?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Selenium-enriched yeast can transform toxic inorganic selenium into absorbable organic selenium, which is of great significance for human health and pharmaceutical industry. A yeast Rhodotorula glutinis X-20 we obtained before has good selenium-enriched ability, but its selenium content is still low for industrial application. In this study, strategies of process optimization and transport regulation of selenium were thus employed to further improve the cell growth and selenium enrichment. Through engineering phosphate transporters from Saccharomyces cerevisiae into R. glutinis X-20, the selenium content was increased by 21.1%. Through using mixed carbon culture (20 g L−1, glycerol: glucose 3:7), both biomass and selenium content were finally increased to 5.3 g L−1 and 5349.6 µg g−1 (cell dry weight, DWC), which were 1.14 folds and 6.77 folds compared to their original values, respectively. Our results indicate that high selenium-enrichment ability and biomass production can be achieved through combining process optimization and regulation of selenium transport.
Graphical abstract
Introduction
Selenium (Se) is a trace element essential for human health [Citation1–Citation3]. The World Health Organization (WHO) has reported that selenium deficiency is one of the most universal nutritional deficiencies in the world [Citation4]. Selenium deficiency affects approximately 0.5–1 billion people around the world and is prone to causing health disorders such as cancer, Keshan disease, Kashin-Beck disease, muscular syndrome, and even death [Citation3–Citation5]. Selenium in the body is mainly supplied through diet [Citation5], however, many foods are selenium deficient due to their planting environment, e.g. approximate 72% of Chinese cultivated land is selenium deficient (<0.04 mg kg−1), with at least 30% being severely selenium deficient. Currently, the average dietary intake of selenium by Chinese adults is only 26–32 µg per day, which is much less than the WHO-recommended intake of 40–200 µg per day [Citation2]. Therefore, developing selenium-enriched products is of great significance to improve health standards, especially of communities whose nutrition is absent or deficient in organic selenium.
Among the various selenium-enriched products, selenium-enriched yeast has been considered a safe and effective nutrient fortifier. Previous studies have verified that the dietary supplementation of selenium-enriched yeast greatly improved health compared to those who were administered inorganic selenium products [Citation6]. Moreover, yeast’s ability to incorporate metals has been exploited as a delivery vehicle for many minerals [Citation7]. However, the imbalance between cell growth and selenium accumulation usually attenuates the quality of selenium-enriched yeast [Citation8–Citation10]. Excessive amounts of selenium prevent enzymatic oxidation of yeast macromolecules [Citation11], and high concentrations of the selenide form of selenium can be particularly toxic to cells [Citation12,Citation13]. Although several approaches have been tested to solve this problem, there are still tremendous challenges. For example, treating yeast with a pulsed electric field increased selenium accumulation by 65%, but resulted in a threefold reduction in biomass, possibly due to cell toxicity or changed enzymatic activity [Citation14]. Although supplementing selenium at different stages in the cell cycle can reduce the toxicity of higher concentrations, biomass still decreased by up to 20% [Citation15]. Because the mechanism of coordinating selenium accumulation and yeast growth is not fully understood, developing a method that can balance these processes is extremely important.
Theoretically, yeast accumulates selenium via physicochemical and biological mechanisms, including extracellular binding by metabolites and biopolymers, binding to the specific polypeptides, and metabolism-dependent accumulation, however, the capability of yeast to enrich selenium largely depends on their endogenous characteristics [Citation7]. The most classical and effective way to obtain a highly selenium-enriched yeast is still screening the strain from a selenium-enriched environment. In 2008, a geological survey of China discovered a large area containing selenium-enriched soil (selenium content ranging from 0.14–2.75 mg kg−1) on the north slope of the Tianshan Mountains in Xinjiang, providing a possible environment for selenium-enriched microorganisms [Citation16,Citation17]. From this area, we have identified a strain of yeast, Rhodotorula glutinis X-20, which shows good selenium enrichment and growth performance [Citation18]. The objective of the present study is to optimize fermentation conditions and to balance cell growth and selenium enrichment. Basic culture conditions are established and process optimization is performed. The selenium-enrichment capacity of R. glutinis X-20 is greatly improved by regulating the phosphate transport system, and selenium enrichment and cell growth is also balanced by using a mixed carbon source in culture. This study provides a foundation for the application of R. glutinis X-20.
