ABSTRACT
Chemoresistance is a major obstacle to effective breast cancer chemotherapy. However, the underlying molecular mechanisms remain unclear. The long noncoding RNA H19 (H19) is involved in various stages of tumorigenesis, however, its role in doxorubicin resistance remains unknown. The goal of this study was to evaluate the role of H19 in the development of doxorubicin-resistant breast cancer. Quantitative real-time PCR (qRT-PCR) analyzed H19 expression in chemotherapy-resistant and sensitive breast cancer tissues. Both knockdown and overexpression of H19 were used to assess the sensitivity to doxorubicin in breast cancer cells in vitro and in vivo. qRT-PCR and Western blot were used to explore the doxorubicin resistance mechanism of H19. We observed that the H19 expression was significantly upregulated in chemotherapy-resistant breast cancer tissues and doxorubicin-resistant breast cancer cell lines. Knockdown of H19 enhanced the sensitivity to doxorubicin both in vitro and in vivo. While H19 overexpression developed doxorubicin-resistant in breast cancer cells both in vitro and in vivo. Furthermore, it was revealed that H19 negatively regulated PARP1 expression in breast cancer cells following doxorubicin treatment. Knockdown of H19 sensitized breast cancer cells to doxorubicin by promoting PARP1 upregulation. H19 overexpression could recapitulate doxorubicin resistance by PARP1 downregulation. Our findings revealed that H19 plays a leading role in breast cancer chemoresistance development, mediated mainly through a H19-PARP1 pathway.
Introduction
Breast cancer was regarded as the second leading cause of death caused by cancer among women after lung cancer [Citation1]. Currently, chemotherapy is the treatment of choice for breast cancer which initiates tumor cell apoptosis while other therapeutic modalities highlight works on hormone receptor resistance and targeted therapy. After receiving treatment, patients with breast cancer may have long-term survival with almost 90% of them showing a 5-year survival rate [Citation2]. However, intrinsic or acquired drug resistance is a major impediment to the successful treatment of women with breast cancer using chemotherapy. With the progress of treatment, patients often become resistant to chemotherapy and result in treatment failure [Citation3,Citation4]. Therefore, it is necessary to explore the molecular mechanisms that mediate breast cancer formation and chemotherapy resistance that is useful in treating the disease.
Long noncoding RNAs (lncRNAs) have recently gained considerable attention as key players in biological regulation; however, the mechanisms by which lncRNAs govern various disease processes remain mysterious and are just beginning to be understood [Citation5]. Some lncRNAs have been proved to play an important role in the tumor drug resistance [Citation6], such as lncRNA MIR100HG which mediates cetuximab resistance via Wnt/beta-catenin signaling [Citation7]. lncRNA HULC triggers autophagy attenuates the chemosensitivity of HCC cells [Citation8]. A large number of clinical studies have suggested that long noncoding RNA H19 (H19) can serve as a potential biomarker for the diagnosis of breast cancer. High expression levels of H19 increase the drug resistance of breast cancer cells and is associated with poor prognosis within patients with breast cancer [Citation9]. For example, circulating H19 has been proved to predict presurgical response to neoadjuvant chemotherapy in breast cancer, high H19 levels had poor response to neoadjuvant chemotherapy [Citation10]. H19 overexpression was negatively correlated to the trastuzumab-therapy response, and H19 downregulation could reverse trastuzumab resistance and enhance the inhibitory function of this drug [Citation11]. H19 overexpression was observed in doxorubicin-resistant breast cancer cells, and H19 downexpression significantly decreased doxorubicin resistance by reducing cell viability and inducing apoptosis [Citation12]. However, the biological mechanism of H19 in regulating doxorubicin sensitivity in breast cancer has not been fully elucidated. Si et al. [Citation13] reported that H19 attenuated cell apoptosis in response to PTX treatment by inhibiting transcription of pro-apoptotic genes BIK and NOXA. Zhu et al. [Citation14] reported that H19 mediated breast cancer chemoresistance mainly through a H19-CUL4A-ABCB1/MDR1 pathway. Han et al. [Citation15] reported that targeting H19 might restore chemo-sensitivity in paclitaxel-resistant breast cancer by mediating the AKT signaling pathway. However, the biological role of H19 in predicting postsurgical chemotherapy response in patients with breast cancer and on doxorubicin chemoresistance in breast cancer cells remains poorly understood.
