ABSTRACT
The deep-sea bacterium strain FA13 was isolated from the sediment of the South Atlantic Ocean and identified as Bacillus circulans based on 16S ribosomal DNA sequence. Through liquid fermentation with five media, the cell-free supernatant fermented with ISP2 showed the highest inhibition activities against mycelial growth of Aspergillus parasiticus mutant strain NFRI-95 and accumulation of norsolorinic acid, a precursor for aflatoxin production. Based on ISP2, uniform design was used to optimize medium formula and fermentation conditions. After optimization, the inhibition efficacy of the 20-time diluted supernatant against A. parasiticus NFRI-95 mycelial growth and aflatoxin production was increased from 0–23.1% to 100%. Moreover, compared to the original protocol, medium cost and fermentation temperature were significantly reduced, and dependence on seawater was completely relieved, thus preventing the fermentor from corrosion. This is the first report of a deep-sea microorganism which can inhibit A. parasiticus NFRI-95 mycelial growth and aflatoxin production.
Introduction
Aflatoxins are highly toxic and carcinogenic secondary metabolites that produced by certain strains of Aspergillus flavus and Aspergillus parasiticus [Citation1]. They are one of the major mycotoxins that contaminate grains, foods and feeds, and causes substantial economic losses worldwide [Citation2,Citation3]. Therefore, control of aflatoxin contamination has always been the highlights of researches. Compared with physical and chemical methods, biological control using bacteria, yeast and non-aflatoxigenic strains of A. flavus has become a non-toxic and efficient alternative [Citation4–Citation8].
The special environment of the deep sea has endowed deep-sea microorganisms with unique physiological structures and metabolic systems, producing diverse metabolites with novel structures and various functions [Citation9]. At present, many studies on deep-sea microorganisms and their bioactive metabolites have been reported [Citation10]. Among them, some deep-sea bacteria and their novel metabolites could effectively suppress terrestrial fungal phytopathogens, such as Fusarium, Rhizoctonia, Valsa and Phytophthora [Citation11–Citation14].
Currently, there are only few reports on offshore microorganisms that inhibit aflatoxin production [Citation15–Citation18]. Researches on deep-sea microorganisms that inhibit aflatoxin production have not yet been reported. We isolated a bacterium, designated FA13, from the deep-sea sediment of the South Atlantic Ocean, during our research on deep-sea microorganism resources that inhibit aflatoxin production. Using visual agar plate assay, it has been confirmed that this bacterium had the ability to remarkably inhibit A. parasiticus NFRI-95 mycelial growth and aflatoxin production. The objectives of the present study were to identify the FA13 strain, to screen for suitable fermentation medium, to optimize fermentation conditions for its enhanced production of active substances that inhibit aflatoxin, and to study the stability of the active substances that inhibit aflatoxin, so as to lay a foundation for the separation, purification and application of the active compounds.
Materials and methods
Media
The five cultivation media and their compositions are as follows: (1) A1 medium: 10.0g soluble starch, 2.0g peptone, 4.0g yeast extract, 750mL seawater and 250mL deionized water;(2)Starch casein medium:10g soluble starch, 4g yeast extract, 2g casein, 1000mL seawater, pH7.2–7.4;(3)ISP2 medium: 4g yeast extract, 10g maltose, 4g glucose, 1000mL seawater, pH7.2–7.4;(4)PDA medium: 30g potato, 20g glucose, 1000mL deionized water; (5)Gause No.1 medium: 20g soluble starch, 1g KNO3, 0.5g NaCl, 0.5g K2HPO4, 0.5g MgSO4, 0.01g FeSO4, 1000mL deionized water, pH7.2–7.4.
Isolation and identification of the FA13 bacterium
The deep-sea sediment sample was collected from a depth of 3203 m of the South Atlantic Ocean (W14.5°, S13.6°) on Jul. 25th, 2012. The sample was kept at 4°C in sterile plastic bag in a refrigerator and carried to the laboratory. In Nov., 2012, the sediment was diluted 10 times by sterile seawater to make a suspension and heated for 6 min at 55°C by using water bath in the lab. Then, one hundred micro liter of the supernatant was taken and spread on Gause No.1 medium supplemented with potassium dichromate(0.05g/L). After 3 days’ incubation at 28°C, the colonies were picked. The FA13 strain was obtained after repeated streaking.
