ABSTRACT
Effects of antimicrobial compounds on dry anaerobic digestion (dry-AD) processes were investigated. Four compounds with known inhibition effects on traditional wet digestion, i.e. car-3-ene, hexanal, 1-octanol and phenol were selected and investigated at concentrations of 0.005%, 0.05% and 0.5%. Food waste (FW) and Paper waste (PW) were used as model substrates, all assays were running with the substrate to inoculum ratio of 1:1 (VS basis) corresponding to 15% TS in reactors. Generally, increasing concentrations of inhibitors resulted in decreasing methane yields with a few exceptions; in all these specific cases, long, lag phase periods (60 days) were observed. These adaptation periods made possible for the microbial systems to acclimatize to otherwise not preferred conditions leading to higher methane yields. Comparing the effects of the four different groups, phenols had the highest inhibitory effects, with no methane production at the highest amount added, while the lowest effects were obtained in cases of car-3-ene. Furthermore, the results showed that adding inhibitors up to a certain concentrations can repair the balance in AD process, slowing down the degradation steps, hence making it possible for the methanogens to produce a higher amount of methane. This phenomenon was not observed in case of PW, which is already a slow degradable substrate in its nature.
1. Introduction
Anaerobic digestion (AD) to produce biogas has not only been a successful treatment of different organic wastes, but has also provided a solution for solving environmental, health and energy challenges [Citation1–Citation3]. The digestion process could either be wet (wet-AD) or dry (dry-AD) depending on the total solids (TS) content of the feedstock; wet-AD has a lower TS content, i.e. between 0.5% and 15% [Citation4] and in dry-AD a higher TS content above 20% is applied [Citation5,Citation6]. Wet-AD has been the common process for digesting organic wastes, but the development of horizontal continuous dry-AD in the last decade made dry-AD an attractive choice of the technology due to the high solid content of the treated wastes, low water usage, low digestate water content and other economic benefits [Citation7–Citation9]. However, dry-AD processes might require a longer retention time compared to that of wet-AD especially when the feedstocks contain substances that could be inhibitory to the digestion process; the methanogens are the most sensitive group of microorganisms involved and as such require a longer time for acclimatization to avoid process failure [Citation10,Citation11].
Wastes from Food and Paper industries are carbon-rich feedstocks with high TS content which makes them suitable for dry-AD processes. The production of pulp and paper involves several process steps, like material preparation, pulping, bleaching, washing/filtering, screening, and finally drying or paper making aiming to get pulp or paper, respectively [Citation12]. From this manufacturing process, a large amount of different waste streams are generated, such as wastewater and solid wastes [Citation13]. Dry-AD process is suitable for treating the solid wastes generated while meeting the industry’s energy demand. However, the generated paper waste (PW) contains mostly hemicellulose and cellulose which are difficult for the microorganisms to degrade and as such the degradation process is slow.
On the other hand, about one-third of food produced is being loosed or wasted, counting up about 1.3 billion tones per year [Citation14]. The reduction of food loss and waste is also addressed by the sustainable development goal 12.3, aiming to halve the amount of capita by 2030 [Citation15]. Food loss occurs at the post-harvest level caused by a decrease in quality or quantity of food during the supply chain, while food waste is a part of food loss caused by discarding food which otherwise would be suitable for human consumption [Citation15]. Food waste (FW) is also contributing to environmental pollution and the depletion of our natural resources. FW is a carbon-rich organic waste with suitable carbon-nitrogen ratio for AD and often with high solid content making it suitable for dry-AD process. Nevertheless, the process tends to be susceptible to acid accumulation due to the easy degradation of these types of feedstocks [Citation16–Citation18]. Hence, there is a need for stable process conditions and for control of the digestion process to achieve an enhanced methane yield.
