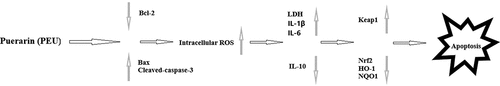
ABSTRACT
In this study, we examined the antitumor effects of Puerarin (PEU) on androgen-independent (DU145 and PC-3) and androgen-dependent (LNCaP) prostate cancer cells, and explored its potential mechanisms. Supplement with PEU (2.5 μM, 5 μM, and 10 μM) exhibited a marked inhibitory effect against the growth of DU145 and PC-3 cells, especially beyond 24 h, whereas there is only slight growth inhibitory effect on LNCaP cells at the high concentration of 10 μM at 72 h. This loss of cell viability in DU145 and PC-3 cells by PEU was mediated by the induction of apoptosis via up-regulation of Bax and cleaved-caspase-3, but downregulation of Bcl-2. Moreover, more intracellular ROS and LDH production were observed in DU145 and PC-3 cells upon PEU treatment. Meanwhile, the amount of pro-inflammatory cytokines (IL-1β and IL-6) was increased, but the content of anti-inflammatory cytokines IL-10 was attenuated. Additionally, PEU pretreatment resulted in an increase of Keap1 protein expression, and a decline of Nrf2, HO-1 and NQO1 protein expression in DU145 and PC3 cells. The present findings indicated that PEU exerted its antitumor activities toward androgen-independent prostate cancer cells via inactivation of Keap1/NrF2/ARE signaling pathway.
1. Introduction
Prostate cancer, as one most prevalent urinary malignancy of the male, ranked the second place in the cause of cancer death among aged men all over the world, trailing only lung cancer [Citation1,Citation2]. Although its incidence remains relatively high in western countries, there is a sharp rise in China for the aging Chinese population over the past decade, which has caught China’s healthcare attention [Citation3]. A growing recognition disclosed that a life Western diets and lifestyles change, together with gene mutation arising from environmental factors, contributed to the pathogenic factors of this frequently occurring disease [Citation4]. As of now, the exact pathogenesis behind prostate cancer is still an urgent concern and determining the molecular mechanism is crucial for facilitating early clinical diagnosis and therapy. At present, chemotherapy, androgen ablation therapy, radiotherapy and radical prostatectomy are most commonly employed to manage localized disease of some patients with androgen-dependent prostate cancer at an early stage [Citation5]. However, these therapy approaches are unsatisfactory for patients with advanced and aggressive prostate cancer, especially when this disease eventually progress into incurable hormone‑resistant subtype [Citation6,Citation7]. The transformation of prostate cancer at androgen-dependent to androgen-independent state is a lethal deterioration, which accelerate its metastasis and become resistant to currently used chemotherapeutic agents [Citation8,Citation9]. Moreover, chemotherapy and radiation therapy are commonly accompanied with serious toxic side effects to prostate cancer patients [Citation10]. Thus, a deeper understanding of the mechanism underlying prostate cancer development and developing novel therapeutic agents targeting androgen-insensitive prostate cancer cells are highly desirable to treat and prevent this advanced malignancy.
To date, many natural occurring products have drawn extensive attention as anticancer agents in the management of cancer development, because of their potential tumor selectivity and low cytotoxicity [Citation11]. Puerarin (PEU), a much famous isoflavone‑C‑glycoside, is the principal bioactive ingredient from the traditional Chinese herb Radix puerariae, which owns a wide range of biological activities, including cardioprotection, neuroprotection, anti-inflammatory, antioxidant, alleviating pain, inhibiting alcohol intake, regulation of bone metabolism and reduction of intraocular pressure [Citation12–14]. Furthermore, an increasing body of evidence has demonstrated PEU also showed anticancer effects in a variety of cancer cell lines, such as lung cancer [Citation15], cervical cancer [Citation16], esophageal cancer [Citation17], bladder cancer [Citation18], gastric cancer [Citation19], colon cancer [Citation20], and so on. However, despite this background, the antitumor effect of puerarin on prostate cancer is not thoroughly investigated until now.
Considering this condition, the present study was thus aimed to examine the antitumor effects of PEU on the androgen-dependent or independent prostate cancer cells and to unveil the underlying mechanisms beneath this action.