Identification of the FA13 bacterium was performed by morphological observation and 16s rRNA sequence analysis. Template DNA was extracted according to the protocol recommended by the kit. Amplification of the 16s rRNA gene was carried out by PCR using universal primers 27 F(5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ) and 1492 R(5ʹ-AAGGAGGTGATCCAGCCGCA-3ʹ) in a final volume of 50 μL. The reaction solution contained 10× PCR Buffer 5 μL, 10 mM dNTPmix 4 μL, 10 μM 27F 1 μL, 10 μM 1492R 1 μL, template DNA 1 μL, 5 U/μl Taq polymerase 0.5 μL, distilled water 37.5 μL. The thermal cycling was done with the following protocol: 5 min at 94°C; followed by 30 cycles of 1 min denaturation at 94°C, 40 s annealing at 55°C, and 40 s extension at 72°C; and a final extension of 10 min at 72°C. The PCR product was separated on 1% agarose gel using electrophoresis, before sending to BGI (Beijing) Co. Ltd. for sequencing. Sequence similarity was determined using NCBI Blast and EzTaxon. Phylogenetic analysis was performed using MEGA software version 5.0 with distance options according to Kimura’s two-parameter model and clustering with the neighbour-joining method, and supported with bootstrap values based on 1000 replications.
Cultivation and measurement of anti-fungal/anti-aflatoxigenic activity
The overnight liquid seed culture of FA13 was inoculated into 50 ml Erlenmeyer flasks, containing 20 ml of the above-mentioned 5 kinds of liquid media, respectively. The inoculation size was 1% (v/v). The cultures were cultivated at 28°C with shaking at 120rpm for 6 days. Each experiment was carried out in three replicates. After the cultivation, the cell suspension was centrifuged at 8,000 × g for 20 min at room temperature. The supernatant was supplemented with GY (2% glucose and 0.5% yeast extract) to compensate for the consumption of nutrients by bacterial growth, and the pH of the medium was adjusted to approximately 6.0. After filter sterilization with a 0.22-μm-pore-size Millipore membrane, the resulting solution was used as a cell-free supernatant for anti-fungal and anti-aflatoxigenic bioassays.
A. parasiticus mutant strain NFRI-95was used as an indicator in the anti-fungal and anti-aflatoxigenic bioassays. Strain NFRI-95 did not produce aflatoxin, but accumulate norsolorinic acid, the first stable precursor in the synthetic pathway of aflatoxin, in the mycelia. Norsolorinic acid is vivid red and visible to human eyes. For determination of the anti-fungal activity, tip culture method was used [Citation19]. To test the anti-aflatoxigenic activity (suppression of red pigment production), norsolorinic acid in the mycelia was extracted with a solution containing 1mol/L NaOH and methanol (1:9, v/v). Then, OD560nm of the extract was measured. Uncultivated media supplemented with GY and inoculated with A. parasiticus NFRI-95 were used as controls for tip culture. The inhibition ratio of mycelial growth and aflatoxin were calculated according to the following formulas:
Mycelial growth inhibition ratio(%) = (W2 – W1)/W2 × 100% with W2: fresh weight of the mycelia in the control tip and W1: fresh weight of the mycelia in the experiment tip
Aflatoxin inhibition ratio(%) = (A2-A1)/A2 × 100% with A2: OD560nm of the control tip and A1: OD560nm of the experiment tip
Uniform design of the medium composition and cultivation conditions
Hybrid uniform design method in the DPS was used to optimize the medium compositions and the cultivation conditions. Three nutritional components in the ISP2 medium (yeast extract, maltose and glucose) and five cultivation conditions (inoculation size, cultivation temperature, cultivation time, pH of the medium and seawater content) were chosen in this study(refer to for detailed factors and their values). Sixteen experiments were generated according to the hybrid uniform design. Based on the medium composition and cultivation conditions of the 16 designed experiment schemes, cultivation and measurement of the anti-fungal and anti-aflatoxigenic activity of the cell-free supernatant were conducted by using the methods described above.
Table 1. Factors and their levels in the uniform design.
Stability of the active metabolites in the supernatants
Effects of temperature, acid-alkali, zymolysis and storage period on the stability of the anti-fungal and anti-aflatoxigenic active substances in the supernatants fermented with optimized protocol 1 were evaluated.
