ABSTRACT
The episomal vector cannot integrate into the host cell chromosome, which has no potential risk in gene therapy. However, the low level of transgene expression driven by episomal vectors needs to be solved. In this study, we investigated the effects of enhancers, promoters and promoter variants on transgene expression levels driven by episomal vectors in HEK293, Chang liver and primary cells. Results showed that all eight cis-acting elements used could increase transfection efficiency and transient eGFP expression in transfected HEK293 and Chang liver cells. In stably transfected mammalian cells, the elongation factor-1 alpha (EF-1α) promoter and mutant-404 showed high and stable transgene expression. The mechanisms might be related to the type and quantity of transcription factor regulatory elements. Moreover, quantitative reverse transcription polymerase chain reaction analysis showed that mRNA expression levels were not directly proportional to protein expression levels. Furthermore, the EF-1α promoter conferred high transgene expression levels in primary cells, and the plasmid was also present in the episomal state. Taken together, these results provided valuable information for improving transgene expression with episomal vectors in mammalian cells.
Graphical abstract
Introduction
Gene therapy involves the delivery of a therapeutic gene into a patient’s cells as a drug to treat diseases. Vector systems play the key role in driving the expression of transgenes in gene therapy [Citation1,Citation2]. Episomal vectors can replicate in synchrony with each cell cycle division within the host genome for sustained, nonviral and nonintegrating transgene expression in vitro and in vivo [Citation3–Citation5], which does not cause insertional mutagenesis in gene therapy [Citation6]. Therefore, the use of episomal vectors has obvious advantages in gene therapy. The first nonviral and episomal plasmid vector pEPI based on the matrix attachment region (MAR), which can replicate autonomously with low copy numbers in all cells tested, was developed by Piechaczek et al. [Citation7]. However, the pEPI vector has several limitations, such as low copy number, unstable expression and low expression level [Citation8,Citation9].
Many studies have attempted to achieve high transgene expression levels via optimizing cis-acting elements in episomal vectors [Citation10–Citation12]. In our previous study, we constructed an episomal vector harboring a 387-bp DNA sequence containing a characteristic MAR motif driven by the cytomegalovirus (CMV) promoter; the construct was expressed in Chinese hamster ovary (CHO) cells in the episomal state [Citation13]. However, the episomal vectors currently used for transgene expression often result in low levels of expression [Citation14]. Therefore, the vector needs to be further optimized to achieve high expression level and stability.
Plasmid vectors contain some cis-acting elements, including promoters, enhancers, polyadenylation signals and other expression elements, which all affect transgene expression levels. Hagedorn et al. showed that the insulator sequence (cHS4) and a ubiquitous chromatin-opening element (UCOE) can improve expression and facilitate the establishment of a nonviral vector [Citation15]. Benjamin et al. demonstrated that the CMV promoter mutants show a reduced propensity for productivity loss in CHO cells [Citation16]. Although CMV is a strong promoter, some studies have shown that this promoter is intrinsically susceptible to transcriptional silencing associated with DNA methylation [Citation17,Citation18]. Alternatively, a variety of strong promoters, including human elongation factor-1 alpha (EF-1α) promoter and CAG promoter (a combination of the CMV immediate early enhancer and the chicken β-actin promoter), have been exploited to achieve the high-level expression of various genes [Citation19,Citation20].
In our previous study, we investigated enhancers, various promoters and promoter variants on transgene expression in CHO-K1 cells and found that the EF-1a promoter is a potent regulatory sequence for episomal vectors and maintains high transgene expression [Citation21]. However, only the CHO-K1 cell line, which was unfit for gene therapy owing to its differences from human cells, was tested. In this study, we will optimize the episomal vectors with different cis-acting elements, including enhancer elements, EF-1α and CAG promoters and CMV promoter mutant, to explore their effects on transgene expression in humanized HEK293, Chang liver and primary cells and their molecular mechanisms.
Materials and methods
Vector construction
The CMV promoter mutants (including cytosines at positions 404 and 542 point-mutated to guanosines) were artificially synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The synthesized sequences replaced the CMV promoter (i.e. the CMV promoter was excised and replaced with CMV promoter mutants) in the previously described pEM vector ()) [Citation21], and they were named as mutant-404 and mutant-542 ()). By contrast, the EF-1α and CAG promoters were generated using polymerase chain reaction (PCR). To achieve directional cloning, we introduced the Ase I/Nhe I enzyme site at the 5´ ends of primers. The PCR program was as follows: 95°C pre-denaturation for 3 min, 94°C for 40 s, 56°C for 30 s, 72°C for 30 s, 30 cycles and a final step at 72°C for 3 min. The PCR products were recovered, and their sequences were confirmed, followed by digestion with the Ase I/Nhe I enzyme (TaKaRa Biotechnology Co. Ltd., Dalian, China). The products were then ligated into the pEM vector to produce vectors containing the EF-1α and CAG promoters (,). Three different enhancer elements were also artificially synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and added upstream of the CMV promoters to produce three vectors containing different enhancers ()). The three enhancers, which contained different combinations of nuclear factor (NF)-κB, E-box, GC-box and CCAAT-enhancer-binding protein alpha (C/EBPα) transcription factor regulatory elements (TFREs), were designed according to a previous report [Citation22] and named as enhancer-NGE, enhancer-EEN and enhancer-NNG. The sequence of cis-acting elements used in this study is shown in Supplementary Figure S1.