Poly (ADP-ribose) polymerase (PARP) catalyzes the attachment of ADP-ribose units to itself and its target proteins to recruit the DNA repair machinery to the site of DNA damage. PARP1 also functions in other cellular processes, including transcriptional regulation and cell cycles [Citation16]. Recent clinical data confirmed that the cytoplasmic PARP-1 expression in the needle core biopsies of breast cancer was low, and low PARP-1 expression was associated with no or poor response to chemotherapy [Citation17]. Haynes et al. [Citation18] reported that PARP-1 stabilization/hyperactivation induced cell death and reversed chemo-resistance in breast cancer cells. Thus, we speculated that PARP1 is involved in chemotherapy response in breast cancer.
Previous studies demonstrated that loss of some long non-coding RN (lncRNA) leads to PARP1 up-regulation in cells or tissues, such as lncRNA MALAT1 [Citation19], lncRNA Mhrt [Citation20], lncRNA GAS5 [Citation21] and lncRNA FOXD3-AS1 [Citation22]. However, whether H19 takes part in the dysregulation of PARP1 expression in breast cancer cells remains unknown.
In the present study, we determined the importance of H19 in breast cancer chemoresistance using doxorubicin as a model chemotherapeutic agent in vitro and in vivo.
Materials and methods
Patients samples
Breast cancer samples were collected from Department of Breast surgery, the affiliated Hospital of Qingdao University (Qingdao, China) between 2015 and 2016. A total of 63 pairs of breast cancer tissues and adjacent normal tissues were examined in the study. All patients underwent conventional doxorubicin (Dox)-based chemotherapy. Patients with disease progression or recurrence 6 months or less after Dox therapy were defined as Dox resistance, whereas those without recurrence or recurrence 24 months after Dox therapy were defined as Dox sensitivity. After systemic Dox treatments, 25 Dox-sensitive patients contributed to 25 pairs of breast tumor tissues and 25 of normal tissues, and 38 Dox-resistant patients contributed 38 pairs of breast tumor and normal tissues. All of the samples were retrieved within 15 min after the surgery and immediately snap-frozen in liquid nitrogen and stored at −80°C until used for gene expression analysis. This study was approved by the Clinical Ethics Review Board at the affiliated hospital of Qingdao University and written informed consents were from all patients at their recruitment time.
Cell lines and cell culture
The human breast cancer MCF-7 cells were purchased from Institute of Cell Research, Chinese Academy of Sciences (Beijing, China). Doxorubicin (Dox)-resistant MCF-7/Dox cells were established by induction with gradient concentrations of Dox in vitro. Cells were cultured in DMEM (Sigma) supplemented with 10% fetal bovine serum (Gibco), 100 units/ml of penicillin, 100 μ g/ml, streptomycin in a 5% CO2 atmosphere at 37°C. MCF-7/Dox cells were cultured at a final concentration of 1 μM.
Construction of the H19 shRNA lentivirus
The lentivirus expressed short hairpin RNA (shRNA) targeting the H19 gene was constructed by GeneCopoeia (Shanghai, China), with the lentivirus-mediated scrambled shRNA generated as a negative control. The interfering sequences specifically targeting the H19 gene was 5′-CGTCATGTTCTGGTTTGAGAT-3′. The lentivirus particles were produced with a third-generation lentivirus packaging system. Briefly, three auxiliary plasmids (pRRE, pRSV-Rev, and pCMV-VSVG) and the core plasmid were transfected into human embryonic kidney 293 T cells (HEK293 T) using the Lipo6000 transfection reagent (Beyotime Biotechnology, Shanghai, China). The viral particles were extracted 72 h after transfection, and lentivirus particles were further concentrated. Six hundred sixty-one W cells were then infected with virus particles, and polybrene (Beyotime Biotechnology, Shanghai, China) was added to enhance infection efficiency. To avoid virulence, media were replaced with fresh complete DMEM media 24 h later. To obtain the cells that were consistently knocking down the target gene, cells were screened with hygromycin (Sangon Biotech, Shanghai, China) or puromycin (Beyotime Biotechnology, Shanghai, China) at an initial concentration of 8 μg/mL 2 days after infection of viral particles. Two weeks after screening, cells with stable H19 knockdowns were obtained. The stably transfected colonies were named Lv-H19 shRNA and Lv-NC shRNA.