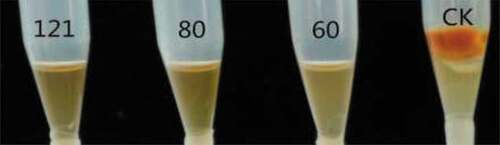
Thermal stability: to determine the thermal stability, aliquots of the cell-free supernatants were maintained at 60°C and 80°C for 2 h, and autoclaved at 121°C for 30 min respectively, and naturally cooled to room temperature. Cell-free supernatant without heat treatment was used as the control. Thermal stability of the active substances was tested with tip culture assay.
Effect of acid-alkali: The pH of the cell-free supernatants was adjusted to 2, 4, 8, 10 and 12, respectively, using 1 mol/L NaOH or 1 mol/L HCl. Then, after 6 h of incubation at room temperature, the pH of the culture supernatants was adjusted to approximately 6.0. The untreated culture supernatant served as the control. The effect of acid-alkali on the stability of the active substances was tested with tip culture assay.
Effect of zymolysis: the cell-free supernatants were well mixed with 1 g/L trypsin, chymotrypsin or pepsin and incubated for 6 h by using 37°C water bath. Then, the proteases were denatured with boiling water for 30 min, and cooled naturally to room temperature. The culture supernatant without enzymatic treatment served as the control. The effect of zymolysis on the stability of the active substances was tested with tip culture assay.
Effect of storage period: the cell-free supernatants were stored at room temperature and protected from light for 6 months. After that, the effect of storage period on the stability of the active substances was tested with tip culture assay.
Results
Isolation and identification of the FA13 strain
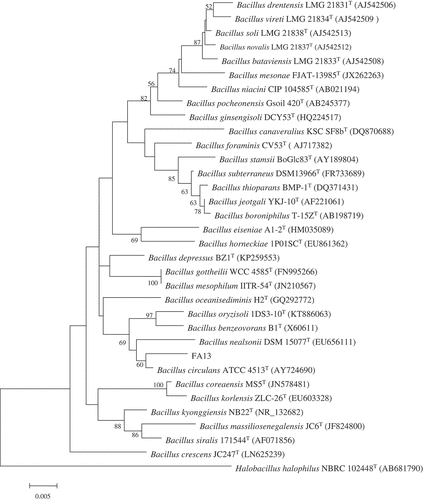
Totally, fifteen bacterial strains were isolated from the deep-sea sediments of the South Atlantic Ocean, by using seawater Gause No.1 agar media. Preliminary screening was carried out for all the strains that inhibit the mycelia growth of A. parasiticus NFRI-95. The result indicated that the FA13 strain exhibited a strong inhibitory effect. Microscopic observation demonstrated that cells of the FA13 strain were short rod-shaped and spore-forming. The 16S rRNA gene of the FA13 strain was (GenBank accession no. MF574386) compared with available 16S rRNA gene sequences in the EzTaxon server and the result indicated that it showed the highest sequence similarity to B. circulans ATCC 4513 (98.55%). The phylogenetic tree () also demonstrated that the FA13 strain and B. circulans ATCC 4513 grouped together. Therefore, the FA13 strain was designated B. circulans FA13.
Screening of suitable cultivation media
Results of supernatants from five fermented media showed that the cell-free supernatant cultivated with Gause No.1 medium exhibited the lowest anti-fungal and anti-aflatoxigenic activities, with an inhibition ratio of 19.08% and 76.80%, respectively. On the other hand, cell-free supernatants cultivated with the other four media (A1, starch casein, PDA and ISP2) could inhibit the spore germination of A. parasiticus NFRI-95 completely, as the culture broth in the tip was transparent, suggesting no mycelia growth.
In order to further define the suitable cultivation media, the cultivated supernatants obtained from the above-mentioned four media were diluted 10 times, respectively. Results showed that the inhibition ratios of mycelia growth of those cultivated by A1, starch casein and PDA were decreased to 52.20%, 39.10% and 41.90%, while the aflatoxin inhibition ratio was decreased to 85.60%, 94.90% and 87.80%, respectively. However, the inhibition ratios of mycelia growth and aflatoxin were maintained at 100% for the diluted culture obtained from the ISP2 medium. Therefore, ISP2 was confirmed to be the suitable cultivation medium and chosen for further optimization of the medium compositions and cultivation conditions by uniform design.