Cell culture and transfection
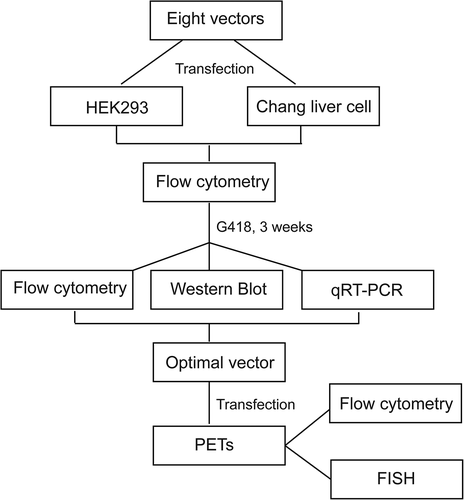
Human embryonic kidney cells (HEK293 cells; Institute of Laboratory Animal Sciences, Beijing, China) were grown in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. Human Chang liver cells (Institute of Laboratory Animal Sciences, Beijing, China) were maintained in Roswell Park Memorial Institute 1640 (RPMI 1640) medium (Invitrogen, MA, USA) supplemented with 10% FBS and 1% penicillin–streptomycin in a humidified incubator at 37°C with 5% CO2. Porcine fetal fibroblasts (PEFs) were isolated and cultured in DMEM containing 10% FBS, 1% glutamine (Gibco, MA, USA), 1% non-essential amino acids and 1% penicillin–streptomycin at 37°C and 5% CO2. After digestion, cells were centrifuged and seeded in 12-well plates. After reaching 80%–90% confluence, triplicate transfections were performed for each vector using Lipofectamine 2000 (Invitrogen) and PolyJet (SignaGen Laboratories, Rockville, MD, USA) according to the manufacturer’s instructions. Experiments were performed twice to ensure the reproducibility of the results (experiments 1 and 2 were performed using Lipofectamine 2000 and PolyJet transfection reagents, respectively). The cells in each well were transfected with different vectors using 2 µl Lipofectamine 2000 transfection reagent per 1 µg linearized plasmid DNA (plasmid DNA was digested with Nhe I) or 2.25 µl PolyJet transfection reagent per 0.75 µg linearized plasmid DNA. This study was conducted in accordance with the declaration of Helsinki and with approval from the Ethics Committee of Xinxiang Medical University.
Transfection efficiency and transient expression
At 48 h post-transfection, the transfection efficiency and transient expression were analyzed by flow cytometry. HEK293, Chang liver and PEF cells were obtained and analyzed using a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). A total of 100,000 fluorescent events were acquired using a 530/15 bandpass filter for the green fluorescent protein signal acquired with a fluorescence emission wavelength of 530 nm.
Recombinant protein expression in stably transfected cells
Forty-eight hours after transfection, stably transfected cells were selected in the medium containing 800 µg/ml of Geneticin (G418, Invitrogen). Non-transfected cells died after 7–10 days of selection, and stably transfected pools were obtained after 3 weeks. The cells were further cultured in medium supplemented with 400 µg/ml G418 for 20 generations, and the eGFP stability expression was analyzed by flow cytometry. All transfections were carried out in triplicate.
Western blot
Western blot was used to further analyze eGFP protein expression in stably transfected cells. A total of 5 × 106 cells were collected and lysed with RIPA Lysis buffer (Beyotime, Shanghai, China). Approximately 10 µl of cell lysate was electrophoresed on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Subsequently, the proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad, USA) and reacted with 1:5000 diluted eGFP antibody (Beyotime, Shanghai, China) at 4°C overnight and then with goat anti-rabbit IgG antibody conjugated with horseradish peroxidase. After washing with PBS for 5 min, the protein bands were visualized using an enhanced chemiluminescence substrate kit (Amersham, GE Healthcare, Chicago, IL, USA). The relative expression values of protein were quantitatively determined using ImageJ software (version 1.41, National Institutes of Health, USA).