Plasmids transfection
H19 expression vector pCMV6-H19 (clone ID: 4993796), PARP-1 expression vector pCMV6- PARP-1 and the vector construct (pCMV6-Entry) was obtained from OriGene Technologies, Rockville, MD. Breast cancer cells were transfected with pCMV6-H19, pCMV6-PARP-1 and pCMV6 using Lipofectamine LTX (Life Technologies) following the manufacturer’s instructions. Briefly, cells in a 6-well plate were transfected with 1 µg plasmid using Lipofectamine LTX (Life Technologies). 48 h post-transfection, cells were trypsinized and plated in 10 cm dishes to establish stable clones. Colonies that survive after a 2-3-week selection in 400 µg/ml G418 were isolated under fluorescence microscope. Stable cells in six-well plates were transfected with 10 ng plasmid.
siRNA transfection
siRNAs targeting human H19 (H19 siRNA), siRNAs targeting human PARP-1 (PARP-1 siRNA), and non-targeting siRNAs (NC siRNA) were obtained from Santa Cruz Biotechnology (Shanghai, China). For siRNA-mediated knockdown studies, cells were transfected with 500 pmol of siRNA specific for H19 or PARP-1 or NC siRNA. After 8 h, the transfection medium was replaced with DM. Subsequent assays were conducted 24 to 48 h after transfection.
Drugs treatment
Doxorubicin was obtained from Sigma (Shanghai, China). MCF-7 and MCF-7/Dox cells were treated for 72 h with doxorubicin (0 to 120 μM). Cytotoxicity was measured using MTT. To observe the effect of H19 or PARP-1 on Dox-induced cytotoxicity, cells were treated with 1.2uΜDox for 72 h.
Cellular viability assay
Cell viability was monitored by 2-(4, 5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) colorimetric assay. Briefly, 20 μL of MTT (5 mg/ml) was added to each well. After 4 hr of incubation at 37°C, cell supernatants were discarded. MTT crystals were dissolved with DMSO, while absorbance was measured at 570 nm. All experiments were done with 6–8 wells per experiment and repeated at least three times.
Flow cytometry analysis of apoptosis
The apoptosis assay was performed using ApoTargetTM annexin V-FITC kit (BioSource International, Inc., Camarillo, CA, USA) and following the manufacturer’s protocol. At each indicated time point, the cells were harvested using Trypsin-EDTA (Sigma-Aldrich) and washed once in 1 × PBS. Then, the cell apoptosis was analyzed using Annexin V and propidium iodide (PI). Apoptotic cells were identified as Annexin V-FITC‐positive and PI-positive cells.
qRT-PCR
Total RNA was isolated from cells or frozen tissue with the RNA extraction kit (Nanjing, China) according to the manufacturer’s instructions. RNA quality was measured using NanoDrop (Thermo Scientific). Reverse transcription reaction for H19 and PARP-1 was performed using a SuperScript® III First-Strand Synthesis System (Invitrogen). H19 expression levels were quantified by real-time qRT-PCR using miScript Primer assay (Qiagen) and normalized to RNU6. PARP-1 expression levels were normalized to GAPDH.
Western blotting analysis
Whole-cell lysates were prepared using 2% SDS, sonicated, and centrifuged (12,000 rpm) at 4°C for 15 min. The supernatants were boiled for 5 min and size-fractionated by SDS-PAGE (7.5% acrylamide). After transferring proteins onto nitrocellulose filters, the blots were incubated with primary PARP-1 andβ-actin antibody following incubations with secondary antibody, immunocomplexes were developed by using chemiluminescence.
Animal experiment
Female BALB/c nude mice (5–6 weeks of age, 16–18 g) were obtained from National Rodent Shanghai Experimental Branch Center, Chinese Academy of Sciences (Shanghai, China). All experimental procedures involving animals were conducted in accordance with the institutional guidelines by the Affiliated hospital of Qingdao University.