Optimization of the medium composition and cultivation conditions and experimental verification
Anti-fungal and anti-aflatoxigenic effects of experiment schemes proposed by uniform design
Results showed that all the cell-free supernatants from the 16 experiment schemes could completely inhibit the spore germination of A. parasiticus NFRI-95. Therefore, the supernastants were further diluted 10 times and tested for their anti-fungal and anti-aflatoxigenic activities. As can be seen from the , there exists some differences in the anti-fungal and anti-aflatoxigenic activities among 16 experiment schemes. Therefore, aiming at increasing the inhibition ratios of mycelia growth and aflatoxin, the DPS uniform design software was used to optimize the experimental factors.
Table 2. Anti-fungal and anti-aflatoxigenic activities of the 10 times diluted supernatants obtained from different experiment runs designed by uniform design.
Optimization with mycelia growth inhibition ratio as the target
To enhance the mycelia growth inhibition ratio, the quadratic polynomial stepwise regression, multifactor and square regression analysis, and partial least squares quadratic polynomial stepwise regression analysis were performed. However, their analysis results were unsatisfactory, as the objective function value did not fit well with the predicted value. On the other hand, the multifactor and reciprocal regression analysis was better. The result is shown in . The resulting regression equation was:
Table 3. Results of the multifactor and reciprocal regression analysis with the highest mycelia growth inhibition ratio.
Y = – 8.04669817 + 0.5346961966 * X3 + 1.6553901303 * X4 + 6.124822367 * X6 – 0.23904773878 * X1 * X3 + 0.024164154042 * X1 * X5 – 0.09943444422 * X2 * X3 – 0.003946526800 * X2 * X5 + 0.06766702078 * X3 * X5 + 0.24283270303 * X3 * X6 – 0.4653554272 * X4 * X6 – 0.08358333841 * X5 * X6 – 0.06415126959 * X6 * X7 + 0.30180697630 * X6 * X8.
The relation coefficiency of the regression equation was R = 0.999996, the coefficient of determination was R2 = 0.999993, F value was 21,386.3090, p value was 0.0000 and the minimum residual path coefficient was 0.05138. Thus, the regression equation was significant and acceptable (p < 0.01). This indicates that this equation can fit very well with the effects of various factors on the inhibition ratio of mycelia growth. The optimized fermentation protocol (protocol 1) was to ferment at 21.24°C, pH 6.192 for 10.1799 days using 9.369g/L yeast extract, 2.082g/L maltose, 13.806g/L glucose, 0% seawater with an inoculation size of 1.06%. The predicted maximum value of the mycelia growth inhibition ratio was 73.231%.
Optimization with aflatoxin inhibition ratio as the target
To enhance the toxin inhibition ratio, quadratic polynomial stepwise regression, multifactor and reciprocal regression analysis, and partial least squares quadratic polynomial stepwise regression analysis were performed. However, their analysis result was not good, as the objective function value did not fit well with the predicted value. The multifactor and square regression analysis and partial least squares reciprocal regression analysis were better. The result of multifactor and square regression analysis is shown in . It is indicated that the aflatoxin inhibition ratio is remarkably affected by the linear terms of Factor X1, X2, X3, X6, X7 and X8 the quadratic terms of Factor X1, X2, X3, X4, X5, X6, X7 and X8. The regression equation was as follows:
Table 4. Results of multifactor and square regression analysis with the highest aflatoxin inhibition ratio.
Y = – 229.5178760 + 20.559861205 * X1 + 10.453408696 * X2 + 11.255727827 * X3 + 16.055962203 * X6 + 5.306840400 * X7 + 3.801218843 * X8 – 2.5003807349 * X1 * X1 – 0.16891870667 * X2 * X2 – 0.7120144801 * X3 * X3 + 0.4150864301 * X4 * X4–0.003391938195 * X5 * X5 – 1.0788601903 * X6 * X6 – 0.25796625568 * X7 * X7 – 0.3858903884 * X8 * X8.