Materials and methods
Strain and medium
R. glutinis X-20 colonies were inoculated in yeast extract peptone dextrose medium (YPD medium, containing 1% yeast extract, 2% peptone, and 2% glucose) with 30 mg·L−1 sodium selenite. After culturing 36 h at 30°C, their selenium-enrichment potential was determined with Hydride Generation Atomic Fluorescence Spectrometry (HG-AFS).
Determination of selenium content in yeast
The total selenium content in yeast was determined by Hydride Generation Atomic Fluorescence Spectrometry (HG-AFS) [Citation19–Citation21]. One millilitre of yeast broth was centrifuged at 8,000 rpm for 10 min and washed 3 times with deionized water to remove selenium ions and selenium salt that were unbound to yeast cells. The collected cells were transferred into a Teflon crucible. 10 mL mixed acid (9 mL HNO3 and 1 mL perchloric acid) and few glass beads were added sequentially. The cells were digested at 200°C using an electric heating plate until the solution volume was reduced to 2 mL. The digested solution was cooled to room temperature, and 5 mL hydrochloric acid (6 mol L−1) was added to reduce selenium (VI) into selenium (IV) before hydride generation. The digested solution was then transferred into volumetric flasks and diluted with ultrapure water to 50 mL. A blank control solution was prepared using the same method. The digested product was used for total selenium determination according to the HG-AFS conditions described in .
Table 1. HG-AFS conditions.
For extraction and determination of inorganic selenium, cells were mixed with ultrapure water and boiled for 1 h. After centrifugation at 8,000 rpm for 15 min, the supernatant was filtrated and used to evaluate the inorganic selenium content. Organic selenium content in yeast was calculated by taking the difference between total selenium content minus inorganic selenium content [Citation22].
Assay of biomass
Biomass was estimated through measuring the optical density at 600 nm. The dry weight of the cell (DWC) was calculated according to the relationship between the DWC and OD600. To determine the DWC, 5 mL yeast culture was added into a weighed centrifuge tube, centrifuged for 5 min at 10,000 rpm and the supernatant was discarded. After three washes with sterile water, the centrifuge tube containing yeast cells was dried at 105°C until it reached a constant weight (the final weight). The DWC was obtained by subtracting the blank tube weight from the final weight. Meanwhile, the optical density at 600 nm of the cells was recorded. Each experiment was performed in triplicate. The linear relationship between DWC and OD600 is shown in EquationEquation 1(1)
(1) .
Plasmid construction
To study the effect of selenium transport on yeast growth and selenium enrichment, four phosphate transporters Pho84, Pho87, Pho89, and Pho90, were selected by searching the National Centre for Biotechnology Information (NCBI) database. Pho84, Pho87, Pho89, Pho90, promoter TYS1, and the ATP15 terminator were cloned via PCR from the S. cerevisiae CEN.PK2-1C genome. Promoter TYS1, PhoX, and terminator ATP15 were ligated by OE-PCR. After Hind III and Sal I digestion, the ligated products were inserted into the pRS42K plasmid linearized by Hind III and Sal I to form the new plasmid pRS42K-phoX. Recombinant plasmids were transformed into yeast by an improved lithium acetate transformation method. Transformants were screened through resistance screening and colony PCR.
Quantitative PCR
Total RNA extraction, first-strand cDNA synthesis, and qPCR analysis were performed as previously described [Citation23]. PCR conditions consisted of 40 cycles at 95°C for 5 sec, 60°C for 20 sec, and 72°C for 1 min, with a final extension at 72°C for 10 min. ACT1 was used as the reference gene, and relative transcription levels were calculated based on the level of ACT1 mRNA.
