ABSTRACT
Glyphosate has been frequently detected in water environments because of the wide use for controlling weed in farm lands and urban areas. Presently, the focus of the majority of studies is placed on the toxicity of glyphosate on humans and animals. However, the effects of glyphosate on horizontal transfer of conjugative plasmid carrying antibiotic resistance gene (ARG) are largely unknown. Here, we explored the ability and potential mechanism of glyphosate for accelerating horizontal transfer of conjugative plasmid-mediated ARG. The results showed that glyphosate can effectively boost horizontal transfer rate of conjugative plasmid carrying ARG. The possible mechanism analysis demonstrated that over-production of reactive oxygen species and reactive nitrogen species effectively regulated expression levels of bacterial outer membrane protein and conjugative transfer-related genes, thereby resulting into elevated horizontal transfer rate of plasmid-mediated ARG. In conclusion, this study casts new understanding into the biological effects of glyphosate on ARG.
1. Introduction
In recent years, a variety of antibiotic resistance genes (ARG) have been frequently detected in different environments, such as water, soil, and air [Citation1,Citation2]. The ever-growing dissemination of ARG has compromised therapeutic efficacy of antibiotics [Citation3,Citation4], therefore increasing health care costs [Citation5,Citation6] and posing a huge threat to the public health [Citation7,Citation8]. The wide use of antibiotics accelerates occurrence and spread of antibiotics resistance pathogens [Citation5,Citation9,Citation10]. The horizontal transfer of conjugative plasmid is a crucial contributor to ARG spread [Citation11,Citation12].
At present, mounting evidence has revealed that besides diverse antibiotics, environmental pollutants, including heavy metals, nanoparticles, disinfectants and non-antibiotic pharmaceuticals, can contribute to ARG transmission [Citation13–18]. It is noteworthy that environmental relevant concentrations of glyphosate can alter bacterial sensitivity to various antibiotics [Citation19].
Glyphosate, the most common active ingredient of herbicides, is widely applied to control weed in farm lands, urban areas, and gardens [Citation20–23]. Presently, glyphosate is frequently detected in various environments, foodstuff and even in the blood and urine of human beings [Citation8,Citation24–29]. Presently, the focus of the majority of studies is placed on the toxicity of glyphosate on humans and animals [Citation30–33], but the effects of glyphosate on horizontal transfer of conjugative plasmid carrying ARG are largely unknown.
To fill the gap, we determined the effect of glyphosate on horizontal transfer rates of conjugative plasmid carrying ARG between Escherichia coli (E. coli) strains, and then explored the potential molecular mechanism. The results will cast new understanding into the biological effects of glyphosate on ARG spread.
2. Materials and methods
2.1. Bacteria and glyphosate
The donor bacterium was E. coli SD-1 harboring a conjugative plasmid, which has ampicillin (Amp), kanamycin (Kan) and tetracycline (Tet) resistance genes. The E. coli SD-2 was the recipient with chloramphenicol (Chl) resistance gene.
Glyphosate (N-[phosphonomethyl]-glycine, >99.7% purity, C3H8NO5P) and four antibiotics (Amp, Chl, Kan and Tet) were bought from Jinan Lvba Bio-Tech CO., LTD (Jinan, Shandong, China).
2.2. Influence of glyphosate on bacterial propagation
Luria-Bertani (LB, pH 7.4) broth was used to incubate the donor and recipient bacteria overnight (180 rpm) at 36°C, and then the bacteria were isolated through centrifugation (6 000 rpm) for 5 min. After supernatants were removed, phosphate-buffered saline (PBS, pH 7.2) was used to wash and suspend the pellets. The the donor and recipient bacteria were, respectively, inoculated into LB broth with different environmental-relevant concentrations of glyphosate (0 mg/L, 0.1 mg/L, 0.3 mg/L and 0.6 mg/L) to analyze the growth curve [Citation34]. The selection of glyphosate levels used in this study was based on concentrations of glyphosate in water environments (0.10–0.70 mg/L) [Citation35].
2.3. Conjugative transfer rates
After the donor and the recipient bacteria were mixed (1:1 ratio), and the mixture was treated with different concentrations of glyphosate (0 mg/L, 0.1 mg/L, 0.3 mg/L and 0.6 mg/L), respectively [Citation18]. The transconjugants were measured as previously described [Citation14]. In brief, after incubation overnight at 37°C, the mixture (50 μL) were, respectively, plated on LB medium including 4 antibiotics (120.0 mg/L Amp, 40.0 mg/L Kan, 20.5 mg/L Chl and 20 mg/L Tet) for 48 h to count the colonies. The total recipient numbers were determined by LB agar medium containing Chl (20.5 mg/L). The transfer rate was computed by the number of transconjugants to that of the recipients.