The relation coefficiency of the regression equation was R = 0.999999, the coefficient of determination R2 = 0.999997, F value = 27,150, p value = 0.0048 and the minimum residual path coefficient was equal to 0.0102. Thus, the regression equation was significant and acceptable (p < 0.01). This indicates that this equation can fit very well with the effect of various factors on the aflatoxin inhibition ratio. The optimized fermentation protocol (protocol 2) was to ferment at 34.399°C, pH 11.704 for 8.703 days using 7.914g/L yeast extract, 12.639g/L maltose, 6.545g/L glucose, 4.577% seawater with an inoculation size of 4.430%. The predicted maximum value of the toxin inhibition ratio obtained by software simulation was 137.1%.
The regression equation obtained by partial least squares reciprocal regression analysis was as follows:
Y = 134.5594691–4.212325 × 1 + 0.452986 X2 – 5.431081 × 3–1.919745 × 4 – 0.147515 × 5–3.530894 × 6–2.236530 × 7–12.590841 × 8 + 0.173792 X1 * X2 – 0.206707 × 1 * X3 + 0.189688 X1 * X4 – 0.023535 × 1 * X5 + 0.037325 X1 * X6 – 0.212785 × 1 * X7 + 0.342111 X1 * X8 + 0.009111 X2 * X3 – 0.208044 × 2 * X4 – 0.001214 × 2 * X5 – 0.050361 × 2 * X6 + 0.067487 X2 * X7 + 0.122191 X2 * X8 + 0.674344 X3 * X4 + 0.013326 X3 * X5 + 0.346344 X3 * X6 – 0.125220 × 3 * X7 + 0.070801 X3 * X8 – 0.004496 × 4 * X5 + 0.259328 X4 * X6 + 0.040652 X4 * X7 + 0.559272 X4 * X8 – 0.017031 × 5 * X6 + 0.010180 X5 * X7 + 0.020150 X5 * X8 + 0.145807 X6 * X7 + 0.265836 X6 * X8 + 0.036184 X7 * X8.
The optimized fermentation protocol (protocol 3) was to ferment at 41.457°C, pH 10.990 for 10.370 days using 9.873g/L yeast extract, 2.198g/L maltose, 12.456g/L glucose, 0% seawater with an inoculation size of 8.504%. The predicted maximum value of the toxin inhibition ratio obtained by software simulation was 151.57%.
Experimental verification of the optimized fermentation protocols
Strian FA13 was cultivated according to the above-mentioned optimized fermentation protocols. The anti-fungal and anti-aflatoxigenic activities of the 20 times diluted cell-free supernatants were shown in . The predicted and measured mycelia growth inhibition ratios were similar for the supernatant obtained from the optimized fermentation protocol 1 targeting at mycelia growth inhibition ratio. The optimization purpose was achieved. On the other hand, for the supernatant obtained from the optimized fermentation protocol 2 and 3 targeting at toxin inhibition ratio, the measured aflatoxin inhibition ratios had poor relevance with the predicted values. The actual aflatoxin inhibition ratios did not reach the expected values. The results demonstrate the importance of validating the predicted mathematic regression models through real experiments.
Table 5. Comparison of predicted values and measured values of different optimized fermentation protocols.
To further validate the feasibility of protocol 1, three batches of experiments were carried out. Results indicated that the inhibition ratios of mycelia growth and aflatoxin of the 20 times diluted cell-free supernatants obtained from all three batches were 100% ( and ). Therefore, protocol 1 was proved to be reproducible and feasible.
Table 6. Inhibition ratios of mycelia growth and aflatoxin of the 20 times diluted supernatants obtained from three batches of the protocol 1.
Comparison between the optimized protocol and the original protocol
Compared with the original cultivation protocol (), the optimized protocol 1 was remarkably improved in terms of cultivation temperature, seawater content and medium cost, except for an increase in cultivation time. In particular, the medium cost was reduced by 64.3%, the cultivation temperature was decreased from 28°C to 21°C and the dependence on seawater was completely relieved, thus preventing the fermentor from corrosion.
Table 7. Comparison of various factors between the optimized protocol and the original protocol.
Stability of the anti-fungal and anti-aflatoxigenic substances
Effect of heat on the active substances
After heat treatments, there were no changes in the mycelia growth and aflatoxin inhibition ratios of the supernatants (). They all remained 100%, the same as those of the controls without heat treatment. This revealed that the active substances in the supernatants were resistant to high temperature and high pressure.