2. Materials and methods
2.1. Materials and chemicals
Puerarin (PEU, purity >99.5%), dimethyl sulfoxide (DMSO), 2,7-dichorofluorescin diacetate (DCFH-DA), and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St. Louis, MO, U.S.A.). Dulbecco’s modified Eagle medium (DMEM) and fetalbovine serum (FBS) were purchased from Life Technologies Inc (Gaithersburg,MD, USA). The levels of interleukin 1 beta (IL-1β), interleukin-6 (IL-6) and cytokines IL-10 commercially available enzyme-linked immunosorbent assay (ELISA) kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Primary antibodies against Bcl-2, cleaved-caspase-3, Keap1, Bax, Nrf2, HO-1, NQO1and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). BCA protein assay kit, Horseradish peroxidase (HRP) conjugated secondary antibody and enhanced chemiluminescence (ECL) reagent were from Pierce Chemical (Rockford, IL, USA). Annexin V‑fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit and the lactate dehydrogenase (LDH) assay kit were from BD Biosciences (San Jose, CA, USA). All other reagents used in this experiment were of analytical grade commercially available.
2.2. Cell lines and cultures
Human androgen-dependent LNCaP prostate cancer cell line, androgen-independent (DU145 and PC-3) prostate cancer cell lines and the normal PrEC prostate cell line were from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All human prostate cancer cell lines were cultured in DMEM supplemented with 10% (v/v) heat–inactivated FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified condition with 5% CO2 at 37°C. Normal PrEC cells were cultivated in RPMI 1640 medium under the same conditions.
2.3. Cell viability
Cell viability was examined with MTT assay. In brief, prostate cancer cells and normal prostate PrEC cells (1 × 104 cells/well) were seeded into the flat-bottomed 96-well plates overnight, and then treated with different concentrations of PEU (0, 2.5 μM, 5 μM, 10 μM) for 24, 48 or 72 h. Thereafter, 20 μl of stock solution of MTT (0.5 mg/ml) was added into each well for another 4 h’s incubation. After that, culture medium was aspirated and DMSO (150 μl) was poured to each well with mild oscillation to dissolve formazan crystals. Finally, the absorbance of sample in each well was measured using a microplate reader (Bio-Rad, Nebraska, USA) at the wavelength of 490 nm. Cell viability (%) was calculated as a percentage of control group (set as 100%).
2.4. 5-ethynyl-2'-deoxyuridine (EdU) incorporation assay
EdU staining was used to assess the change of cells proliferation. Briefly, treated cells were first washed twice with ice-cold PBS, and then fixed with 4% freshly prepared formaldehyde for 20 min at 37°C. After that, the fixed cells were rinsed thrice with PBS and stained with EdU staining kit according to the manufacturer’s protocol. Finally, the cells were photographed under a fluorescence microscope (Agilent 1200; Agilent Technologies).
2.5. LDH assay
Cell toxicity was evaluated by measuring the release of LDH from cells. Following different treatment, cells (2 × 105 cells/well) were inoculated into 6-well plate and kept for 24 h to adhere. Thereafter, the cells were subject to centrifugation (10,000 × g) for 10 min. The released LDH of resulting cell supernate was examined using a commercial LDH detection kit as per manufacturer’s instructions.
2.6. Annexin V-FITC/PI double-staining analysis
The percentage of apoptosis in cancer cells was performed using an Annexin V-FITC Apoptosis Detection Kit as per the manufacturer’s instructions. In brief, treated cells were collected, rinsed twice with ice-cold PBS, and suspended in binding buffer (500 μl) containing Annexin V-FITC and PI each in 5 μl in the dark at 37°C. After 15 min, these cells were washed twice again with PBS and subject to FACSCalibur flow cytometer (Becton & Dickinson Co., USA) for data collection and analysis. To quantify the cell apoptosis rate, each measurement was recorded statistically at least using 1 × 104 cells with CellQuest software (BD Biosciences).