qRT-PCR analysis
eGFP transgene expression at the mRNA level was detected in stably transfected cells and analyzed by quantitative reverse transcription PCR (qRT-PCR). From 5 × 106 stably transfected cells, the total RNAs were isolated using TaKaRa RNAiso Reagent according to the manufacturer’s instructions (TaKaRa Company). RNA was converted to cDNA using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, UK). qRT-PCR was performed on a Light Cycler 480 system (Roche) using the Roche LightCycler® 480 SYBR Green Master Mix. The eGFP primers were as follows: 5'-CTACGTCCAGGAGCGCACCATCT-3' (forward), 5'-GTTCTTCTGCTTGTCGGCCATGATAT-3' (reverse). GAPDH was used as an internal reference, and the primer sequences were as follows: 5'-CGACCCCTTCATTGACCTC-3' (forward), 5'-CTCCACGACATACTCAGCACC-3' (reverse). The qPCR procedure consisted of 40 cycles using the manufacturer’s recommended parameters. Relative eGFP mRNA was calculated using the 2−∆∆ Ct method. All experiments were repeated three times.
Analysis of the status of vectors
To verify the status of vectors within the selected stable PEF cells, FISH was performed as described previously at 20 generation post-transfection [Citation13]. eGFP probe was labeled using a digoxigenin-nick translation kit (Roche, Mannheim, Germany). The samples were counterstained with 1 μg/mL of 4',6'-diamidino-2-phenylindole and further analyzed using a Leica DMRB fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Approximately 10 fields were observed.
Bioinformatics analysis
TFREs were identified using the MatInspector software (http://www.genomatix.de/products/index.html) [Citation23].
Statistical analysis
All experimental data were analyzed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Data were reported as means ± standard deviations. A post-analysis of variance multiple comparison procedure was further performed to assess pairwise differences in expression confirmed by analysis of variance. Results with P values less than 0.05 were considered statistically significant.
Results
Transfection efficiency in HEK293 and Chang liver cells
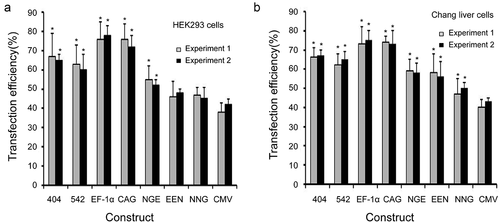
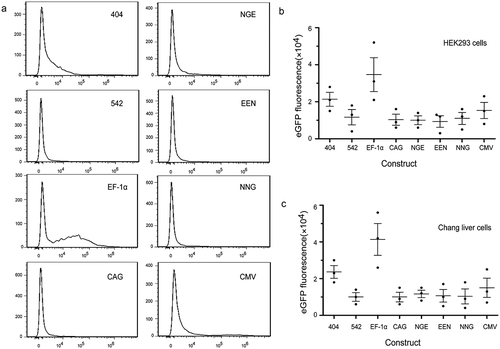
We first evaluated the transfection efficiency of different enhancers, promoters and promoter variants in HEK293 and Chang liver cells. The transfection efficiency was the highest for plasmids containing the EF-1α promoter, followed by the CAG promoter, mutant-404, mutant-542, enhancer-NGE, enhancer-EEN, enhancer-NNG and CMV promoter (). The transfection efficiencies of the plasmids under the EF-1α and CAG promoters, mutant-404, mutant-542 and enhancer-NGE were significantly higher than those under the CMV promoter in HEK293 cells (P < 0.05, )). By contrast, in Chang liver cells, the transfection efficiencies under all elements used in this study were significantly higher than those under the CMV promoter (P < 0.05, )). Moreover, no difference was observed between experiments 1 and 2, suggesting that the transfection efficiency was not affected by the transfection reagent.