Another major concern in the digestion of these wastes streams has been the presence of chemical compounds that may inhibit the digestion process; sometimes these contaminants enter the process as a result of improper sorting. The effect of these substances on the digestion process vary depending on the type and concentration, as well as operation parameters, such as retention time, pH, TS content of the feedstock, temperature and available microorganisms [Citation10,Citation19]. Phenolic compounds are well known as contaminants found in agricultural and industrial wastes [Citation20], such as pulp & paper mill industries [Citation21,Citation22]. Terpenes are process emissions from kraft pulp industry, especially 3-carene and α-pinene [Citation23]; terpenes have been reported as the dominating volatile organic compounds in the biogas reactor when digesting food waste [Citation24]. In addition, terpenes are well known in herbs, car-3-ene are found in rosemary and p-cymene in cumin spice [Citation25]. Chemical compounds including terpenes (e.g. car-3-ene), aldehydes (e.g. hexanal) and alcohols (e.g. 1-octanol) have been reported as flavors in various fruits. These chemical compounds are antimicrobial substances [Citation26,Citation27] that protect the fruits against microbial invasion, and as such the presence of these flavours can results in process instability during AD processes [Citation28,Citation29].
Several researchers have previously reported these chemical compounds (car-3-ene, hexanal, 1-octanol and phenol) to affect methane yield during wet digestion processes [Citation20,Citation29,Citation30]. Wikandari et al.[Citation29] investigated the effect of aldehydes (hexanal, nonanal, and E-2-hexenal), terpenes (car-3-ene, α-pinene, and myrcene), and alcohol (octanol) at concentrations of 0.005%, 0.05%, and 0.5% in a synthetic medium under thermophilic wet digestion processes. Their result showed all these compounds to have inhibitory effect on methane yield. However, the effect of these chemical compounds on dry-AD of FW and PW under mesophilic conditions has not been clearly stated.
This work was aimed to examine the effect of car-3-ene, hexanal, 1-octanol and phenol on dry-AD to see if they show similar inhibition effects as reported on wet-AD. Two substrates of easily degradable and hard to degrade, i.e. food and paper industry wastes were selected for the study.
2. Material and methods
2.1 Inoculum, substrates and chemicals
The inoculum obtained from a dry anaerobic digester plant, treating municipal solid waste (Västblekinge Miljö AB, Mörrum, Sweden) operating at mesophilic conditions was used. The inoculum was filtered through a 5-mm porosity sieve after removing the plastic particles manually and then it was kept at 37°C before use in batch assays. The total solid (TS) content of the inoculum was 13%.
Paper waste (PW) and synthetic food waste (FW) were used as substrates in this work. The paper waste with a TS content of 27% was supplied by pulp and paper industry Södra AB (Varberg, Sweden). The TS content of the synthetic food wastes used was 22%, as a results of the addition of more bread, rice and pasta, compared to as it was reported in a previous work of [Citation1]. The chemical compounds examined were from four groups i.e. terpenoid (car-3-ene), aldehyde (hexanal), alcohol (1-octanol) and aromatic organic compound (phenol); all provided by Sigma-Aldirch (Germany). Chemical solutions were prepared by diluting pure liquid chemical compounds of car-3-ene, hexanal and 1-octanol with distilled water in order to achieve concentrations of 0.005%, 0.05% and 0.5% (w/v) [Citation31]. The chemical solution of phenol was prepared by mixing a solid compound of phenol with methanol in order to prepare concentrations of 0.005%, 0.05% and 0.5% (w/v) [Citation32].