2.7. Intracellular ROS measurement
Intracellular ROS content was analyzed using the ROS-specific probe DCFH-DA. Briefly, cells suffering different treatment were incubated with DCFH-DA (10 μM) for 30 min in the dark, washed twich with PBS, detached by trypsinization, resuspended in cold PBS, and immediately imaged on a fluorescence microscope. The DCF fluorescence intensity was analyzed using a FACSCalibur flow cytometer (Becton & Dickinson Co., USA) with an excitation wavelength at 488 nm and an emission wavelength at 525 nm. The results were normalized to the control group and expressed as a percentage with relative to control group (set as 100%)
2.8. Cytokine analysis
The levels of pro-inflammatory cytokines (IL-1β and IL-6) and anti-inflammatory cytokines (IL-10) were measured using respective ELISA kits according to the manufacturer’s instruction.
2.9. Western blot assay
Following treatment, cancer cells were harvested, rinsed twice with cold PBS and extracted with ice-cold lysis buffer for 10 min in ice. Then, the cell lysate was centrifuged at 12,000 rpm at 4°C for 10 min and the protein concentration in each resulting supernatant was determined using a BCA protein assay kit. Subsequently, each sample with 20 μg of total protein was decentralized by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then electrophoretically transferred to nitrocellulose membrane. The bolts on membranes were blocked with non‑fat milk (5%) for 1 h at 37°C and then probed overnight with specific primary antibodies (Bax, Bcl-2, cleaved caspase-3, Keap1, Nrf2, NQO1 and HO1) each at 1:1000 dilution, followed by the addition of appropriate HRP-linked secondary antibody (1:5,000) at 37°C for 1 h. Following washing three times, the blot image were developed with an enhanced chemiluminescence (ECL) detection kit and the target protein expression levels were normalized to an internal reference control β‑actin.
2.10. Statistical analysis
All data were expressed as mean ± standard deviation (SD) of three independent experiments and all statistical analyses were performed by GraphPad Prism software version 5.0. Statistically significant differences was carried out using the two-tailed Student’s t-test or one-way analysis of variance (ANOVA). The p-value of less than 0.05 was accepted to be significantly different. All experiments were performed at least in triplicates.
3. Results
Although previous emerging evidence has suggested that PEU exhibited strong antitumor effect toward different cancer, the inhibitory activities of PEU on prostate cancer are not thoroughly investigated and the underlying mechanism remains elusive. Thus, the aim of the present study was to explore the effects of PEU on the apoptosis in human androgen-dependent prostate cancer cell line LNCaP and androgen-independent prostate cancer cell lines (DU145 and PC-3), as well as elucidate potential mechanisms underlying this effect.
3.1. Effect of PEU on the cell growth of prostate cancer cells
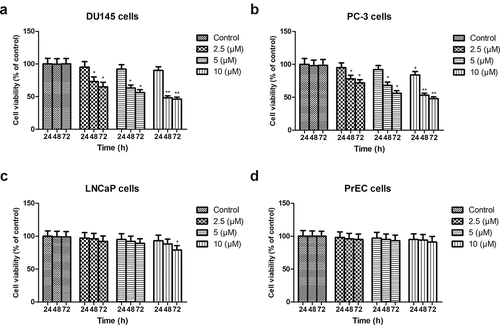
To examine the effect of PEU on the cell viability of prostate cancer cells, two kinds of cell lines (androgen-dependent: LNCaP; androgens independent: DU145 and PC-3) were used in MTT assay. As showed in ()), PEU (2.5 μM, 5 μM, 10 μM) treatment beyond 24 h exhibited a dose – and time-dependent inhibitory effect on the androgens independent cell growth (DU145 and PC-3 cells), which was statically significantly different from control cells (P < 0.05 or P < 0.01). The cell growth inhibitory efficacy at 48 h by PEU at different concentration was close to that at 72 h. On the contrary, only a moderate suppressing effect occurred in LNCaP cells at high concentration of PEU (10 μM) at 72 h as compared with control group (, P < 0.05). Moreover, there was no any toxicity in normal human prostate epithelial PrEC cells in response to PEU treatment at all concentrations at each period (, P > 0.05). The current findings demonstrated that human androgen-independent prostate cancer DU145 and PC3 cells were more susceptible to PEU addition than androgen-dependent LNCaP cells. For further examine this special antitumor selectivity by PEU, the concentration of 2.5 and 10 μM and 48 h incubation time were selected as optimal parameters in the following assays.