Effect of acid-alkali on the active substance
After incubation at different pH, the mycelia growth and aflatoxin inhibition ratios of the supernatants remained 100%, the same as those of the untreated controls. It can be inferred that the active substances in the supernatants were resistant to acid-alkali, being stable at pH 2–12.
Effect of zymolysis on the active substance
The mycelia growth and aflatoxin inhibition ratios remained 100% after pepsin, α-chymotrypsin and trypsin treatment, the same as those of the controls without protease treatment. This suggested that the active substances in the supernatants were resistant to protease hydrolysis.
Effect of storage period on the active substance
After 6 months storage, the mycelia growth and aflatoxin inhibition ratios were still 100%, indicating that the active substances in the supernatants were quite stable.
Discussion
To our knowledge, there have been no reports on deep-sea microorganisms that inhibit aflatoxin production. Bacillus circulans FA13 strain isolated from deep-sea sediment of the South Atlantic Ocean in this study showed significant inhibition activity. The supernatants of the five media used to cultivate the FA13 strain could all efficiently suppress the production of norsolorinic acid, indicating that the FA13 strain could grow on a broad range of nutrient substances and produce active metabolites that inhibit the mycelial growth of A. parasiticus and production of aflatoxin.
In order to enhance the yield of anti-fungal substances, many researchers used response surface methodology to optimize cultivation media and cultivation conditions [Citation20–Citation22]. Uniform design was first proposed by Wang yuan and Fang kaitai in 1978 [Citation23]. Compared to the response surface methodology, uniform design is superior in that when dealing with multi-factors and multi-levels, fewer number of essential experiments are needed to achieve satisfactory results [Citation23]. In this research, we firstly used the uniform design method to optimize the concentrations of different nutrient components in ISP2 medium and the fermentation conditions for production of anti-aflatoxigenic metabolites by the FA13 strain. However, only for optimized protocol 1, experimental verification demonstrated that the measured mycelia growth inhibition ratio fitted well with that of the predicted value. Furthermore, compared with the original protocol, although the cultivation time of the optimized protocol 1 was extended, the medium cost was reduced by 64.3% and the cultivation temperature was decreased from 28°C to 21°C. To some extent, this reduces energy consumption. The optimized protocol 1 was completely free from seawater, thereby reducing corrosion damages of the cultivation equipment caused by it and extending equipment lifespan. Therefore, the optimized protocol 1 designed in this study was obviously practicable and feasible.
The anti-aflatoxin substances produced by the deep-sea B. circulans FA13 strain exist in the cultivated supernatants. They are resistant to high temperature and high pressure, which is different from the Bacillus megaterium strain isolated from the fish intestines. The anti-aflatoxin activity of the B. megaterium strain culture was lost after autoclave treatment [Citation16]. The active substances in the supernatant of the FA13 culture were resistant to acid-alkali, zymolysis and long-term preservation, as well. The high stability of the active substances produced by FA13 provides favorable conditions for preparation of biocontrol agents through fermentation and spray drying and extension of shelf life. In our future study, isolation, purification and identification of the active compounds and clarification of the toxicity and inhibition mechanism of identified compounds will be conducted, so as to accelerate and ensure its application in the biological control of aflatoxin contaminated food and feeds.
In conclusion, a novel deep-sea B. circulans FA13 was isolated from the sediment of the South Atlantic Ocean. The fermented cell-free supernatants of B. circulans FA13 could remarkably inhibit mycelial growth of the A. parasiticus and accumulation of norsolorinic acid. The inhibition efficacy of the 20 times diluted cell-free supernatant was increased from 0–23.1% to 100% after fermentation optimization by uniform design. In addition, the active substances in the cell-free supernatant obtained from the optimal protocol are highly stable and resistant to high temperature, high pressure, acid-alkali and zymolysis. The novel deep-sea B. circulans FA13 strain and its optimal fermentation protocol established in this study offer a basis for further study with large scale fermentation and application in control of aflatoxin contamination of food and feed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ono EY, Ono MA, Funo FY, et al. Evaluation of fumonisin-aflatoxin co-occurrence in Brazilian corn hybrids by ELISA. Food Addit Contam. 2001;18:719–729.
- Roze LV, Hong SY, Linz JE. Aflatoxin biosynthesis: current frontiers. Ann Rev Food Sci Tech. 2013;4:293–311.
