publications. The information will be tailored to the perspectives of inventors and entrepreneurs; academic researchers; manufacturers; distributors; commercial, clinical, biocide and consumer affairs regulators; medicines agencies; clinical microbiologists; attending physicians; healthcare facility managers and procurement officers; environmental monitoring specialists and environmental protection agencies; hygiene companies; and, of course, patients and their carers.
Conclusion
Antimicrobial coatings represent innovation in response to an impending healthcare challenge that is unprecedented. Antimicrobial drugs of last resort are beginning to fail, and outbreaks of multidrug-resistant bacteria are increasingly reported.Citation37-Citation40 This is despite the enhanced resourcing of infection prevention and control teams and greater emphasis on antimicrobial stewardship, albeit sometimes retrospectively. As healthcare costs constitute significant percentages of national budgets, and capital investments in physical infrastructure and staff recruitment attempt to cope with aging populations and higher levels of patient throughput than was previously anticipated, there are opportunities for the deploying of new technologies to delay or halt generation of antimicrobial resistance.
Initiatives such as One Health are important at national and international scale. However, within individual healthcare systems or local healthcare facilities, there will be opportunities to instigate changes that will be impactful. These will relate to the integration of medical, management and hygiene staff into teams that understand and deal collectively with infection prevention and control and with outbreaks or affected patients, both from clinical and human perspectives; there will also be decisions made regarding the appropriateness of adoption or otherwise of new technologies, such as antimicrobial coatings.
AMiCI aims to aid these decisions through provision of credible information that recognizes the sometimes aligned, but sometimes disparate, perspectives of stakeholders and target groups.
Disclosure of potential conflict of interests
No potential conflicts of interests were disclosed.
Funding
This article derives from COST Action AMiCI (CA15114), supported by COST (European Cooperation in Science and Technology).
References
- World Health Organization. (2009) WHO guidelines for hand hygiene in health care, Geneva, Switzerland, World Health Organization Press.
- World Health Organisation. (2011) WHO Report on the burden of endemic health care-associated infection worldwide, Geneva: World Health Organisation.
- Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011; 377(9761):228-41; PMID:21146207; https://doi.org/10.1016/S0140-6736(10)61458-4
- Stone P. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon and Outcomes Res 2009; 9(5):417-22; https://doi.org/10.1586/erp.09.53
- European Centre for Disease Prevention and Control. (2013) Summary: Point prevalence survey of healthcare-associated infections and antimicrobial use in European hospitals 2011–2012, Stockholm: European Centre for Disease Prevention and Control.
- European Centre for Disease Prevention and Control. (2014) Point prevalence survey of healthcare-associated infections and antimicrobial use in European long-term care facilities. April-May 2013, Stockholm: European Centre for Disease Prevention and Control.
- Health Protection Surveillance Centre. (2014) Point Prevalence Survey of Healthcare‐Associated Infections & Antimicrobial Use in Long‐Term Care Facilities (HALT): May 2013, Republic of Ireland: National Report, Dublin: Health Protection Surveillance Centre.
- O'Connor C, Kiernan MG, Finnegan C, Powell J, Power L, O'Connell NH, Dunne CP. Colonisation with extended-spectrum beta-lactamase (ESBL) not detected in a prevalence study. Ir J Med Sci 2016; Online first 24th September. https://doi.org/10.1007/s11845-016-1505-8; https://www.ncbi.nlm.nih.gov/pubmed/27761798
- Kingston L, O'Connell NH, Dunne CP. Hand hygiene-related clinical trials reported since 2010: a systematic review. J Hosp Infect 2016; 92(4):309-20; PMID:26853369; https://doi.org/10.1016/j.jhin.2015.11.012
- Cole M. Exploring the hand hygiene competence of student nurses: A case of flawed self-assessment. Nurse Educ Today 2009; 29(4):380-8; https://doi.org/10.1016/j.nedt.2008.10.010
- Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, Perneger TV. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet 2000; 356:1307-12.