MCF-7/Lv-H19, MCF-7/Lv-NC, MCF-7/Dox/H19 shRNA and MCF-7/Dox/NC shRNA cells [0.2 mL (2 × 106)] were subcutaneously injected into the right back of the mice, respectively. Once tumor volume grew to (4 weeks) about 100 cm3, the mice (n = 8 per group) were, respectively, treated with PBS (0.1 mL, tail i.v. injection), Dox (0.1 mL, 10 mg/kg, tail i.v. injection, 4 times/week) and housed for another 3 consecutive weeks. Tumor volume was measured once every 3 days and volumes were evaluated by the following formula: tumor volume = (length × width2)/2. All mice were killed by intraperitoneal injection of 200 mg/kg pentobarbital at the end of the experiment. The tumor specimens were carefully excised and stored at −80°C for further use.
Statistical analyses
Statistical analyses were carried out by using SPSS 22.0 soft (IBM, SPSS, Chicago, IL, USA). The data are presented as the mean ± SD. Differences between groups were analyzed by one-way analysis of variance (ANOVA) and Student’s t-test. A p-value <0.05 was considered statistically significant.
Results
H19 overexpression in breast cancer tissue correlates with chemoresistance
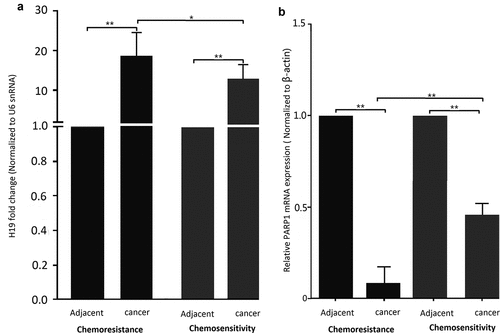
To assess whether H19 has a role in breast cancer resistance to chemotherapy, qRT-PCR was used to analyze H19 expression in breast cancer patients. A total of 25 specimens from patients with chemotherapy sensitivity and 38 specimens from patients with chemotherapy resistance were included in this study (). Our results showed chemotherapy-resistant breast cancer tissue specimens exhibited generally higher H19 levels compared with chemotherapy-sensitive tissues ()). In addition, H19 levels were significantly upregulated in both chemotherapy-sensitive and chemotherapy-resistant cancer tissues relative to their adjacent normal tissues ()).
Table 1. Clinical information of the 63 patients included in this study
Figure 1. H19 contributes to chemoresistance in breast cancer tissues and cell lines. (a) qPCR analysis showed that H19 expression levels were higher in patients with chemotherapy resistance than that of patients with chemotherapy sensitivity, H19 expression levels were higher in patients with breast cancer than that of their parental. (b) qPCR analysis showed that PARP1 expression levels were lower in patients with chemotherapy resistance than that of patients with chemotherapy sensitivity, PARP1 expression levels were lower in patients with breast cancer than that of their parental. *P < 0.01,**P < 0.001
PARP1 downexpression in breast cancer tissue correlates with chemoresistance
In addition, we detected the PARP1 mRNA levels in the adjacent normal tissues of above breast cancer tissues. As shown in ), PARP1 mRNA levels were significantly downregulated in both chemotherapy-sensitive and chemotherapy-resistant cancer tissues relative to their adjacent normal tissues.
H19 is overexpressed and PARP1 is downexpressed in the Dox-resistant MCF-7 cells
MCF-7 cells were continuously exposed to increasing concentrations of Doxorubicin (Dox) for 6 months. As shown in ), the IC50 of MCF-7 in Dox was 1.2 μM and 29.6 μM in the established MCF-7/Dox cells.
Figure 2. Overexpression of H19 enhances breast cancer resistance to Dox. (a) The IC50 values of MCF-7/Dox cells were higher than that of their parental MCF-7 cells. (b) qRT-PCR analysis of relative H19 expression in MCF-7/Dox cells and MCF-7 cells; (c), qRT-PCR analysis of relative PARP1 expression in MCF-7/Dox cells and MCF-7 cells. *P < 0.01
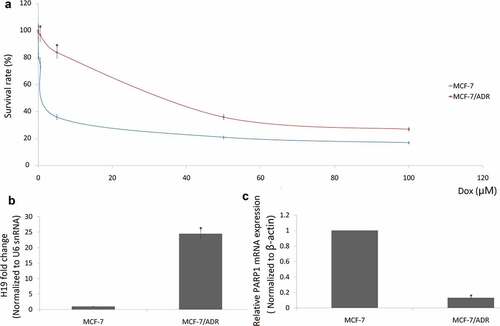
Consistent with the results from breast cancer tissues, high mRNA levels of H19 were found in doxorubicin-resistant MCF-7/Dox cells compared with the doxorubicin-sensitive MCF-7 cells ()). On the contrary, low mRNA levels of PARP1 were found in doxorubicin-resistant MCF-7/Dox cells compared with the doxorubicin-sensitive MCF-7 cells ()).