2.2 Dry anaerobic digestion assays
Paper waste and synthetic food waste were used as substrates. Additionally, microcrystalline cellulose with a particle size of 50 µm (Sigma Aldrich, Germany) was used as positive control aiming to determine the activity of the inoculum and the dry anaerobic digestion assays were performed according to Angelidaki et al. [Citation33]; however, with no addition of water keeping high solids content within the assays. The experiments were carried out at mesophilic conditions (37 ± 1°C)using 118 mL serum glass bottles as reactors. Substrates with a loading of 3.0 g VS were used with an inoculum of 3.0 g VS keeping the VS ratio (VSsubstrateto VSinoculum) at 1:1. Thereafter, the chemical compounds at concentrations of 0.005%, 0.05% and 0.5% (w/v) were added making a total TS of 15% and 16% in reactor mixtures of food and paper wastes, respectively. In order to measure the methane production of only the inoculum, the inoculum was incubated without the addition of substrates and chemical compounds; i.e. distilled water or methanol was only added in those blank setups. Finally, rubber septum and aluminum caps were used to seal the reactors, and then a gas mixture, containing 80% N2 and 20% CO2,was used for flushing through the headspace of all reactors under 2 min in order to achieve anaerobic conditions. The reactors were then kept in an incubator (Venticell 707, MMM Medcenter Einrichtungen GmbH, Munich, Germany)
At a temperature of 37 ± 1°C to ensure constant temperature conditions and they were sheaken manually prior to each gas measurement during an experimental period of 112 days. Gas samples were taken from the headspace of each reactor in every second and third day at the beginning and once a week toward the end of the digestion period to follow up the biogas production. A 250-μl pressure-lock gas syringe (VICI, precious sampling Inc., USA) was used for the sampling and the measurements were performed according to Teghammar et al. [Citation34], and the gas volumes were then recalculated and presented at standard conditions (0°C and 1 atm). All experimental setups were performed in triplicates.
2.3. Analytical methods
Total solids (TS), volatile solids (VS), moisture content and pH for the substrates and the inoculum were determined according to Standard methods for water and wastewater [Citation35]. The concentration of the total nitrogen (TN) content was analyzed by using Kjeldahl nitrogen method [Citation36], while the total carbon was obtained by correcting the total dry weight carbon value for the ash content [Citation18,Citation37].
The compositions (methane, hydrogen and carbon dioxide) of the produced gas were determined using GC (Perkin-Elmer 590, Perkin-Elmer, USA) equipped with a packed column (6′ × 1.8″ OD, 80/100, Mesh, Perkin Elmer, USA), and a thermal conductivity detector (Perkin-Elmer, USA), with an inject temperature of 150°C. The carrier gas was nitrogen operated with a flow rate of 20 ml/min at 60°C.
2.4. Statistical analysis
The experiments were performed in triplicates, and all error bars and intervals reported represent 95% confidence intervals.
3. Results and discussion
In order to determine the effects of some inhibitory compounds, found in food waste (FW) and paper waste (PW), batch dry-AD assays were carried out in the presence of four different types of inhibitors at three different concentrations.
Increasing the concentrations of these chemical compounds resulted in decreased methane yields with a few exceptions as shown in . In case of food waste, the results however showed that the addition of inhibitors up to a concentration of 0.05% would rather improve the performance of the system leading to up to 84% more methane yield compared to the control (). Since all assays were running with the substrate to inoculum ratio of 1:1 (VS basis) to achieve high initial solids of 15% TS in the system, the assays running with FW were seemed to be overloaded. This assumption is confirmed by the low methane yield (223 mL/g VS) observed for the control (no inhibitor addition). Other studies reported higher methane yields for food wastes [Citation38–Citation40]. Overloading can be a challenge, when larger amount of easy degradable compounds, as in food waste, are present in AD systems. Since the first degradation steps (i.e. hydrolysis and acidogenesis) perform faster, it will lead to the accumulation of intermediary products, mainly volatile fatty acids, during these conditions, which in turn will lead to a decrease in pH. Methanogens, the group of microorganisms producing methane in the last step, will negatively be affected by this pH change, resulting in a decreased methane yield [Citation39,Citation40]. The results of this study most likely suggest that adding inhibitors up to a certain concentrations can repair the balance in the degradation process, slowing down the initially degradation steps, hence making it possible for the methanogens to produce a higher amount of methane in the end. Nevertheless, further increase in the concentration of inhibitors up to 0.5%, led to a clear inhibition.