Transient expression in HEK293 and Chang liver cells
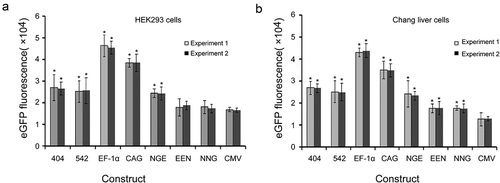
We evaluated the different elements individually to determine their abilities to enhance transient gene expression levels in HEK293 and Chang liver cells using eGFP as the reporter gene. The EF-1α promoter exhibited substantial enhancing effects in HEK293 and Chang liver cells, and transient eGFP levels were increased by approximately threefold. For the other cis-acting elements, the enhancing effects were observed in the following order: CAG promoter > mutant-404 > mutant-542 > enhancer-NGE > enhancer-EEN > enhancer-NNG > CMV promoter. Similarly, transient expressions of the eGFP gene under the EF-1α and CAG promoters, mutant-404, mutant- 542 and enhancer-NGE were also significantly higher than those under the CMV promoter in HEK293 cells (P < 0.05, )). All elements used in this study induced significantly higher eGFP expression compared with that under the CMV promoter in Chang liver cells (P < 0.05, )). When the eGFP expression level from plasmid pEM containing the CMV promoter was set to 1.0, the expression levels in HEK293 cells transfected with plasmid containing the mutant-404, mutant-542, EF-1α promoter, CAG promoter, enhancer-NGE, enhancer-EEN and enhancer-NNG were 1.61 ± 0.22, 1.55 ± 0.13, 2.76 ± 0.12, 2.33 ± 0.25, 1.51 ± 0.16, 1.18 ± 0.24 and 1.05 ± 0.11, respectively, and those in Chang liver cells were 2.08 ± 0.45, 1.97 ± 0.23, 3.40 ± 0.35, 2.71 ± 0.21, 1.78 ± 0.11, 1.37 ± 0.17 and 1.36 ± 0.13, respectively. Moreover, the eGFP transient expression showed no difference between experiments 1 and 2, suggesting that eGFP transient expression was not affected by the transfection reagent.
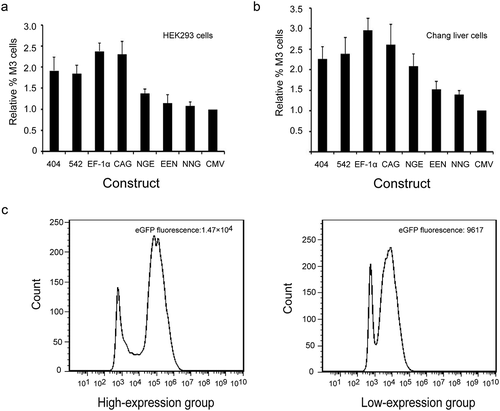
To further evaluate the effects of different elements on eGFP transgene expression levels in HEK293 and Chang liver cells, the percentage of high producers (% M3, fluorescence values greater than 104) was calculated. We analyzed the total population of eGFP-expressing cells and found that the EF-1α promoters resulted in the highest percentages of highly expressing cells (% M3) in transfected HEK293 and Chang liver cells (,). Next, the cells were divided into high-expression (fluorescence values greater than >104) and low-expression groups (fluorescence values smaller than <102) by flow sorting in the plasmid with EF-1α promoters, and eGFP expression was detected by flow cytometry. The results showed that eGFP expression levels in the high-expression group was significantly higher than those in the low-expression group in HEK293 and Chang liver cells (P < 0.05). The representative results are shown in ).
Stable expression in transfected HEK293 and Chang liver cells
Forty-eight hours after transfection, the HEK293 and Chang liver cells were subjected to drug selection to establish stable transfectants. We cultured three colonies of stably transfected cells with drug agents for 20 generations, and eGFP expression was measured. The EF-1α promoter had the most stable expression, followed by mutant-404. The other elements showed less stable expression compared with the CMV promoter (one example is shown in ). The eGFP expression levels under the EF-1α promoter in HEK293 and Chang liver cells were 3.47 ± 0.62 and 4.13 ± 0.54, respectively, which were higher than those under the CMV promoter (1.53 ± 0.27 and 1.50 ± 0.31, ,). Moreover, the eGFP expression levels with mutant-404 were also higher than those of the CMV promoter in HEK293 and Chang liver cells (2.03 ± 0.22 vs. 1.53 ± 0.27, 2.37 ± 0.40 vs. 1.50 ± 0.31; ,). These results suggested that the EF-1α promoter and mutant-404 played a role in strengthening and maintaining eGFP expression and that the EF-1α promoter was the most effective in maintaining recombinant protein expression stability.
Analysis of eGFP protein expression
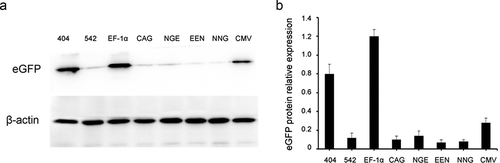
To further investigate the effects of different cis-acting elements on recombinant protein expression, stably transfected cells were collected, and eGFP was detected by Western blot analysis. The results indicated that the eGFP expression levels by Western blot assay were consistent with those of flow cytometry. The EF-1α promoter had the highest eGFP expression, followed by mutant-404 and CMV promoter. The eGFP expression levels with EF-1α promoter and mutant-404 were higher than those of the CMV promoter in HEK293 and Chang liver cells. The representative results are shown in .
mRNA expression in transfected HEK293 and Chang liver cells
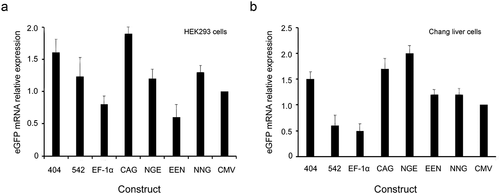
We further analyzed the mRNA expression levels of stably transfected cells. The results showed that the mRNA expression was the highest in cells transfected with the CAG promoter, followed by cells transfected with mutant-404. The mRNA expression levels were not directly proportional to protein expression levels, suggesting that the regulatory mechanism after transcription may influence transgene expression ().