Figure 1. Effect of PEU (0, 2.5, 5, and 10 μM) on the cell viability of different prostate cancer cells. (a) Effect of PEU (0, 2.5, 5, and 10 μM) on the cell viability of androgen-independent DU145 cells; (b) Effect of PEU (0, 2.5, 5, and 10 μM) on the cell viability of androgen-independent PC-3 cells; (c) Effect of PEU (0, 2.5, 5, and 10 μM) on the cell viability of androgen-dependent LNCaP cells; (d) Effect of PEU (0, 2.5, 5, and 10 μM) on the cell viability of normal human prostate epithelial PrEC cells. Results are expressed as the means ± SD of three separate determinations. *P < 0.05 and **P < 0.01 compared with control cells
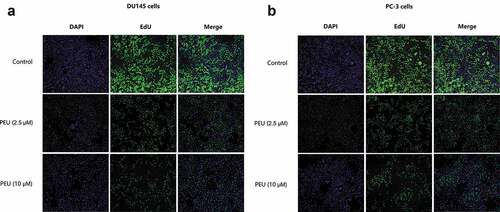
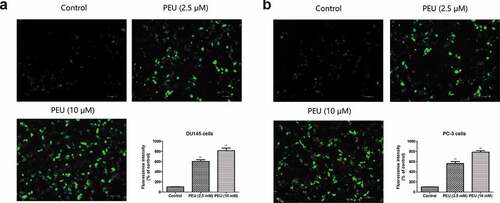
In line with this result, EdU incorporation assay () demonstrated that the addition of PEU with two concentrations (2.5 and 10 μM) led to a lower green fluorescence intensity in DU145 and PC-3 cells relative to higher green fluorescence intensity of the untreated cells, which indicated cell loss executed by PEU for both cancer cells.
Figure 2. Effect of PEU (0, 2.5 and 10 μM) on the cell viability of prostate cancer cells evaluated by BrdU cell proliferation assay kit; (a) Effect of PEU (0, 2.5 and 10 μM) on the cell viability of androgen-independent DU145 cells evaluated by BrdU cell proliferation assay kit; (b) Effect of PEU (0, 2.5 and 10 μM) on the cell viability of androgen-independent PC-3 cells evaluated by BrdU cell proliferation assay kit
3.2. Effect of PEU on the apoptosis and apoptosis-related protein expression of prostate cancer cells
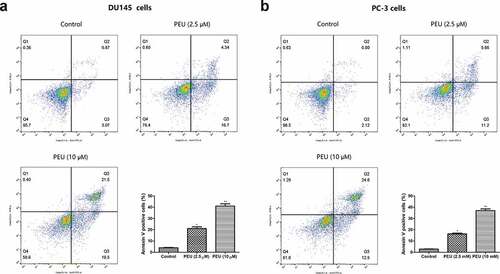
Next, Annexin V-FITC/PI dual-labeling technique was employed to reveal whether this cell viability loss was pertaining to the induction of apoptosis. Flow cytometry analysis ()) showed that the percentage of annexin V-FITC binding cells increased from 3.94% in untreated cells to 21.1% at 2.5 μM of PEU and 41.0% at 10 μM of PEU for DU145 cells, as well as 3.94% in untreated cells to 16.32% at 2.5 μM of PEU and 37.0% at 10 μM of PEU for PC-3 cells, respectively, which are all statically significant from control cells (P < 0.05 or P < 0.01). This finding suggested that PEU can promote the apoptosis of DU145 and PC3 cells.
Figure 3. Effect of PEU (0, 2.5 and 10 μM) on the apoptotic cell death of prostate cancer cells. (a) Effect of PEU (0, 2.5 and 10 μM) on the apoptotic cell death of androgen-independent DU145 cells; (b) Effect of PEU (0, 2.5 and 10 μM) on the apoptotic cell death of androgen-independent PC-3 cells. Results are expressed as the means ± SD of three separate determinations. *P < 0.05 and **P < 0.01 compared with control cells
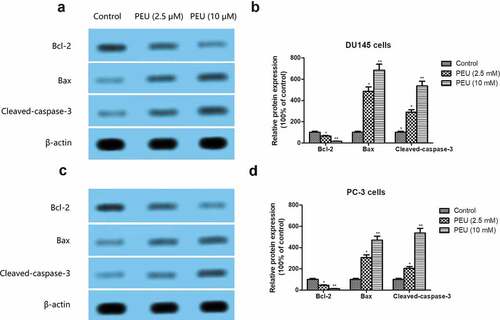
Apoptosis-related protein, including Bax, Bcl-2, and cleaved-caspase-3, was further examined by Western blot analysis. As illustrated in )), pretreatment with PEU caused a significant reduction in Bcl-2 expression, but dramatically increased Bax and cleaved-caspase-3 protein expression in DU145 and PC3 cells when compared with those in control cells (P < 0.05 or P < 0.01).