Table 1. Effect of the additions of chemical compounds on biogas production at different concentrations
Long lag phase periods of 15–20 days were observed in all cases (). For similar experiments with wet-AD, Wikandari et al. [Citation29] also reported a lag phase of 9 days when similar inhibitors were added in the digestion in batch assays. These long adaptation periods made it possible for the microbial systems to acclimatize to otherwise not preferred conditions leading to higher cumulative methane yields, even with a higher concentration of inhibitory substances. Comparing the effects of the four different groups, the results of this study showed that phenols had the highest inhibitory effects, resulting in no methane production at the highest amount added, while the lowest effects were obtained in cases of the addition of car-3-ene ().
Figure 1. Effect of chemical compounds on anaerobic dry digestion of food waste: (a) car-3-ene (b) hexanal (c) 1-octanol (d) phenol, at different concentrations of 0.005%, 0.05%, 0.5%
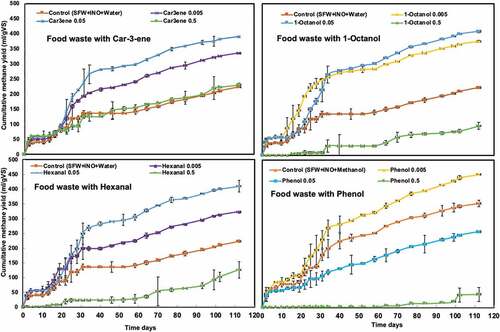
The performance of the system was affected differently in case of dry digestion of PW ( and ). PW consists of slowly degradable lignocellulosic fibers, making the hydrolysis step as the rate-limiting step in its anaerobic degradation process. This system most likely was already in balance despite of the high solid loading of 15% TS, showing a methane yield of 240 NmL/g VS in the control (). This is comparable with other values reported previously for AD of PW [Citation3,Citation41,Citation42]. Addition of inhibitors except in the case of car-3-ene, resulted in lower methane yields than that obtained from only PW (). Similarly, as in the case of FW, the addition of car-3-ene resulted in the lowest effects. Surprisingly, the highest methane yield of 317 NmL/g VS was observed when the highest concentration of car-3-ene was added; however, this was reached after a very long period, i.e. 60 days, of lag phase (). Jansson et al. [Citation43] also previously reported a long lag phase of 70 days for PW used in similar batch assays at S/I ratio (VS basis) of one with a TS of 16%. In an earlier report [Citation44], the inhibitory effects of car-3-ene was examined on 14 different bacterial strains and the results showed antimicrobial effects with a minimum inhibitory concentration (MIC) of between 50–800 mg/L. Other terpenoid compounds, such as D-limonene was also reported to have a negative impact on mesophilic and termophilic anaerobic digestion at concentrations of 400 or 450–900 mg/L [Citation29,Citation44,Citation45].
Figure 2. Effect of chemical compounds on anaerobic dry digestion of paper waste: (a) car-3-ene (b) hexanal (c) 1-octanol (d) phenol, at different concentrations of 0.005%, 0.05%, 0.5%
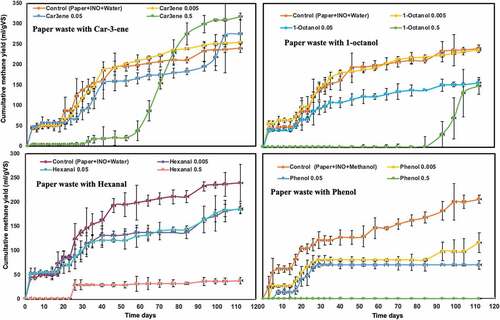
When it comes to the effects of the other inhibitors, several studies reported negative effects of hexanal (aldehyds) and 1-octanol (alcohol). Hexanal showed to have a negative impact on Listeria monocytogenesand also, E.coli and Salmonella enteritidiswhen the growth of these strains were investigated on fresh apple slices in presence of inhibitor concentrations of up to 100, 250 and 200 mg/L. The results showed a similar tendency with a long lag phase of up to 50 days detected [Citation46].