- Wei Z, Xue B, Li M, et al. Screening a strain of Aspergillus niger and optimization of fermentation conditions for degradation of aflatoxin B1. Toxins (Basel). 2014;6:3157–3172.
- Jermnak U, Chinaphuti A, Poapolathep A, et al. Prevention of aflatoxin contamination by a soil bacterium of Stenotrophomonas sp. that produces aflatoxin production inhibitors. Microbiology. 2013;159:902–912.
- Hua SST, Parfitt DE, Holtz BA. Evaluation of a biopesticide, Pichia anomala WRL-076 to control Aspergillus flavus in a commercial orchard. Proceedings of the Fifth California Conference of Biological Control; 2006 Jul 25–27; Riverside, USA. p. 152–155.
- Kenneth DJ. Atoxigenic Aspergillus flavus biological control of aflatoxin contamination: what is the mechanism? World Mycotoxin J. 2015;8:235–244.
- Alaniz Zanon MS, Barros GG, Chulze SN. Non-aflatoxigenic Aspergillus flavus as potential biocontrol agents to reduce aflatoxin contamination in peanuts harvested in Northern Argentina. Int J Food Microbiol. 2016;231:63–68.
- Mannaa M, Kim KD. Microbe-mediated control of mycotoxigenic grain fungi in stored rice with focus on aflatoxin biodegradation and biosynthesis inhibition. Mycobiology. 2016;44:67–78.
- Blunt JW, Copp BR, Keyzers RA, et al. Marine natural products. Nat Prod Rep. 2014;31:160–258.
- Pettit RK. Culturability and Secondary Metabolite Diversity of Extreme Microbes: Expanding Contribution of Deep Sea and Deep-Sea Vent Microbes to Natural Product Discovery. Mar Biotechnol. 2001;13:1–11.
- Chen LL, Wang N, Wang XM, et al. Characterization of two antifungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresour Technol. 2010;101:8822–8827.
- Romanenko LA, Tanaka N, Kalinovskaya NI, et al. Antimicrobial potential of deep surface sediment associated bacteria from the Sea of Japan. World J Microbiol Biotechnol. 2013;29:1169–1177.
- Ma ZW, Hu JC, Wang XM, et al. NMR spectroscopic and MS/MS spectrometric characterization of a new lipopeptide antibiotic bacillopeptin B1 produced by a marine sediment-derived Bacillus amyloliquefaciens SH-B74. J Antibiot (Tokyo). 2014;67:175–178.
- Tareq FS, Lee M, Lee HS, et al. noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org Lett. 2014;16:928–931.
- Yan PS, Xu Z, Wang K, et al. Marine Bacterial Strains with broad anti-fungal and anti-bacterial activities. Proceedings of the 5th International Symposium on Biocontrol and Biotechnology; 2007 Nov 1–3; NongKhai, Thailand. p. 139.
- Kong Q, Shan SH, Liu QZ, et al. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int J Food Microbiol. 2010;139:31–35.
- Kong Q, Chi C, Yu JJ, et al. The inhibitory effect of Bacillus megaterium on aflatoxin and cyclopiazonic acid biosynthetic pathway gene expression in Aspergillus flavus. Appl Microbiol Biotechnol. 2014;98:5161–5172.
- Li P, Yan PS. Inhibition of Aspergillus parasiticus and cancer cells by marine actinomycete strains. J Ocean Univ China. 2014;13:985–994.
- Yan PS, Song Y, Sakuno E, et al. Cyclo(L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl Environ Microbiol. 2004;70:7466–7473.
- Mezghanni H, Khedher SB, Tounsi S, et al. Medium optimization of antifungal activity production by Bacillus amyloliquefaciens using statistical experimental design. Prep Biochem Biotechnol. 2012;42:267–278.
- Grahovac J, Grahovac M, Dodi J, et al. Optimization of cultivation medium for enhanced production of antifungal metabolites by Streptomyces hygroscopicus. Crop Prot. 2014;65:143–152.
- Kilani-Feki O, Khedher SB, Dammak M, et al. Improvement of antifungal metabolites production by Bacillus subtilis V26 for biocontrol of tomato postharvest disease. Biol Control. 2016;95:73–82.
- Li RZ, Lin DKJ, Chen Y. Uniform design: design, analysis and applications. Int J Mater Prod Tec. 2007;20:101–114.