Results and discussion
Selenium yeast is important for health and the pharmaceutical industry. Previously, yeast strain R. glutinis X-20, which showed good selenium enrichment and growth performance, was screened from selenium-enriched soil in Xinjiang, China. Optimal culture conditions were determined through single experimental factors [Citation18]. To further optimize the selenium enrichment of this yeast, basic culture conditions including temperature, pH, culture time, and liquid volume were explored. Because selenite absorption depends on transporters of phosphate or monocarboxylate [Citation7], phosphorus stimulation and a transformed phosphate transport system were employed to improve selenium enrichment. Different carbon sources can have different effects on cell growth and selenium metabolism, thus mixed carbon culture was employed to balance cell growth and selenium enrichment. After optimization, a selenium content of 5349.6 µg g-1(DWC) was obtained.
Basic culture conditions and process optimization
Temperature and pH
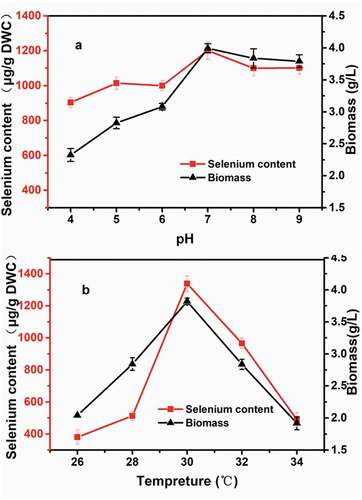
Temperature and pH are highly significant for yeast growth and selenium enrichment. ) shows that the biomass and selenium-enrichment potential of strain X-20 are highly pH dependent. The X-20 strain accumulated a maximum amount of selenium when grown at pH 7.0. However, earlier studies have reported that selenium accumulation in yeast was more efficient at lower pH values [Citation24]. Yin et al. also demonstrated that the optimal pH for selenium enrichment in S. cerevisiae was 5.822. Using Rhodotorula, the optimal pH for enrichment of other ions was also between 6.0 and 7.0 [Citation25]. Efficient selenium enrichment at pH 7.0 implies an underlying difference in mechanism of selenium accumulation in R. glutinis X-20, which should be further examined. The cell growth and selenium accumulation are substantially increased when the culture temperature raised from 26°C to 30°C ()). Over 30°C, there is a steep decrease in both selenium content and biomass. The selenium content and DWC of R. glutinis X-20 at 30°C reached 1338.9 µg g−1 and 3.82 g L−1, respectively. This culture temperature is similar as the other selenium-enrichment yeasts such as S. cerevisiae [Citation26,Citation27].
Culture time
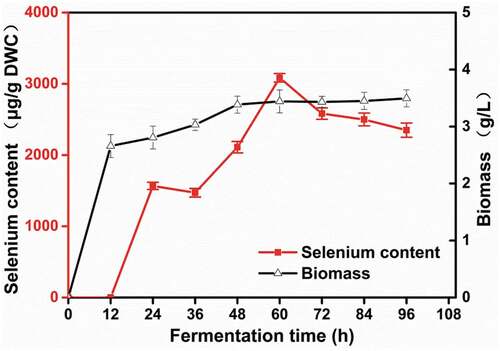
Fermentation time not only affects selenium enrichment but also influences production cost. To explore the optimal fermentation time, R. glutinis X-20 was inoculated in 50 mL fresh YPD medium (with 2% glycerol as a carbon source and peptone as a nitrogen source) in flasks with 1% inoculation. After 12 h culture at 30°C and 200 rpm, 50 g/mL sodium selenite was added into the flasks. From 24 h to 60 h, selenium content and yeast biomass increased by 96.919% and 63.629%. With the prolonged culture time, selenium content decreased sharply while yeast biomass remained stable (). These results suggest that selenium accumulation in R. glutinis X-20 is growth-related. From an economic perspective, fermentation time is a very important factor for production [Citation28]. Shortening fermentation time can shorten the production cycle of industrial selenium-enriched yeast and increase utilization of the instrument. Thus, in follow-up experiments we decided to use 60 h as an endpoint for fermentation.