Figure 4. Effect of PEU (0, 2.5 and 10 μM) on the apoptosis-related protein expression of prostate cancer cells. (a) Effect of PEU (0, 2.5 and 10 μM) on the expression of Bax, Bcl-2 and cleaved-caspase-3 protein of androgen-independent DU145 cells; (b) Quantification of Bax, Bcl-2 and cleaved-caspase-3 protein expression of androgen-independent DU145 cells; (c) Effect of PEU (0, 2.5 and 10 μM) on the expression of Bax, Bcl-2 and cleaved-caspase-3 protein of androgen-independent PC-3 cells; (d) Quantification of Bax, Bcl-2 and cleaved-caspase-3 protein expression of androgen-independent PC-3 cells. Results are expressed as the means ± SD of three separate determinations. *P < 0.05 and **P < 0.01 compared with control cells
3.3. Effect of PEU on the intracellular ROS production of prostate cancer cells
Furthermore, we examined the intracellular ROS production by using the fluorescent probe HDCF-DA on flow cytometry. As shown in ()), PEU (2.5 and 10 μM) stimulation resulted in 6.1 and 8.2 folds increase of DCF fluorescence intensity for DU145 cells, 5.7 and 7.9 folds increase for PC3 cells, respectively, which was statistically different with the vehicle-treated control cells (P < 0.01).
Figure 5. Effect of PEU (0, 2.5 and 10 μM) on the intracellular ROS production of prostate cancer cells. (a) Effect of PEU (0, 2.5 and 10 μM) on the intracellular ROS production of androgen-independent DU145 cells; (b) Effect of PEU (0, 2.5 and 10 μM) on the intracellular ROS production of androgen-independent PC-3 cells. Results are expressed as the means ± SD of three separate determinations. **P < 0.01 compared with control cells
3.4. Effect of PEU on the LDH and inflammatory factors release of prostate cancer cells
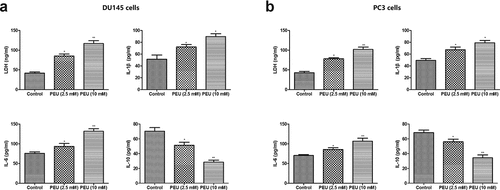
The LDH release and the content of inflammatory factors (TNF-α, IL-1β, and IL-6) were examined by using respective ELISA kits. As indicated in )), pretreatment with PEU (2.5 and 10 μM) induced a significant up-regulation of LDH, IL-1β, and IL-6 production, but overtly decreased the IL-10 release, when compared with the untreated control group (P < 0.05 or P < 0.01). This observation indicated PEU can cause LDH release and inflammatory response to trigger cell death of androgen-independent DU145 and PC3 cells.
Figure 6. Effect of PEU (0, 2.5 and 10 μM) on the LDH and inflammatory factors release of prostate cancer cells. (a) Effect of PEU (0, 2.5 and 10 μM) on the LDH, IL-1β, IL-6 and IL-10 release of androgen-independent DU145 cells; (b) Effect of PEU (0, 2.5 and 10 μM) on the LDH, IL-1β, IL-6 and IL-10 release of androgen-independent PC-3 cells. Results are expressed as the means ± SD of three separate determinations. **P < 0.01 compared with control cells
3.5. Effect of PEU on the Keap1/Nrf2/ARE signaling pathway of prostate cancer cells
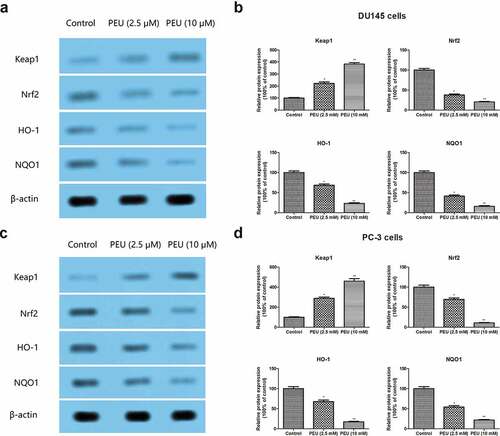
To explore the potential mechanism underlying, we investigated the effect of PEU on the Keap1/Nrf2/ARE signal pathway in DU145 and PC3 cells. Western blot analysis revealed that the addition of PEU (2.5 and 10 μM) to DU145 and PC-3 cells for 48 h resulted in the downregulation of Keap1 protein expression, whereas the Nrf2, HO-1 and NQO1 protein expression was substantially ameliorated in DU145 and PC-3 cells (). All values were statistically different from untreated control cells (P < 0.05 or P < 0.01). These observing definitely indicated that PEU exhibited its antitumor effect toward DU145 and PC3 cells via inactivation of Keap1/Nrf2/ARE signaling pathway.