In another study, the effect of hexanal was investigated on the growth of several different microorganisms (both bacteria and fungi), where Bacillus subtilis, Brevibacterium ammoniagenes, Staphylococcus aureus, Streptococcus mutans, Propionibacterium acnes, Psedomonas aeruginosa, E. coli, Enterobacter aerogenes, Proteus vulgaris, S. cereviseae, Candida utilis, Pityrosporum ovale, Penicillium chrysogenum, Thricophyton mentagrophytes were tested. It was found that the minimum inhibitory concentration of hexanal was about 800 mg/L for all strains tested [Citation44]. The inhibitory mechanism of hexanal was examined later by Patrignani et al. [Citation47] stating that it has a negative impact on the membrane fatty acids modulation.
When it comes to the inhibitory effects of alcohols, the results of Ingram [Citation48], indicated that toxicity of alcohols is related to the length of the molecular chain, with a longer chain leading to a higher inhibitory activity. As it was mentioned above, the addition of phenols has resulted in the highest toxicity in case of both FW and PW [Citation49,Citation50]. There are a few previous studies that reported the effects of phenols on anaerobic digestion processes. Levén et al. [Citation49] investigated anaerobic degradation of phenols and they found that the degradation depends on process temperature with higher degradation efficiency at mesophilic than at termophilic conditions. However, they have just reviewed the degradation of phenolic compounds naturally presented in different fractions of organic solid wastes, but they have not reported inhibitory levels of phenols. Another study investigated the effects of phenols added at 10 different concentration [up to 5 g/L) in anaerobic batch digestion assays. It was found that although the performance of the process was not affected up to an initial concentration of phenol of 1 g/L, there was a progressive shift obtained within the microbial population from phenol concentrations above 0.5 g/L, showing the high level of adaptability of the microbial communities [50].
Conclusion
The results of this work showed that the organic compounds investigated (car-3-ene, hexanal, 1-octanol, and phenol) have both synergetic and antagonistic effects on the methane production from dry-AD of FW and PW. Comparing the effects of these four different chemical compounds for both substrates investigated, phenols had the highest antagonistic effects at concentrations of 0.05% and 0.5% while synergetic effects were observed at all concentrations when car-3-ene was added. Addition of chemical compounds up to a concentration of 0.05% improved the performance of the dry-AD process (in case of FW), an increase in methane yield from 45% to 84% was obtained compared to the control. However, the process was highly inhibited as the concentration increased to 0.5%. On the other hand, high inbition was observed in the case of PW at all concentrations irrespective of the chemical compound used except for car-3-ene that resulted in an increase from 6% to 32% as concentration increases.
Acknowledgement
This work was financially supported by Linnaeus University and University of Borås in Sweden.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Ariunbaatar J, Esposito G, Yeh DH, et al. Enhanced anaerobic digestion of food waste by supplementing trace elements: role of Selenium (VI) and Iron (II). Front Environ Sci. 2016;4:8.
- Lou X, Nair J. The impact of landfilling and composting on greenhouse gas emissions–a review. Bioresour Technol. 2009;100(16):3792–3798.
- Rodriguez C, Alaswad A, El-Hassan Z, et al. Mechanical pretreatment of waste paper for biogas production. Waste Manage. 2017;68:157–164.
- Li Y, Park SY, Zhu J. Solid-state anaerobic digestion for methane production from organic waste. Renew Sust Energ Rev. 2011;15(1):821–826.
- Bolzonella D, Innocenti L, Pavan P, et al. Semi-dry thermophilic anaerobic digestion of the organic fraction of municipal solid waste: focusing on the start-up phase. Bioresour Technol. 2003;86(2):123–129.
- Yi J, Dong B, Jin J, et al. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: performance and microbial characteristics analysis. PLoS One. 2014;9(7):e102548.
- Karthikeyan OP, Visvanathan C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: a review. Rev Environ Sci Bio. 2013;12(3):257–284.