The volume of liquid
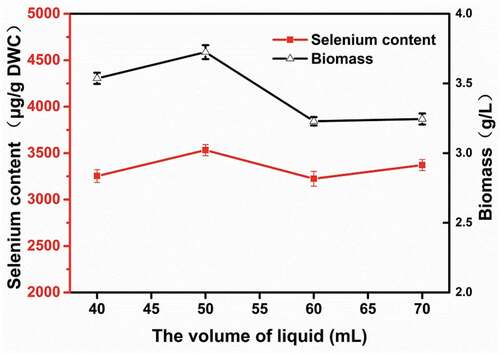
To explore the effect of dissolved oxygen on selenium enrichment, different volumes of YPD media were added to 250 mL shake flasks. shows that larger liquid volumes had a negative effect on selenium enrichment, while smaller liquid volumes were better for the growth and selenium enrichment of R. glutinis X-20, with 50 mL media in a 250 mL flask as the optimal volume. These results are consistent with previous work [Citation29]. Fermentation in shaking flasks with less liquid has higher a volumetric dissolved oxygen coefficient value but increases the evaporation of the fermentation broth. Excessive oxygen is not conducive to the propagation and growth of yeast and will inhibit its selenium-enrichment capabilities [Citation30]. Therefore, 50 mL/250 mL was used for subsequent experiments.
Improving selenium enrichment by regulating phosphate transport system
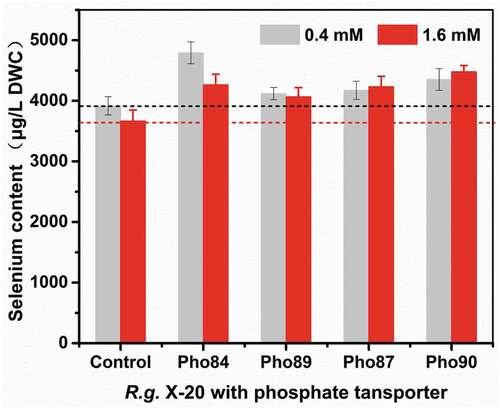
In yeast, selenite is absorbed in a metabolism-dependent manner using transporters of phosphate or monocarboxylate [Citation7]. In our previous work, we demonstrated that the addition of phosphorus increases the selenium content of yeast [Citation18]. When R. glutinis X-20 was grown in medium containing 0.4 mM phosphate, it increased selenium enrichment to 3909 µg g−1 DWC. Less than 0.4 mM phosphate promoted cell growth and selenium enrichment while more than 0.4 mM phosphate inhibited it. These findings suggest that a low concentration of phosphate enhances the uptake and tolerance of selenium in yeast [Citation31,Citation32]. Phosphorus’ ability to regulate selenium enrichment may be related to selenium transport in yeast. According to reports, both high and low affinity phosphate transporters are supposedly involved in the transport of selenium [Citation33]. The low affinity transport system, composed of Pho87 and Pho90, operates at higher phosphate concentrations (>1 mM), while at low concentrations, it is post-transcriptionally downregulated through vacuolar targeting by Spl2 (also a member of the PHO regulon) [Citation34,Citation35]; the high affinity transport system composed of Pho84 and Pho89 functions at both low and high phosphate concentrations, depending on the PHO pathway [Citation36]. Therefore, under high phosphate conditions, Pho87 and Pho90 are the main transporters of selenite, while at low phosphate condition Pho84 and Pho89 are the main selenite transporters.