2.4. Examination of ROS and RNS levels
The production of reactive oxidative species (ROS) and reactive nitrogen species (RNS) was respectively examined using the 2′,7′-Dichlorofluorescein Diacetate (DCFH-DA) (Invitrogen, California, USA) and 4-Amino-5-Methylamino-2′,7′-Difluorofluorescein Diacetate (DAF-FMDA) (Thermo Fisher, Carolina, USA) [Citation36].
2.5. Expression levels of genes associated with conjugation
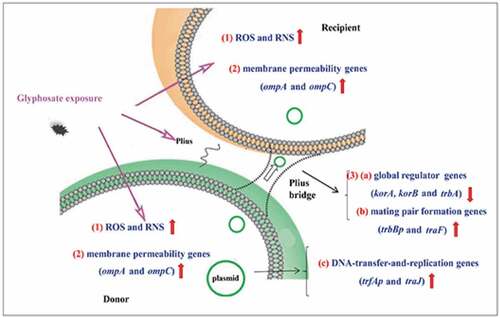
After the mating for 6 h, total RNA was extracted and then cDNA was prepared (TaKaRa, Dalian, China). As previously described [Citation37] (), quantitative polymerase chain reaction (Q-PCR) was performed to examine expression levels of outer membrane protein (OMP) genes (ompA and ompC), global regulator genes (GRG) (trbA, korA, and korB), mating pair formation genes (MPFG) (trbBp and traF), and DNA-transfer-and-replication genes (DTARG) (trfAp and traJ).
Table 1. Primer sequences of outer membrane protein genes and ones associated with conjugation transfer
2.6. Statistical analysis
In this study, SPSS 19.0 software was employed to analyze data. Analysis of variance (ANOVA) was used to compare differences among different groups. When P values were less than 0.05, the difference denoted significant.
3. Results
3.1. Conjugative transfer rate and production of ROS and RNS
To truly reflect the influence of glyphosate contamination on antibiotics resistance, the concentrations of glyphosate used in this study followed possible exposure dose human and animal intake from surface water [Citation35]. In addition, the formation of ROS and RNS was examined in the conjugative transfer process.
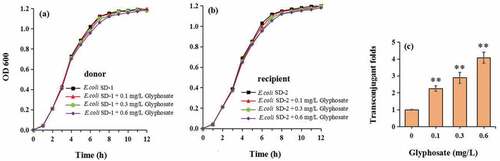
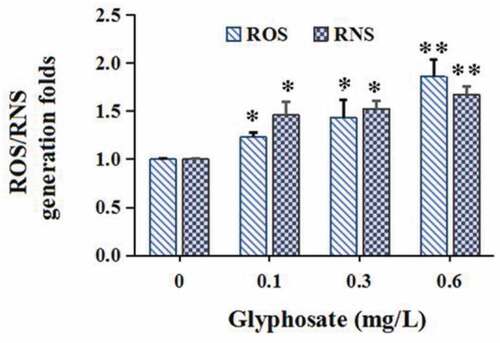
Glyphosate concentrations of glyphosate (0.1 mg/L, 0.3 mg/L and 0.6 mg/L) do not affect the propagation of the donor ()) and recipient strains ()). Glyphosate exposure definitely increased conjugative transfer rate by 2.26–4.08 times in a concentration-dependant manner ()). In addition, compared with the control, the ROS and RNS levels were, respectively, increased to 1.23–1.86 and 1.46–1.67 times ().
3.2. Influence of glyphosate on levels of genes related with conjugation
To explain the possible mechanism of glyphosate for promoting horizontal transfer of plasmid-mediated ARG, we examined expression levels of genes associated with conjugation, including OMP genes, GRG, MPFG, and DTARG.
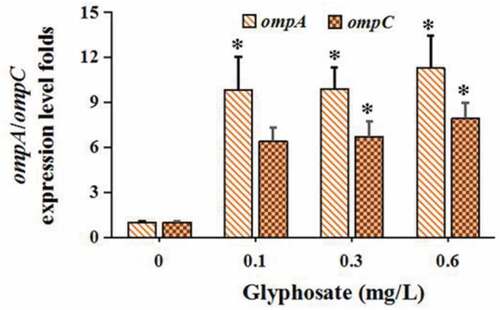
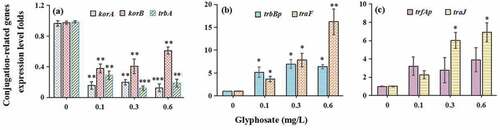
The levels of ompA and ompC were, respectively, increased by 9.82–11.26 and 6.38–7.91 folds after the treatment of glyphosate (). In terms of genes related to conjugation, we found that the GRG (korA, korB, and trbA) expression levels were suppressed markedly by glyphosate ()). Two MPFG members trbBp and traF were all up-regulated significantly after the treatment of glyphosate ()). In addition, glyphosate up-regulated DTARG levels (trfAp and traJ) ()).