H19 overexpression induces the chemoresistance of MCF-7 cells
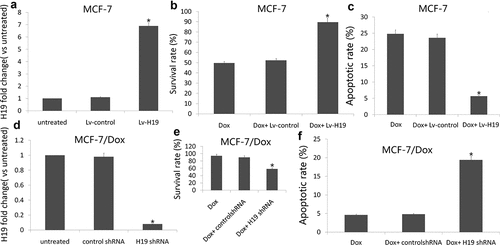
We further investigated whether inhibiting or increasing H19 expression could modulate cell survival and the sensitivity of MCF-7 and MCF-7/Dox cells to Dox, which is currently used for the treatment of breast cancer. Following transfection of an pCMV6-H19 into MCF-7, we treated the cells with IC50 of Dox 1.2 uM for 72 h. Introduction of H19 notably increased H19 expression ()) and reduced Dox (1.2 uM) induced cell apoptosis and increased cell viability (). In addition, following transfection of an H19 siRNA into MCF-7/Dox cells, we treated the cells with IC50 of Dox 1.2 uM for 72 h. Introduction of H19 siRNA notably decreased H19 expression ()) and increased Dox (1.2 uM) induced cell apoptosis and decreased cell viability ().
Figure 3. Targeting H19 enhances breast cancer sensitivity to Dox
Targeting H19 sensitizes breast cancer cells to doxorubicin through upregulating PARP1
MCF-7 cells were treated with Dox (1.2 uM) for 72 h, PARP1 mRNA and protein was significantly upregulated compared to the controls (). After MCF-7 cells were transfected with PARP1 siRNA, Dox (1.2 uM) induced cell apoptosis was significantly decreased and cell proliferation was significantly increased, followed by decreased PARP1 mRNA and protein expression (,)).
Figure 4. Targeting H19 reduced cell viability and induction of apoptosis by PARP1 upregulation in Dox-treated breast cancer cells
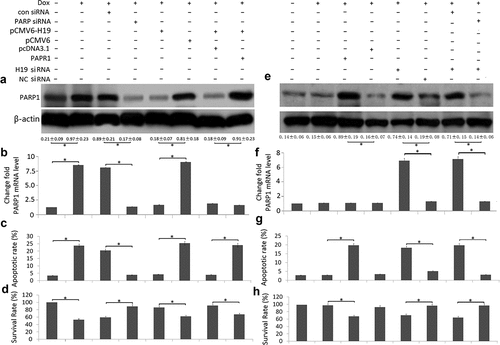
MCF-7 cells were treated with Dox (1.2 uM) for 72 h, H19 mRNA was significantly downregulated compared to the controls. After MCF-7 cells were transfected with pCMV6-H19, Dox-induced PARP1 mRNA and protein expression was decreased compared to the controls (,)), followed by decreased cell apoptosis and increased cell viability (,)). When MCF-7 cells were co-transfected with pCMV6-H19 and pCMV6-PARP1, PARP1 protein expression was increased (), Dox-induced cell apoptosis was increased and cell viability was decreased (,)).
MCF-7/Dox cells were treated with Dox (1.2 uM) for 72 h, PARP1 mRNA and protein did not change significantly compared to the controls (,)). After MCF-7/Dox cells were transfected with pCMV6-PARP1, PARP1 protein expression was upregulated ), Dox (1.2 uM) induced cell apoptosis was significantly increased and cell proliferation was significantly decreased compared to the controls (,)).
MCF-7/Dox cells were transfected with H19 siRNA, PARP1 mRNA and protein expression was upregulated compared to the control shRNA (). When MCF-7/Dox cells were co-transfected with H19 siRNA and PARP1 siRNA, PARP1 protein expression was decreased (,)), Dox-induced cell apoptosis was decreased and cell viability was increased (,)).