Transient and stability expression in PEF cell
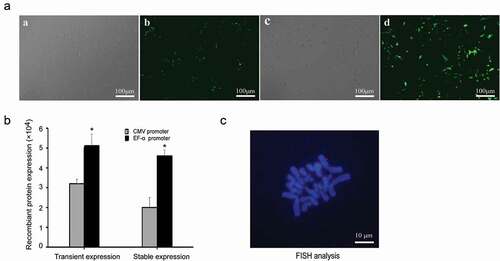
Given that the EF-1α promoter showed the best performance in terms of yielding both high expression levels and transfection efficiency, we chose the EF-1α promoter vector for transfection of primary cells. Forty-eight hours after transfection, eGFP gene expression was observed using an OLYMPUS IX71 fluorescence microscope, and the expression of eGFP was measured by flow cytometry at 48 h and 20 generations after transfection. In PEF cells, the EF-1α promoter produced relatively higher eGFP expression compared with the CMV promoter ()). Flow cytometry showed that the eGFP expression levels with EF-1α and CMV promoters were 5.12 ± 0.53 and 3.26 ± 0.22 in transient expression and 4.67 ± 0.3 and 2.05 ± 0.6 in stability expression, respectively ()). The EF-1α promoter increased the eGFP transgene expression by 1.6-fold and 2.3-fold than the CMV promoter in transient and stable cell pools, respectively.
Figure 8. Recombinant protein expression and status of vectors in PEF cells. (a) eGFP expressions were observed under an epifluorescence microscope after 48 h of transfection. Cells transfected with plasmid containing the CMV promoter under white light (a) and fluorescence (b). Cells transfected with plasmid containing the EF-1α promoter under white light (c) and fluorescence (d). (b) eGFP expressions were determined by flow cytometry after 48 h transfection and at generation 20. Standard error of the mean (SEM) is indicated. * indicates that transgene expression with EF-1α promoter was significantly higher than that from vectors containing the CMV promoter (Student’s t-test, P < 0.05). (c) PEF cells transfected with plasmid containing EF-1α promoter at generation 20 were analyzed by FISH to assess whether the vectors were present as integrated copies. The episome (red) was visualized by eGFP FISH (Blue: metaphase chromosomes; red: vectors).
FISH analysis
FISH analysis was performed on PEF cells at generation 20, and 10 metaphase spreads were analyzed. FISH results revealed that the observed mitotic stability of the vector containing the EF-1α promoter was a result of the vector existing episomally on metaphase chromosomes in PEF cells. The representative results are shown in ).
Analysis of the TFREs
Promoter activity is related to the transcription factor-binding sites and TFREs [Citation24,Citation25]. The distributions of seven TFREs (NF-κB, E-box, GC-box, C/EBPα, E4F1 and CRE) were assessed for the EF-1α, CAG and CMV promoters and enhancer-NGE, enhancer-EEN and enhancer-NNG. The NF-κB, E-box, C/EBPα, E4F1 and CRE TFREs were abundant in the enhancer-NGE, enhancer-EEN and enhancer-NNG (). The EF-1α promoter containing NF-κB had lower E4F1and CRE TFREs (). These findings led us to conclude that NF-κB, E-box, E4F1 and CRE TFREs relate to promoter activity the most.
Table 1. Locations of various transcription factor binding motifs within the promoters and enhancers.
Discussion
An ideal episomal vector for human gene therapy must provide high levels of transgene expression. In this work, we evaluated the effects of different cis-acting elements on transgene expression levels in HEK293, Chang liver, and primary cells (PEF cells). We demonstrated that enhancer elements, mutant CMV and CAG and EF-1α promoters increased transfection efficiency and recombinant protein transient expression. Moreover, the mutant-404 and EF-1α promoters increased recombinant protein stable expression in HEK293 and Chang liver cells. In PEF cells, the EF-1α promoter produced relatively higher eGFP expression compared with the CMV promoter, and FISH analyses indicated that the vector can replicate episomally with cell division.