4. Discussion
In the current study, the over-formation of ROS and RNS was found, which is in agreement with previous studies to a large extent, which indicated that the over-formation of intracellular ROS or RNS plays a crucial role in accelerating conjugative transfer rate of antibiotic resistance plasmid [Citation18,Citation37–40]. ROS formation can promote conjugative transfer rate of ARG via improving intrachromosomal recombination rates [Citation41]. In addition, nitrite can contribute to peroxynitrite formation, which, as a kind of RNS, can induce SOS response, improving conjugative transfer of ARG [Citation42].
The OMP genes of bacteria, such as OmpA and OmpC exert a major role in gene transfer and membrane permeability [Citation43–44]. The levels of ompA and ompC were increased significantly in this study. Many studies have shown that increased OMP expression level can facilitate horizontal transfer of ARG among bacteria via reduced membrane permeability and porins expression levels [,Citation45–46].
Conjugation bridge formation between bacteria, an important step for conjugative transfer, is closely related with the GRG, MPFG and DTARG [Citation47]. In this study, we found that expression levels of three GRG members were suppressed markedly. The reduced korA and korB levels can boost conjugative transmission of antibiotic resistance plasmid between bacteria through up-regulating trfAp promoter level [48].
When the mating bridge is formed between bacteria, the MPFG, locating in cell membranes, plays an important role. The bridge can contribute to conjugant formation. In this study, two MPFG members trbBp and traF were all up-regulated significantly after the treatment of glyphosate, which indicated that cell membrane permeability was increased [Citation37].
The DTARG plays a crucial role in relaxosome formation and transfer-replication initiation process, therefore contributing to conjugative transfer of plasmid DNA [49]. Glyphosate up-regulated trfAp and traJ expression levels in this study, which can boost horizontal transfer of plasmid-mediated ARG.
5. Conclusion
Taken together, this paper is the first to analyze the effects of glyphosate on conjugative transmission of antibiotic resistance plasmid in water. The result showed that glyphosate can definitely increase conjugative transfer of ARG. In terms of potential mechanisms, cell membrane permeability and conjugative transfer-related genes were regulated via the over-formation of ROS and RNS during bacteria were exposed to glyphosate, therefore increasing the conjugative transfer rates of plasmid-mediated ARG.
Research highlights
Glyphosate promotes production of ROS and RNS.
ROS and RNS enhance bacterial OMP expression.
ROS and RNS regulate conjugative transfer-related genes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen H, Chen R, Jing L, et al. A metagenomic analysis framework for characterization of antibiotic resistomes in river environment: application to an urban river in Beijing. Environ Pollut. 2019;245:398–407.
- Hendriksen RS, Munk P, Njage P, et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun. 2019;10(1):1124.
- Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P&T: Peer-Rev J Formulary Manage. 2015;40(4):277–283.
- Zaman SB, Hussain MA, Nye R, et al. A review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9(6):e1403.
- Shrestha P, Cooper BS, Coast J, et al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control. 2018;7:98.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493.
- Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098.
- Mills PJ, Kania-Korwel I, Fagan J, et al. Excretion of the herbicide glyphosate in older adults between 1993 and 2016. JAMA. 2017;318(16):1610–1611.
- Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187.
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229–241.
- Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405(6784):299–304.
- von Wintersdorff CJ, Penders J, van Niekerk JM, et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 2016;7:173.
- Jin M, Lu J, Chen Z, et al. Antidepressant fluoxetine induces multiple antibiotics resistance in Escherichia coli via ROS-mediated mutagenesis. Environ Int. 2018;120:421–430.
- Lu J, Wang Y, Li J, et al. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ Int. 2018;121(Pt 2):1217–1226.
- Wang Y, Lu J, Mao L, et al. Antiepileptic drug carbamazepine promotes horizontal transfer of plasmid-borne multi-antibiotic resistance genes within and across bacterial genera. Isme J. 2019;13(2):509–522.
- Zhang S, Wang Y, Song H, et al. Copper nanoparticles and copper ions promote horizontal transfer of plasmid-mediated multi-antibiotic resistance genes across bacterial genera. Environ Int. 2019;129:478–487.
- Zhang Y, Gu AZ, Cen T, et al. Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment. Environ Pollut. 2018;237:74–82.
- Zhang Y, Gu AZ, He M, et al. Subinhibitory concentrations of disinfectants promote the horizontal transfer of multidrug resistance genes within and across genera. Environ Sci Technol. 2017;51(1):570–580.
- Kurenbach B, Gibson PS, Hill AM, et al. Herbicide ingredients change Salmonella enterica sv. Typhimurium and Escherichia coli antibiotic responses. Microbiology (Reading). 2017;163(12):1791–1801.