- Rapport J, Zhang R, Jenkins B, et al. Current anaerobic digestion technologies used for treatment of municipal organic solid waste. Contractor's Report, California Environmental Protection Agency. Sacramento, CA; 2008.
- Watkins K 2006. Human development report 2006-beyond scarcity: power, poverty and the global water crisis. [Cited 2017 Sept 07]. http://www.undp.org/content/dam/undp/library/corporate/HDR/2006%20Global%20HDR/HDR-2006-Beyond%20scarcity-Power-poverty-and-the-global-water-crisis.pdf.
- Schnürer A, Jarvis A 2010. Microbiological handbook for biogas plants. Swedish Gas Centre Report 207: 13–138.
- Veluchamy C, Kalamdhad AS. Influence of pretreatment techniques on anaerobic digestion of pulp and paper mill sludge: A review. Bioresour Technol. 2017;245:1206–1219.
- Bajpai P. Green chemistry and sustainability in pulp and paper industry. Springer International Publishing, Swithzerland; 2015.
- Kamali M, Khodaparast Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotox Environ Safe. 2015;114:326–342.
- FAO. Global food losses and food waste – extent, causes and prevention. 2011. [Cited 2016 Sept 20]. http://www.fao.org/docrep/014/mb060e/mb060e.pdf.
- FAO. 2017. Global initiative on food loss and waste. [Cited 2020 Mar 25]. http://www.fao.org/3/a-i7657e.pdf.
- Demirel B, Scherer P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenerg. 2011;35(3):992–998.
- Liu X, Liu H, Chen Y, et al. Effects of organic matter and initial carbon–nitrogen ratio on the bioconversion of volatile fatty acids from sewage sludge. J Chem Technol Biotechnol. 2008;83(7):1049–1055.
- Zhou C, Liu Z, Huang Z-L, et al. A new strategy for co-composting dairy manure with rice straw: addition of different inocula at three stages of composting. Waste Manage. 2015;40:38–43.
- Yanti H, Wikandari R, Millati R, et al. Effect of ester compounds on biogas production: beneficial or detrimental? Energy Sci Eng. 2014;2(1):22–30.
- Hernández M, Fernández L, Borrás C, et al. Characterization of surfactant/hydrotalcite-like clay/glassy carbon modified electrodes: oxidation of phenol. Anal Chim Acta. 2007;597(2):245–256.
- Chandra R, Raj A, Purohit HJ, et al. Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere. 2007;67(4):839–846.
- Uğurlu M, Gürses A, Doğar Ç, et al. The removal of lignin and phenol from paper mill effluents by electrocoagulation. J Environ Manage. 2008;87(3):420–428.
- Strömvall A-M 1992. Terpenes emitted to air from forestry and the forest industry. PhD thesis, Chalmers University of Technology, Göteborg, Sweden
- Arrhenius K, Holmqvist A, Carlsson M, et al. Terpenes in biogas plants digesting food wastes: study to gain insight into the role of terpenes. Stockholm: Energiforsk; 2017.
- Patel S. Plant essential oils and allied volatile fractions as multifunctional additives in meat and fish-based food products: a review. Food Addit Contam. 2015;32(7):1049–1064.
- Delgado-Adámez J, Garrido M, Bote M, et al. Chemical composition and bioactivity of essential oils from flower and fruit of Thymbra capitata and Thymus species. J Food Sci Tech. 2017;54(7):1857–1865.
- Ferreira Farias A, Da Silva Ramos R. Chemical composition, an antioxidant, cytotoxic and microbiological activity of the essential oil from the leaves of aeollanthus suaveolens Mart. ex Spreng. PLoS One. 2016;11(12):e0166684.
- Negro V, Mancini G, Ruggeri B, et al. Citrus waste as feedstock for bio-based products recovery: review on limonene case study and energy valorization. Bioresour Technol. 2016;214:806–815.