- Page K, Wilson M, Parkin IP. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J Material Chem 2009; 19:3819-31; https://doi.org/10.1039/b818698g
- Molling JW, Seezink JW, Teunissen BE, Muijrers-Chen I, Borm PJ. Antibacterial activity of a panel of commercially available (nanoparticle) coatings in Europe. Nanotechnol Sci Appl 2014; 7:97-104; PMID:25404853; https://doi.org/10.2147/NSA.S70782
- Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006; 6:130; PMID:16914034; https://doi.org/10.1186/1471-2334-6-130
- Boyce JM1, Havill NL, Otter JA, McDonald LC, Adams NM, Cooper T, Thompson A, Wiggs L, Killgore G, Tauman A, et al. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol. 2008; 29:723-9; PMID:18636950; https://doi.org/10.1086/589906
- Morter S, Bennett G, Fish J, Richards J, Allen DJ, Nawaz S, Iturriza-Gómara M, Brolly S, Gray J. Norovirus in the hospital setting: virus introduction and spread within the hospital environment. J Hosp Infect 2011; 77:106-12; https://doi.org/10.1016/j.jhin.2010.09.035
- Otter JA, Cummins M, Ahmad F, van Tonder C, Drabu YJ. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J Hosp Infect 2007; 67:182-8; https://doi.org/10.1016/j.jhin.2007.07.019
- Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 2011; 77(5):1541-7; PMID:21193661; https://doi.org/10.1128/AEM.02766-10
- Chen X, Hirt H, Li Y, Gorr SU, Aparicio C. Antimicrobial GL13K peptide coatings killed and ruptured the wall of Streptococcus gordonii and prevented formation and growth of biofilms. PLoS One 2014; 9(11):e111579; PMID:25372402; https://doi.org/10.1371/journal.pone.0111579
- Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl 2014; 44:278-284; https://doi.org/10.1016/j.msec.2014.08.031
- Muller MP, MacDougall C, Lim M, et al. Antimicrobial surfaces to prevent healthcare-associated infections: a systematic review. J Hosp Infect 2016; 92:7-13; https://doi.org/10.1016/j.jhin.2015.09.008
- Inkinen J, Mäkinen R, Keinänen-Toivola MM, Nordström K, Ahonen M. Copper as an antibacterial material in different facilities. Lett Appl Microbiol 2017; 64(1):19-26; PMID:27718259; https://doi.org/10.1111/lam.12680
- Schmidt MG, von Dessauer B, Benavente C, Benadof D, Cifuentes P, Elgueta A, Duran C, Navarrete MS. Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a pediatric intensive care unit. Am J Infect Control 2016; 44(2):203-9; https://doi.org/10.1016/j.ajic.2015.09.008
- von Dessauer B, Navarrete MS, Benadof D, Benavente C, Schmidt MG. Potential effectiveness of copper surfaces in reducing health care-associated infection rates in a pediatric intensive and intermediate care unit: A nonrandomized controlled trial. Am J Infect Control 2016; 44(8):e133-9; https://doi.org/10.1016/j.ajic.2016.03.053
- Von Wright A, Vilpponen-Salmela T, Pagès Llopis M, Collins K, Kiely B, Shanahan F, Dunne C. The survival and colonic adhesion of Bifidobacterium longum infantis in patients with ulcerative colitis. Int Dairy J 2002; 12:197-200; https://doi.org/10.1016/S0958-6946(01)00162-5
- Svensson S, Suska F, Emanuelsson L, Palmquist A, Norlindh B, Trobos M, Bäckros H, Persson L, Rydja G, Ohrlander M, Lyvén B, Lausmaa J, Thomsen P. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomedicine 2013; 9(7):1048-56; https://doi.org/10.1016/j.nano.2013.04.009