Previous studies indicated that the endogenous mammalian promoters can provide higher expression than viral promoters [Citation26,Citation27]. The EF-1α promoter has been shown to be one of the strongest promoters in various cell lines [Citation28,Citation29]. Indeed, the EF-1α promoter is often active in cells where viral promoters fail to drive downstream gene expression and are gradually silenced [Citation30,Citation31]. Some studies have shown that promoters of endogenous mammalian genes, such as EF-1α, can be more resistant to silencing than viral promoters [Citation31]. The CAG promoter, a combination of the CMV immediate early enhancer and the chicken β-actin promoter, has frequently been used to drive high-level gene expression in mammalian cells [Citation32–Citation34]. Moreover, the reported EF-1α and CAG promoter are mainly used for viral or integration vectors but not for episomal vectors. In this study, we found that the EF-1α promoter yielded the highest expression level in episomal vectors in transfected HEK293 and Chang liver cells, and CAG yielded high transgene levels in transient expression, which is consistent with a previous report [Citation35]. Thus, these promoters may have an application potential in the design of episomal vectors for gene therapy use.
The CMV promoter is one of the strongest promoters in mammalian cells. However, the expression levels of genes from episomal vectors driven by the CMV promoter are often low [Citation36]. As previously reported, the CMV promoter is prone to transcriptional silencing due to DNA methylation [Citation37,Citation38]. Mammalian DNA is predominantly methylated at cytosine bases that are part of CpG dinucleotides [Citation16]. In the CMV promoter, the cytosines at positions 404 and 542 are frequently methylated [Citation16]. To investigate whether the deletion of CpG sites can enhance CMV-driven gene expression in episomal vectors, we performed point mutations of C to G at positions 404 and 542 and studied the effects of these mutations on gene expression levels in transfected HEK293 and Chang liver cells. The results showed that the mutation of the CMV promoter increased the recombinant protein transient expression, which was consistent with the results of Benjamin et al. [Citation16], and mutant-404 increased stable expression. Therefore, we believe that the point-mutated CMV promoter will be of great interest to scientists with broad research interests.
To further enhance the functions of promoters, various enhancer elements have been added upstream of the promoters [Citation39]. The enhancers are cis-acting elements that can increase transcription levels. According to a previous study [Citation22], we synthesized three different enhancer elements, including different combinations of NF-κB, E-box, GC-box and C/EBPα, and cloned these elements upstream of the CMV promoters. The results showed that all enhancers used in this study could increase the recombinant protein transient expression in transfected Chang liver cells. However, only enhancer-NGE could increase recombinant protein transient expression in HEK293 cells, and enhancer-EEN and enhancer-NNG did not promote any transcriptional enhancing activity. These results may be related to the enhancer specificity in different cell lines, but all enhancers could not maintain the long-term transgene expression.
Western blot analysis also confirmed that the EF-1α promoter and mutant-404 could increase stable recombinant protein expression in transfected Chang liver and HEK-293 cells, and no significant enhancement was observed in those with enhancers.
Research shows that the most active promoters contain relatively high numbers of NF-κB and E-box and a correspondingly low number of GC-box and C/EBPα blocks [Citation22]. The distributions of positive (NF-κB, E-box), neutral (GC-box, C/EBPα) and negative (E4F1, CRE) TFREs were assessed for the EF-1α, CAG and CMV promoters and enhancers. The NF-κB and E-box TFREs were abundant in the enhancer-NGE, enhancer-EEN and enhancer-NNG, but the E4F1 and CRE TFREs were also abundant (). A high number of positive sites are apparently counteracted by high numbers of negative sites to produce relatively weak promoters. The EF-1α promoter had lower E4F1 and CRE TFREs, which may be the reason why the EF-1α promoter showed the most universal and highest enhancement of gene expression. These findings led us to conclude that the type and quantity of TFREs may contribute to promoter activity the most. This may be the reason for the different transgene expressions of different promoters and enhancers. However, the translation-promoting effects of enhancers may rely to some extent on elements associated with the host cell line, and they can be used according to different host cell lines in gene therapy and provide the basis for future study and clinical practice.
Moreover, qRT-PCR analysis showed that the eGFP mRNA expression was the highest in cells transfected with CAG promoter, followed by cells transfected with mutant-404. These results are inconsistent with the results of protein expression levels, suggesting that the post-transcription process may affect transgene expression.