Targeting H19 expression sensitizes breast cancer cells to Dox by targeting PARP1 in vivo
MCF-7/Dox cells stably expressing a control vector (Lv-NC shRNA) or Lv-H19 shRNA were subcutaneously injected into the right fat pad of nude mice. The tumor volume was monitored every three days. In pre-experiment, NC shRNA or H19 shRNA alone did not significantly affect tumor (Data no shown). However, the tumor volume of H19 shRNA/Dox group was smaller than that of NC shRNA/Dox group, and significant difference in the tumor volume was observed between the two group ()), suggesting that targeting H19 expression sensitizes breast cancer cells to Dox in vivo.
Figure 5. Targeting H19 decrease resistance of breast tumor to Dox in xenograft tumor models
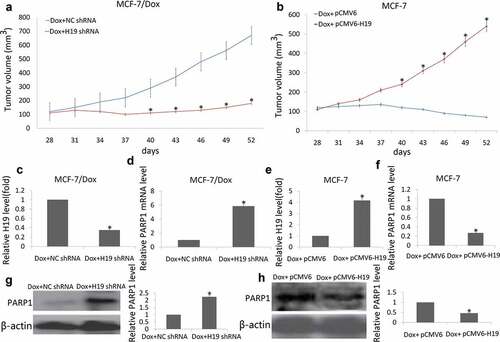
MCF-7 cells stably expressing a control vector (pCMV6) or pCMV6-H19 were subcutaneously injected into the right fat pad of nude mice. In pre-experiment, pCMV6 or pCMV6-H19 alone did not significantly affect tumor (Data no shown). However, the tumor volume of pCMV6-H19/Dox group was smaller than that of pCMV6/Dox group, and significant difference in the tumor volume was observed between the two group ()), suggesting that enforced H19 was resistant to Dox breast cancer cells in vivo.
The mice were sacrificed at 24 days after Dox treatment, and their tumors were harvested. RNA and protein were extracted from the tumor tissues. The qRT-PCR data showed that the H19 levels ()) were significantly decreased and PARP1 mRNA ()) were significantly increased in the H19 shRNA groups; On the contrary, the H19 levels ()) were significantly increased and PARP1 mRNA ()) were significantly decreased in the pCMV6-H19 groups. Western blot data showed that PARP1 protein expression was significantly increased in the H19 shRNA groups ()) and significantly decreased in the pCMV6-H19 groups compared to the controls ()).
Discussion
Drug resistance in cancer patients is a serious clinical problem resulting in treatment failure. The main molecular pathological mechanisms responsible for drug resistance are the dysregulated expression of the drug transporters responsible for anti-cancer drug efflux, and the aberrant activation of anti-apoptotic, cell-survival and oncogenic pathways. During breast tumorigenesis, many miRNAs are aberrantly regulated to promote tumor cell survival [Citation23–Citation25]. Additionally, several miRNAs have been identified to target drug transporter genes in breast cancer cells. For instance, miR-451 and miR-326 are required for the chemosensitivity of breast cancer cells to doxorubicin via direct targeting of ABC family transporter genes ABCB1 and ABCC1 (MRP1), respectively [Citation26,Citation27].
Long noncoding RNAs (lncRNAs) of >200 nucleotides in length have recently emerged as key regulators of developmental processes, including mammary gland development [Citation5]. lncRNA dysregulation has also been implicated in the development of various cancers, including breast cancer [Citation7,Citation8,Citation10]. In breast cancer, aberrant lncRNA expression has been implicated to affect the response to various treatments, including chemotherapy, anti-endocrine therapies, targeted therapies, and radiotherapy [Citation28,Citation29]. In cancer, H19 is frequently overexpressed and it is associated with many aspects of cancer development, including breast cancer [Citation9]. Zhang et al. demonstrated that the expression of H19 was significantly increased in breast cancer biopsies and plasma compared with healthy controls [Citation30], plasma H19 levels were significantly correlated with progesterone and chemotherapy response [Citation11].