- Hasan J, Raj S, Yadav L, Chatterjee K. Engineering a nanostructured “super surface” with superhydrophobic and superkilling properties. RSC Adv 2015; 5:44953-9; https://doi.org/10.1039/C5RA05206H
- Suppi S, Kasemets K, Ivask A, Künnis-Beres K, Sihtmäe M, Kurvet I, Aruoja V, Kahru A. A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. J Hazard Mater 2015; 286:75-84; https://doi.org/10.1016/j.jhazmat.2014.12.027
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 2013; 87:1181-200; PMID:23728526; https://doi.org/10.1007/s00204-013-1079-4
- González-Zorn B, Escudero JA. Ecology of antimicrobial resistance: humans, animals, food and environment. Int Microbiol 2012; 15(3):101-9; https://doi.org/10.2436/20.1501.01.163
- Argudín MA, Lauzat B, Kraushaar B, Alba P, Agerso Y, Cavaco L, Butaye P, Porrero MC, Battisti A, Tenhagen BA, et al. Heavy metal and disinfectant resistance genes among livestock-associated methicillin-resistant Staphylococcus aureus isolates. Vet Microbiol 2016; 191:88-95; https://doi.org/10.1016/j.vetmic.2016.06.004
- Hobman JL, Crossman LC. Bacterial antimicrobial metal ion resistance. J Med Microbiol 2015; 64:471-97; https://doi.org/10.1099/jmm.0.023036-0
- Lima de Silva AA, de Carvalho MAR, de Souza SAL, Dias PMT, da Silva Filho RG, de Meirelles Saramago CS, de Melo Bento CA, Hofer E. Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz J Microbiol 2012; 43:1620-31; PMID:24031994; https://doi.org/10.1590/S1517-83822012000400047
- Randall CP, Gupta A, Jackson N, Busse D, O'Neill AJ. Silver resistance in Gram-negative bacteria: a dissection of endogenous and exogenous mechanisms. J Antimicrob Chemother 2015; 70(4):1037-46; PMID:25567964
- Carling PC, Parry MM, Rupp ME, Po JL, Dick B, Von Beheren S. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect Control Hosp Epidemiol 2008; 29:1035-41; PMID:18851687; https://doi.org/10.1086/591940
- Rossi-Fedele G, Doğramaci EJ, Guastalli AR, Steier L, de Figueiredo JA. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J Endod 2012; 38(4):426-31; https://doi.org/10.1016/j.joen.2012.01.006
- O'Connor C, Cormican M, Boo TW, McGrath E, Slevin B, O'Gorman A, Commane M, Mahony S, O'Donovan E, Powell J, Monahan R, Finnegan C, Kiernan MG, Coffey JC, Power L, O'Connell NH, Dunne CP. An Irish outbreak of New Delhi metallo-beta-lactamase (NDM)-1 carbapenemase-producing Enterobacteriaceae: increasing but unrecognized prevalence. J Hosp Infect 2016; 94(4):351-7; https://doi.org/10.1016/j.jhin.2016.08.005
- O'Connor C, O'Connell NH, Commane M, O'Donovan E, Power L, Dunne CP. Limerick: forever associated with five lines of rhyme or infamous for irrepressible carbapenemase-producing Enterobacteriaceae for all time? J Hosp Infect 2016; 93(2):155-6; https://doi.org/10.1016/j.jhin.2016.03.008
- O'Connor C, Powell J, Finnegan C, O'Gorman A, Barrett S, Hopkins KL, Pichon B, Hill R, Power L, Woodford N, Coffey JC, Kearns A, O'Connell NH, Dunne C. Incidence, management and outcomes of the first cfr-mediated linezolid-resistant Staphylococcus epidermidis outbreak in a tertiary referral centre in the Republic of Ireland. J Hosp Infect 2015; 90(4):316-21; https://doi.org/10.1016/j.jhin.2014.12.013
- O'Connell NH, Power L, O'Gorman A, O'Connor C, Dunne CP. Against the onslaught of endemic carbapenemase-producing Klebsiella pneumoniae, the war is being lost on the Irish Front. J Hosp Infect 2014; 87(4):247-8; PMID:25027225; https://doi.org/10.1016/j.jhin.2014.05.011