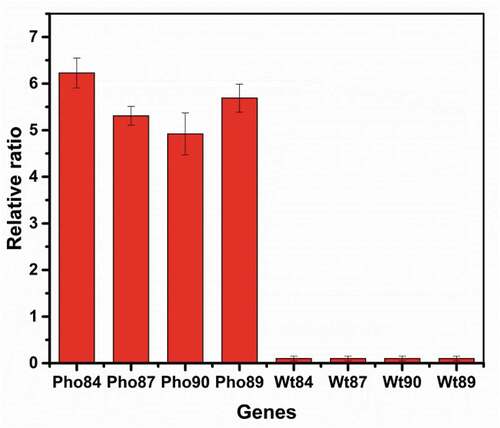
To investigate the relationship between phosphate transporters and selenium transport, we transformed Pho84, Pho89, Pho87, and Pho90 from S. cerevisiae into R. glutinis X-20. As shown in , qPCR confirmed that the heterologous Pho84, Pho89, Pho87, and Pho90 are effectively expressed in R. glutinis X-20. Due to the absence of these genes in R. glutinis X-20, no transcriptional data were detected (wt84, wt87, wt90, wt89). Compared with the control, the selenium content in yeast with these transformed transporter genes increased (). As expected, Pho84 and Pho89 promote selenium enrichment at a low phosphorus concentration (0.4 mM), whereas Pho87 and Pho90 show good effect at a high concentration (1.6 mM). The total selenium reached 4752 µg·g−1 DWC when Pho84 was expressed in R. glutinis X-20. Therefore, selenium enrichment in yeast can be effectively improved by phosphorus stimulation or by regulating the phosphate transport system.
Balancing cell growth and selenium enrichment by mixed carbon culture
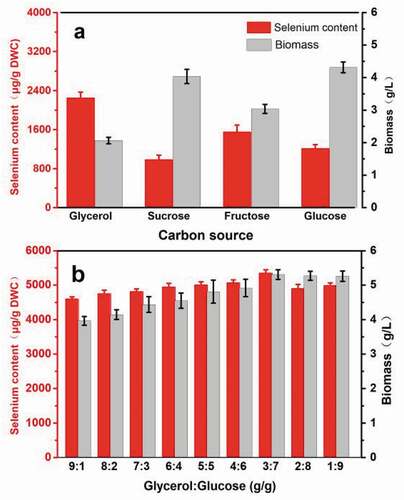
Carbon source identity contributes to the proliferation of yeast cell and can affect selenium accumulation and cell growth. As illustrated in (a), R. glutinis X-20 achieves the highest biomass when cultivated using glucose as the sole carbon source, whereas its selenium content shows maximum when glycerol is the sole carbon source, indicating an imbalance between selenium accumulation and cell growth. Therefore, an alternative culture using a mixed carbon source was further designed. (b) shows the selenium enrichment and biomass production of R. glutinis X-20 after 60 h using mixed glycerol and glucose. Yeast growth increases with increasing of glucose content. When the ratio of glycerol to glucose was 3:7, both biomass and selenium content achieved a maximum, 5.30 g L−1 and 5349.6 µg g−1 DWC, which were 1.14 folds and 6.77 folds compared to their original values, respectively. Ethanol and hydrogen peroxide by-products from glycerol could strengthen the resistance of cells to the external environment and regulate the balance of cell osmotic pressure produced by high concentrations of selenium and their oxidation-reduction potential, improving selenium accumulation [Citation37,Citation38]. The comparative analysis showed that the selenium content of R. glutinis X-20 is 2–5 times higher than most reported yeasts, except for mixed strains (). This evidence indicates that the imbalance between growth and selenium enrichment of R. glutinis X-20 could be resolved using the simple culture method of a mixed carbon source.
Table 2. Selenium-enriched performance in various yeasts.