In the present study, H19 expression was significantly upregulated in breast cancer tissues compared with normal breast tissues. Furthermore, H19 was significantly increased in chemoresistant breast cancer tissues and Doxorubicin-reaiatant MCF-7/Dox cell lines compared with the chemosensitive breast cancer tissues Doxorubicin-sensitive MCF-7 cell lines. These data indicated that H19 overexpression was related with chemoresistance in breast cancer.
A previous study speculated that the overexpression of H19 in breast cancer cells lines facilitates cell cycle transition G1/S while downregulation of H19 by RNA interference impedes S-phase entry and proliferation [Citation31]. Recently H19 has been shown to be upregulated in paclitaxel-resistant breast cancer cells, and knockdown of H19 might restore chemo-sensitivity in paclitaxel-resistant breast cancer cells [Citation15]. One of the most frequently used chemotherapeutics in breast cancer therapy is doxorubicin (Dox). Our in vitro and in vivo data suggested that H19 plays a role in the regulation of Dox-induced cell apoptosis in breast cancer. H19 downexpression increased Dox-induced cell apoptosis and enhanced the Dox response in the Dox-resistant MCF-7/Dox breast cancer. On the contrary, H19 downexpression decreased Dox-induced cell apoptosis and inhibited the Dox response in the Dox-sensitive MCF-7 breast cancer cells. These data indicated that targeting H19 could restore the DOX sensitivity in Dox-resistant breast cancer.
Recent clinical data confirmed the early in vitro studies and suggest that PARP-1 inhibitors could be used not only as chemosensitizers but as well as single agents to selective kill tumors with defective DNA repair by homologous recombination. For example, overexpression of miR-335 decreased the expression of PARP-1 expression, which was contributed to chemo-radiotherapy resistance in SCLC cells [Citation32]. However, PARP1 has been shown to increase the antitumor activity of temozolomide and topotecan in preclinical studies, including models of pediatric cancers [Citation33]. In the present study, PARP-1 expression was significantly downregulated in breast cancer tissues. Furthermore, PARP-1 was significantly increased in chemosensitive breast cancer tissues and Doxorubicin- chemosensitive MCF-7 cell. These data indicated that PARP-1 downexpression was related with chemoresistance in breast cancer. In our present study, PARP-1 expression is dramatically decreased and H19 expression is dramatically increased when MCF-7 cells are induced to acquire Dox resistance (MCF-7/Dox) in culture. When the H19 was knockdown in the MCF-7/Dox cells, PARP-1 expression was upregulated. Targeting H19 restored the sensitivity of MCF-7/Dox cells to Dox. However, the chemosensitivity to Dox was reversed in MCF-7/Dox cells when PARP-1 expression was blocked. In addition, H19 overexpression decreased Dox-induced PARP-1 expression and increased the acquired Dox resistance in Dox-sensitive MCF-7 cells. The role of H19 overexpression in acquired Dox resistance can be reversed by PARP-1 re-expression in the Dox-sensitive MCF-7 cells. Furthermore, blocking the action of H19 alone is sufficient to restore PARP-1 expression by Dox in the resistant cells, and is capable of sensitizing the resistant cells to Dox in vivo, and vice versa.
Conclusion
We identified the function and target of H19 in regulating chemotherapy response both in cell lines and in clinical samples, providing evidence that H19 targets PARP-1 to mediate the chemotherapeutic resistance of breast cancer cells. We extended the current knowledge by highlighting the role of H19 in the chemoresistance of breast cancer for the first time. Although chemotherapy is the backbone of systemic treatment for most malignancies, its efficacy is hindered by the development of drug resistance. Therefore, targeting the mechanisms involved in the chemoresistance of breast cancer will improve treatment efficacy. It is logical to predict that H19 could be a potential biomarker and that the inhibition ofH19 may be a candidate strategy for new breast cancer therapies.
Highlights
H19 overexpression is related with chemoresistance in breast cancer tissues and doxorubicin-resistant cells.
Targeting H19 enhanced the sensitivity to doxorubicin both in vitro and in vivo by PARP1 upregulation.
H19 overexpression recapitulates doxorubicin resistance by PARP1 downregulation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42.
- Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of clinical oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66:43–73.
- Fahad Ullah M. Breast cancer: current perspectives on the disease status. Adv Exp Med Biol. 2019;1152:51–64.
- Nedeljkovic M, Damjanovic A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8:pii: E957.