- Wikandari R, Gudipudi S, Pandiyan I, et al. Inhibitory effects of fruit flavors on methane production during anaerobic digestion. Bioresour Technol. 2013;145:188–192.
- Marone A, Carmona-Martínez AA, Sire Y, et al. Bioelectrochemical treatment of table olive brine processing wastewater for biogas production and phenolic compounds removal. Water Res. 2016;100:316–325.
- OECD. Test No. 224: Determination of the Inhibition of the Activity of Anaerobic Bacteria Reduction of Gas Production from Anaerobically Digesting (sewage) Sludge. Paris: OECD Publishing, Paris.2007.
- ISO 10 634. 2018. Water quality - Guidance for the preparation and treatment of poorly water-soluble organic compounds for the subsequent evaluation of their biodegradability in an aqueous medium ISO, Geneva.
- Angelidaki I, Alves M, Bolzonella D, et al. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol. 2009;59(5):927–934.
- Teghammar A, Yngvesson J, Lundin M, et al. Pretreatment of paper tube residuals for improved biogas production. Bioresour Technol. 2010;101(4):1206–1212.
- APHA. WPCF, Standard methods for the examination of water and wastewater. Washington DC, USA: American Public Health Association/American Water Works Association/Water Environment Federation; 1995.
- Ferree MA, Shannon RD. Evaluation of a second derivative UV/visible spectroscopy technique for nitrate and total nitrogen analysis of wastewater samples. Water Res. 2001;35(1):327–332.
- Haug R. The practical handbook of compost engineering. Boca Raton: Lewis Publishers; 1993.
- Capson-Tojo G, Rouez M, Crest M, et al. Food waste valorization via anaerobic processes: a review. Rev Environ Sci Bio. 2016;15(3):499–547.
- Kawai M, Nagao N, Tajima N, et al. The effect of the labile organic fraction in food waste and the substrate/inoculum ratio on anaerobic digestion for a reliable methane yield. Bioresour Technol. 2014;157:174–180.
- Zhang C, Su H, Baeyens J, et al. Reviewing the anaerobic digestion of food waste for biogas production. Renew Sust Energ Rev. 2014;38:383–392.
- Li WW, Siddhu MAH, Amin FR, et al. Methane production through anaerobic co-digestion of sheep dung and waste paper. Energy Conv Manag. 2018;156:279–287.
- Meyer T, Edwards EA. Anaerobic digestion of pulp and paper mill wastewater and sludge. Water Res. 2014;65:321–349.
- Jansson AT, Patinvoh RJ, Sárvári Horváth I, et al. Dry Anaerobic Digestion of Food and Paper Industry Wastes at Different Solid Contents. Fermentation. 2019;5(2):40.
- Muroi H, Kubo A, Kubo I. Antimicrobial activity of cashew apple flavor compounds. J Agr Food Chem. 1993;41(7):1106–1109.
- Forgács G 2012. Biogas production from citrus wastes and chicken feather: pretreatment and co-digestion. PhD Thesis, Chalmers University of Technology, Göteborg,Sweden
- Lanciotti R, Belletti N, Patrignani F, et al. Application of hexanal,(E)-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J Agr Food Chem. 2003;51(10):2958–2963.
- Patrignani F, Iucci L, Belletti N, et al. Effects of sub-lethal concentrations of hexanal and 2-(E)-hexenal on membrane fatty acid composition and volatile compounds of Listeria monocytogenes, Staphylococcus aureus, Salmonella enteritidis and Escherichia coli. Int J Food Microbiol. 2008;123(1-2):1–8.
- Ingram LO. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976;125(2):670–678.
- Levén L, Nyberg K, Schnürer A. Conversion of phenols during anaerobic digestion of organic solid waste–a review of important microorganisms and impact of temperature. J Environ Manage. 2012;95:S99–S103.
- Poirier S, Bize A, Bureau C, et al. Community shifts within anaerobic digestion microbiota facing phenol inhibition: towards early warning microbial indicators? Water Res. 2016;100:296–305.