Conclusion
Selenium-enriched yeast has been considered a safe and more effective nutrient fortifier, but an imbalance between yeast cell growth and selenium accumulation usually leads to lower product quality. Our study demonstrates that supplementation with phosphate could trigger the uptake of selenite by phosphorus transporters. The culture conditions of R. glutinis X-20 were optimized in terms of pH, culture time, and dissolved oxygen content. Using a simple mixed carbon source (glycerol and glucose in a ratio of 3:7) culture, selenium enrichment and cell growth can be efficiently balanced, finally obtaining 5349.6 µg·g−1 DWC selenium content. These methods can be employed at large scale for selenium enrichment of yeast for health and pharmaceutical settings.
Article Highlights
Explore culture method for balancing cell growth and selenium-enriched capability.
Mixed carbon source and low phosphate to regulate the selenite transportation.
The selenium content reach the maximum is 5349.6 µg/g in Rhodotorula glutinis X-20.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dundas LH, Bentvena S. Selenium: an element for life. Endocrine. 2015;48:756–775.
- Navarro AM, Cabrera VC. Selenium in food and the human body: a review. SciTotal Environ. 2008;400:115–141.
- Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268.
- Wang J, Wang B, Zhang D, et al. Selenium uptake, tolerance and reduction in Flammulina velutipes supplied with selenite. Peer J. 2016;4:e1993.
- Kieliszek M, Błażejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21:609.
- Rayman MP. The use of high-selenium yeast to raise selenium status: how does it measure up? Br J Nutr. 2007;92:557–573.
- Kieliszek M, Błażejak S, Gientka I, et al. Accumulation and metabolism of selenium by yeast cells. Appl Microbiol Biotechnol. 2015;99:5373–5382.
- Ponce de León CA, Bayón MM, Paquin C, et al. Selenium incorporation into Saccharomyces cerevisiae cells: a study of different incorporation methods. J Appl Microbiol. 2002;92:602–610.
- Demirci A, Pometto AL. Production of organically bound selenium yeast by continuous fermentation. J Agric Food Chem. 1999;47:2491–2495.
- Kieliszek M, Błażejak S, Płaczek M. Spectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeast. J Trace Elem Med Biol. 2016;35:90–96.
- Wysocki R, Tamás MJ. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev. 2010;34:925–951.
- Brozmanová J, Mániková D, Vlčková V, et al. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010;84:919–938.
- Herrero E, Wellinger RE. Yeast as a model system to study metabolic impact of selenium compounds. Microb Cell. 2015;2:139–149.
- Yang R, Li SQ, Zhang QH. Effects of pulsed electric fields on the activity and structure of pepsin. J Agric Food Chem. 2004;52:7400–7406.
- Suhajda Á, Hegóczki J, Janzsó B, et al. Preparation of selenium yeasts I. Preparation of selenium-enriched Saccharomyces cerevisiae. J Trace Ele Med Biol. 2000;14:43–47.
- Wei ZS, Tu QJ, Tang SH, et al. A discussion on the geochemical features and origin of selenium-rich soil on the northern slope of the Tianshan mountains from Urumqi to Shawan County. Geophys Geochem Explorat. 2016;40:893–898.
- Wang Q, Liu YH, Yang JN, et al. Analysis on the distribution of selenium resources and its relationships with soil properties of Ili district, Xinjiang Uygur Autonomous Region, China. J Agric Res Environ. 2014;31:555–559.
- Lou XD, Zhang GL, Ye BC. Screening of high selenium-enriched yeast and enhancement of its selenium-enriched capacity. Biotechnol Bulletin. 2017;33:132–137.
- Barrientos EY, Wrobel K, Torres Guzman JC, et al. Determination of SeMet and Se(iv) in bio-fortified yeast by ion-pair reversed phase liquid chromatography-hydride generation-microwave induced nitrogen plasma atomic emission spectrometry (HPLC-HG-MP-AES). J Anal At Spectrom. 2016;31:203–211.
- Xiao ZM, Song R, Jia Z, et al. Quantitative determination of selenium species in se-enriched yeast using liquid chromatography-hydride generation atomic fluorescence spectrometry. Chin J Anal Chem. 2014;42:1314–1319.