- Clements D, Dugdale TM, Butler KL, et al. Herbicide efficacy for aquaticAlternanthera philoxeroidesmanagement in an early stage of invasion: integrating above-ground biomass, below-ground biomass and viable stem fragmentation. Weed Res. 2017;57(4):257–266.
- Goffnett AM, Sprague CL, Mendoza F, et al. Preharvest herbicide treatments affect black bean desiccation, yield, and canned bean color. Crop Sci. 2016;56(4):1962–1969.
- Hanke I, Wittmer I, Bischofberger S, et al. Relevance of urban glyphosate use for surface water quality. Chemosphere. 2010;81(3):422–429.
- Maqueda C, Undabeytia T, Villaverde J, et al. Behaviour of glyphosate in a reservoir and the surrounding agricultural soils. SciTotal Environ. 2017;593-594:787–795.
- Battaglin WA, Meyer MT, Kuivila KM, et al. Glyphosate and its degradation product ampa occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. JAWRA J Am Water Resour Assoc. 2014;50(2):275–290.
- Conrad A, Schroter-Kermani C, Hoppe HW, et al. Glyphosate in German adults-time trend (2001 to 2015) of human exposure to a widely used herbicide. Int J Hyg Environ Health. 2017;220(1):8–16.
- Goen T, Schmidt L, Lichtensteiger W, et al. Efficiency control of dietary pesticide intake reduction by human biomonitoring. Int J Hyg Environ Health. 2017;220(2Pt A):254–260.
- Lanaro R, Costa JL, Cazenave SO, et al. Determination of herbicides paraquat, glyphosate, and aminomethylphosphonic acid in marijuana samples by capillary electrophoresis. J Forensic Sci. 2015;60(Suppl 1):S241–247.
- Mahler BJ, Van Metre PC, Burley TE, et al. Similarities and differences in occurrence and temporal fluctuations in glyphosate and atrazine in small Midwestern streams (USA) during the 2013 growing season. SciTotal Environ. 2017;579:149–158.
- Poiger T, Buerge IJ, Bachli A, et al. Occurrence of the herbicide glyphosate and its metabolite AMPA in surface waters in Switzerland determined with on-line solid phase extraction LC-MS/MS. Environ Sci Pollut Res Int. 2017;24(2):1588–1596.
- Berg G, Grube M, Schloter M, et al. The plant microbiome and its importance for plant and human health. Fronters Microbiol. 2014;5:491.
- Newman MM, Lorenz N, Hoilett N, et al. Changes in rhizosphere bacterial gene expression following glyphosate treatment. SciTotal Environ. 2016;553:32–41.
- O’Doherty KC, Neufeld JD, Brinkman FS, et al. Opinion: conservation and stewardship of the human microbiome. Proc Natl Acad Sci U S A. 2014;111(40):14312–14313.
- Rodriguez-Gil JL, Prosser R, Poirier D, et al. Aquatic hazard assessment of MON 0818, a commercial mixture of alkylamine ethoxylates commonly used in glyphosate-containing herbicide formulations. Part 1: species sensitivity distribution from laboratory acute exposures. Environ Toxicol Chem. 2017;36(2):501–511.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175.
- Peruzzo PJ, Porta AA, Ronco AE. Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ Pollut. 2008;156(1):61–66.
- Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72.
- Cen T, Zhang X, Xie S, et al. Preservatives accelerate the horizontal transfer of plasmid-mediated antimicrobial resistance genes via differential mechanisms. Environ Int. 2020;138:105544.
- Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010;37(3):311–320.
- Qiu Z, Shen Z, Qian D, et al. Effects of nano-TiO2 on antibiotic resistance transfer mediated by RP4 plasmid. Nanotoxicology. 2015;9(7):895–904.
- Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465–478.
- Liu YY, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339(6124):1210–1213.
- Knopp M, Andersson DI. Amelioration of the fitness costs of antibiotic resistance due to reduced outer membrane permeability by upregulation of alternative porins. Mol Biol Evol. 2015;32(12):3252–3263.
- Fernández L, Hancock RE. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25(4):661–681.
- Wang X, Yang F, Zhao J, et al. Bacterial exposure to ZnO nanoparticles facilitates horizontal transfer of antibiotic resistance genes. NanoImpact. 2018;10:61–67.
- Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794(5):808–816.
- Zatyka M, Bingle L, Jones AC, et al. Cooperativity between KorB and TrbA repressors of broad-host-range plasmid RK2. J Bacteriol. 2001;183(3):1022–1031.
- Pansegrau W, Balzer D, Kruft V, et al. In vitro assembly of relaxosomes at the transfer origin of plasmid RP4. Proc Natl Acad Sci U S A. 1990;87(17):6555–6559.