To further verify the effect of episomal vectors in primary cells, they were transfected into PEF cells. The results showed that the EF-1α promoter produced relatively higher eGFP expression compared with the CMV promoter in PEF cells. This finding may be because the CMV promoters used were unsuitable for primary cells, resulting in low transfection efficiency and low expression in primary cells. A previous study demonstrated that the promoter activity is dependent on the cell type [Citation40]. Although CMV promoters are commonly used for the high expression of transgenes in mammalian cells [Citation41,Citation42], they may be inappropriate promoters for strong expression in primary cells. The EF-1α promoter can drive transgene expression in primary cells, but the expression level needs to be further improved. Future studies should attempt to improve the efficiency with other cis-acting elements, such as MARs and UCOEs.
In conclusion, the EF-1α promoter and mutant-404 provide higher enhancement of transient and stable transgene expression levels in two commonly used human cell lines, HEK293 and Chang liver cells. The mechanisms may be related to the type and quantity of TFREs. Moreover, qRT-PCR analysis showed that the mRNA expression levels are not related to protein expression levels in stably transfected mammalian cells. Furthermore, the EF-1α promoter conferred high transgene expression levels in primary cells, and the plasmid was also present in the episomal state. These results provided valuable information for improving transgene expression with episomal vectors in transfected mammalian cells. Therefore, we believe that this contribution is theoretically and practically valuable for gene therapy.
Highlights
EF-1α promoter and CMV promoter mutant greatly enhance transgene expression in HEK293 and Chang liver cells.
In primary cells, High-level transgene expression was from EF-1α promoter.
This study provided valuable information for transgene expression with episomal vector.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download MS Word (17 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Wong SP, Argyros O, Harbottle RP. Sustained expression from DNA vectors. Adv Genet. 2015;89:113–152.
- Xu Z, Chen F, Zhang L, et al. Non-integrating lentiviral vectors based on the minimal S/MAR sequence retain transgene expression in dividing cells. Sci China Life Sci. 2016;59(10):1024–1033.
- Ehrhardt A, Haase R, Schepers A, et al. Episomal vectors for gene therapy. Curr Gene Ther. 2008;8(3):147–161.
- Van Craenenbroeck K, Vanhoenacker P, Haegeman G. Episomal vectors for gene expression in mammalian cells. Eur J Biochem. 2000;267(18):5665–5678.
- Hagedorn C, Wong SP, Harbottle R, et al. Scaffold/matrix attached region-based nonviral episomal vectors. Hum Gene Ther. 2011;22(8):915–923.
- Su RJ, Neises A, Zhang XB. Generation of iPS cells from human peripheral blood mononuclear cells using episomal vectors. Methods Mol Biol. 2016;1357:57–69.
- Piechaczek C, Fetzer C, Baiker A, et al. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999;27:426–428.
- Papapetrou EP, Ziros PG, Micheva ID, et al. Gene transfer into human hematopoietic progenitor cells with an episomal vector carryingan S/MAR element. Gene Ther. 2006;13(1):40–51.
- Calado SM, Oliveira AV, Machado S, et al. Sustained gene expression in the retina by improved episomal vectors. Tissue Eng Part A. 2014;20(19–20):2692–2698.
- Haase R, Magnusson T, Su B, et al. Generation of a tumor- and tissue-specific episomal non-viral vector system. BMC Biotechnol. 2013;13:49.
- Klausner EA, Zhang Z, Wong SP, et al. Corneal gene delivery: chitosan oligomer as a carrier of CpG rich, CpG free or S/MAR plasmid DNA. J Gene Med. 2012;14(2):100–108.
- Stehle IM, Scinteie MF, Baiker A, et al. Exploiting a minimal system to study the epigenetic control of DNA replication: the interplay between ranscription and replication. Chromosome Res. 2003;11(5):413–421.
- Lin Y, Li Z, Wang T, et al. MAR characteristic motifs mediate episomal vector in CHO cells. Gene. 2015;559(2):137–143.
- Zhang X, Wang XY, Jia YL, et al. A vector based on the chicken hypersensitive site 4 insulator element replicates episomally in mammalian cells. Curr Gene Ther. 2017;16(6):410–418.
- Hagedorn C, Antoniou MN, Lipps HJ. Genomic cis- acting Sequences improve expression and establishment of a nonviral vector. Mol Ther Nucleic Acids. 2013;2(8):e118.
- Moritz B, Becker PB, Göpfert U. CMV promoter mutants with a reduced propensity to productivity loss in CHO cells. Sci Rep. 2015;5:16952.
- Hsu CC, Li HP, Hung YH, et al. Targeted methylation of CMV and E1A viral promoters. Biochem Biophys Res Comm. 2010;402(2):228–234.
- Osterlehner A, Simmeth S, Göpfert U. Promoter methylation and transgene copy numbers predict unstable protein production in recombinant Chinese hamster ovary cell lines. Biotechnol Bioeng. 2011;108(11):2670–2681.