- Gui HE, Chen KL, Wang LJ. AFS for Se, As, Sb, Bi and Hg in geochemical samples in the same mother solution. Acta Geologica Sichuan. 2005;25:250–252.
- Yin H, Fan G, Gu Z. Optimization of culture parameters of selenium-enriched yeast (Saccharomyces cerevisiae) by response surface methodology (RSM). LWT-Food Sci Technol. 2010;43:666–669.
- Zhang G, Cao Q, Liu J, et al. Refactoring β-amyrin synthesis in Saccharomyces cerevisiae. AIChE J. 2015;61:3172–3179.
- Marinescu G, Stoicescu AG, Teodorof L. Industrial nutrient medium use for yeast selenium preparation. Ann Univ Dunarea De Jos Galati Fascicle VI Food Technol. 2011;35:45–53.
- Zhang XG, Peng YN, Li XR, et al. Screening of iron-enriched fungus from natural environment and evaluation of organically bound iron bioavailability in rats. Food Sci Technol. 2015;35:58–65.
- Sun PP, Zhang QQ, Zhong S. Preliminary optimization of selenium-enriched fermentation conditions of active dry yeast. J Anhui Polytech Univ. 2011;1:24–27.
- Wang XB, Liu L, Li XR. Breeding of a high selenium-enriched yeast strain. Food Ferment Technol. 2011;5:6–9.
- Hashem M, Zohri ANA, Ali MMA. Optimization of the fermentation conditions for ethanol production by new thermotolerant yeast strains of Kluyveromyces sp. Afr J Microbiol Res. 2013;7:4550–4561.
- Zhu W, Gao ZJ, Yong LI. Screening and optimized culture of selenium-enriched Saccharomyces cerevisiae. J Anhui Agric Sci. 2017;32:75–79.
- Suriyachai N, Weerasaia K, Laosiripojana N, et al. Optimized simultaneous saccharification and co-fermentation of rice straw for ethanol production by Saccharomyces cerevisiae and Scheffersomyces stipitis co-culture using design of experiments. Bioresour Technol. 2013;142:171–178.
- Mouillon JM, Persson BL. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 2010;6:171–176.
- Pérez-Sampietro M, Serra-Cardona A, Canadell D, et al. The yeast Aft2 transcription factor determines selenite toxicity by controlling the low affinity phosphate transport system. Sci Rep. 2016;6:32836.
- Lazard M, Blanquet S, Fisicaro P, et al. Uptake of selenite by Saccharomyces cerevisiae involves the high- and low-affinity orthophosphate transporters. J Biol Chem. 2010;285:32029.
- Easterling ER, French WT, Hernandez R, et al. The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis. Bioresour Technol. 2009;100:356–361.
- Wykoff DD, Rizvi AH, Raser JM, et al. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol Cell. 2007;27:1005–1013.
- Ghillebert R, Swinnen E, De Snijder P, et al. Differential roles for the low-affinity phosphate transporters Pho87 and Pho90 in Saccharomyces cerevisiae. Biochem J. 2011;434:243.
- Kieliszek M, Błażejak S, Kurek E. Binding and conversion of selenium in Candida utilis ATCC 9950 yeasts in bioreactor culture. Molecules. 2017;22:352.
- Babazadeh R, Lahtvee P-J, Adiels CB, et al. The yeast osmostress response is carbon source dependent. Sci Rep. 2017;7:990.
- Zhu CX, Ma AM, Liu DW, et al. Breeding of selenium-enriched yeast strains. Liquor Making Sci Technol. 2007;1:46.
- Zhang GC, Wang DH, Wang DH, et al. The mechanism of improved intracellular organic selenium and glutathione contents in selenium-enriched Candida utilis by acid stress. Appl Microbiol Biotechnol. 2017;101:2131–2141.