- Choudhari R, Sedano MJ, Harrison AL, et al. Long noncoding RNAs in cancer: from discovery to therapeutic targets. Adv Clin Chem. 2020;95:105–147.
- Xiong XD, Ren X, Cai MY, et al. Long non-coding RNAs: an emerging powerhouse in the battle between life and death of tumor cells. Drug Resist Updat. 2016;26:28–42.
- Lu Y, Zhao X, Liu Q, et al. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/ß-catenin signaling. Nat Med. 2017;23:1331–1341.
- Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36:3528–3540.
- Wang J, Sun J, Yang F. The role of long non-coding RNA H19 in breast cancer. Oncol Lett. 2020;19:7–16.
- Özgür E, Ferhatoglu F, Sen F, et al. Circulating lncRNA H19 may be a useful marker of response to neoadjuvant chemotherapy in breast cancer. Cancer Biomark. 2020;27:11–17.
- Sun Z, Zhang C, Wang T, et al. Correlation between long non-coding RNAs (lncRNAs) H19 expression and trastuzumab resistance in breast cancer. J Cancer Res Ther. 2019;15:933–940.
- Wang X, Pei X, Guo G, et al. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J Cell Physiol. 2020 Jan 29. DOI:10.1002/jcp.29585
- Si X, Zang R, Zhang E, et al. LncRNA H19 confers chemoresistance in ERa-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget. 2016;7:81452–81462.
- Zhu QN, Wang G, Guo Y, et al. LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. Oncotarget. 2017;8:91990–92003.
- Han J, Han B, Wu X, et al. Knockdown of lncRNA H19 restores chemo-sensitivity in paclitaxel-resistant triple-negative breast cancer through triggering apoptosis and regulating Akt signaling pathway. Toxicol Appl Pharmacol. 2018;359:55–61.
- Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly (ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424.
- Aiad HA, Kandil MA, El-Tahmody MA, et al. The prognostic and predictive significance of PARP-1 in locally advanced breast cancer of Egyptian patients receiving neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol. 2015;23:571–579.
- Haynes B, Zhang Y, Liu F, et al. Gold nanoparticle conjugated Rad6 inhibitor induces cell death in triple negative breast cancer cells by inducing mitochondrial dysfunction and PARP-1 hyperactivation: synthesis and characterization. Nanomedicine. 2016;12:745–757.
- Hu Y, Lin J, Fang H, et al. Targeting the MALAT1/PARP1/LIG3 complex induces DNA damage and apoptosis in multiple myeloma. Leukemia. 2018;32:2250–2262.
- Han P, Li W, Lin CH, et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–106.
- Long X, Song K, Hu H, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 2019;38:345.
- Zhao X, Li D, Huang D, et al. Risk-associated long noncoding RNA FOXD3-AS1 inhibits neuroblastoma progression by repressing PARP1-mediated activation of CTCF. Mol Ther. 2018;26:755–773.
- Roy SS, Gonugunta VK, Bandyopadhyay A, et al. Significance of PELP1/HDAC2/miR-200 regulatory network in EMT and metastasis of breast cancer. Oncogene. 2014;33:3707–3716.
- Gee HE, Camps C, Buffa FM. MicroRNA-10b and breast cancer metastasis. Nature. 2008;455:E8–9.
- Ryu S, McDonnell K, Choi H. Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell. 2013;23:63–76.
- Kovalchuk O, Filkowski J, Meservy J. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159.
- Liang Z, Wu H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–824.
- Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17:152–163.
- Liu Y, Sharma S, Watabe K. Roles of lncRNA in breast cancer. Front Biosci (Schol Ed). 2015;7:94–108.
- Zhang K, Luo Z, Zhang Y, et al. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016;17:187–194.
- Berteaux N, Lottin S, Monté D, et al. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem. 2005;280:29625–29636.
- Luo Y, Tong L, Meng H, et al. MiR-335 regulates the chemo-radioresistance of small cell lung cancer cells by targeting PARP-1. Gene. 2017;600:9–15.
- Daniel RA, Rozanska AL, Thomas HD, et al. Inhibition of poly(ADP-ribose) polymerase-1 enhances temozolomide and topotecan activity against childhood neuroblastoma. Clin Cancer Res. 2009;15:1241–1249.