- Running Deer J, Allison DS. High-level expression of proteins in mammalian cells using transcription regulatory sequences from the Chinese hamster EF-1alpha gene. Biotechnol Prog. 2004;20(3):880–889.
- Ho SC, Yeo JH, Fang SG, et al. Impact of using different promoters and matrix attachment regions on recombinant protein expression level and stability in stably transfected CHO cells. Mol Biotechnol. 2015;57(2):138–144.
- Wang X, Xu Z, Tian Z, et al. The EF-1a promoter maintains high-level transgene expression from episomal vectors in transfected CHO-K1 cells. J Cell Mol Med. 2017;21(11):3044–3054.
- Brown AJ, Sweeney B, Mainwaring DO, et al. Synthetic promoters for CHO cell engineerin. Biotechnol Bioeng. 2014;111(8):1638–1647.
- Quandt K, Frech K, Karas H, et al. New fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23(23):4878–4884.
- Kuznetsov VA. Mathematical modeling of avidity distribution and estimating general binding properties of transcription factors from genome-wide binding profiles. Methods Mol Biol. 2017;1613:193–276.
- Zamanighomi M, Lin Z, Wang Y, et al. Predicting transcription factor binding motifs from DNA-binding domains, chromatin accessibility and gene expression data. Nucleic Acids Res. 2017;45(10):5666–5677.
- Norrman K, Fischer Y, Bonnamy B, et al. Quantitative comparison of constitutive promoters in human ES cells. PLoS One. 2010;5(8):e12413.
- Ackford JG, Corredor JC, Pei Y, et al. Foreign gene expression and induction of antibody response by recombinant fowl adenovirus-9-based vectors with exogenous promoters. Vaccine. 2017;35(37):4974–4982.
- Suzuki JI, Dezawa M, Kitada M. Prolonged but non-permanent expression of a transgene in ependymal cells of adult rats using an adenovirus-mediated transposon gene transfer system. Brain Res. 2017;1675:20–27.
- Kurosaki F, Uchibori R, Mato N, et al. Optimization of adeno-associated virus vector-mediated gene transfer to the respiratory tract. Gene Ther. 2017;24(5):290–297.
- Byun HM, Suh D, Jeong Y, et al. Plasmid vectors harboring cellular promoters can induce prolonged gene expression in hematopoietic and mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;332(2):518–523.
- Gill DR, Smyth SE, Goddard CA, et al. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8(20):1539–1546.
- Yang CQ, Li XY, Li Q, et al. Evaluation of three different promoters driving gene expression in developing chicken embryo by using in vivo electroporation. Genet Mol Res. 2014;13(1):1270–1277.
- Liu Y, Fu S, Niu R, et al. Transcriptional activity assessment of three different promoters for mouse in utero electroporation system. Plasmid. 2014;74:52–58.
- Luo Y, Liu C, Cerbini T, et al. Stable enhanced green fluorescent protein expression after differentiation and transplantation of reporter human induced pluripotent stem cells generated by AAVS1 transcription activator-like effector nucleases. Stem Cells Transl Med. 2014;3(7):821–835.
- Chan KK, Wu SM, Nissom PM, et al. Generation of high-level stable transgene expressing human embryonic stem cell lines using Chinese hamster elongation factor-1 alpha promoter system. Stem Cells Dev. 2008;17(4):825–836.
- Wang XY, Zhang JH, Zhang X, et al. Impact of different promoters on episomal vectors harbouring characteristic motifs of matrix attachment regions. Sci Rep. 2016;6:26446.
- Yang Y, Chusainow J, Yap MG. DNA methylation contributes to loss in productivity of monoclonal antibody-producing CHO cell lines. J Biotechnol. 2010;147(3–4):180–185.
- Wen S, Zhang H, Li Y, et al. Characterization of constitutive promoters for piggyBac transposon-mediated stable transgene expression in mesenchymal stem cells (MSCs). PLoS One. 2014;9:e94397.
- Wang W, Jia YL, Li YC, et al. Impact of different promoters, promoter mutation, and an enhancer on recombinant protein expression in CHO cells. Sci Rep. 2017;7:10416.
- Zhang XB. Cellular reprogramming of human peripheral blood cells. Genomics Proteomics Bioinformatics. 2013;11(5):264–274.
- Han NR, Lee H, Baek S, et al. Delivery of episomal vectors into primary cells by means of commercial transfection reagents. Biochem Biophys Res Commun. 2015;461(2):348–353.
- Stavrou EF, Lazaris VM, Giannakopoulos A, et al. The ß-globin replicator greatly enhances the potential of S/MAR based episomal vectors for gene transfer into human haematopoietic progenitor cells. Sci Rep. 2017;7:40673.