Figure 7. Effect of PEU (0, 2.5 and 10 μM) on the Keap1/Nrf2/ARE signaling pathway of prostate cancer cells. (a) Effect of PEU (0, 2.5 and 10 μM) on the expression of Keap1, Nrf2, HO-1 and NQO1 protein of androgen-independent DU145 cells; (b) Quantification of Keap1, Nrf2, HO-1 and NQO1 protein expression of androgen-independent DU145 cells; (c) Effect of PEU (0, 2.5 and 10 μM) on the expression of Keap1, Nrf2, HO-1 and NQO1 protein of androgen-independent PC-3 cells; (d) Quantification of Keap1, Nrf2, HO-1 and NQO1 protein expression of androgen-independent PC-3 cells. Results are expressed as the means ± SD of three separate determinations. *P < 0.05 and **P < 0.01 compared with control cells
4. Discussion and conclusion
In the last decades, many naturally occurring ingredients derived from medicinal plants have become clinically attractive due to their tumorselectivity and cytotoxic properties by targeting multiple signaling pathways in multiple tumor models [Citation21,Citation22]. Several of these natural ingredients, such as decursin, curcumin, and epigallocatechin-3 gallate, apigenin have been verified to be useful in the management of prostate cancer development [Citation23]. In an effort to explore a promising anticancer agent for the treatment of this malignancy, this study first performed a MTT assay to evaluate the effect of PEU (2.5 μM, 5 μM, and 10 μM) on the cell viability of different human prostate cancer cell lines, including androgen-independent cell lines (DU145 and PC3) and androgen-dependent cell line (LNCaP), at 24, 48 and 72 h. PEU at all concentrations suppressed the cell growth of androgen-independent DU145 and PC-3 cells, especially beyond 24 h incubation period, but can merely suppress cell viability of androgen-dependent prostate cancer LNCaP cells at high concentration of 10 μM at 72 h. More importantly, not any cell viability loss can be detected in normal human prostate epithelial PrEC cells upon PEU treatment. This result indicated that PEU were prone to inhibit cell viability of androgen-independent DU145 and PC-3 cells than androgen-dependent LNCaP cells. Next, we adopted flow cytometry to assess if this cell loss induced by PEU was related to the induction of apoptosis in DU145 and PC3 cells. As expected, PEU supplement significantly induced the apoptosis of DU145 and PC3 cells in both concentrations, as evidenced by an increased percentage of Annexin V+ positive cells in flow cytometry analysis. The result of western blot analysis demonstrated that the addition of PEU resulted in a significant attenuation of Bcl-2 (an anti-apoptotic protein) protein expression, whereas the protein expression of Bax (an apoptotic protein) and cleaved-caspase-3 was enhanced in DU145 and PC3 cells. It is well-known that upregualtion of caspase-3 and Bax, and downregulation of Bcl-2 is linked to initiation of apoptosis [Citation24]. Hence, this event PEU can induce apoptosis of DU145 and PC3 cells via regulation of Bax, Bcl-2 and cleaved-caspase-3 protein expression.
Recently, growing evidence in cancer research suggested that intracellular ROS are important mediators of cell signaling cascades in the induction of apoptosis [Citation25,Citation26]. Some drugs exhibited an anticancer effect via an increased intracellular ROS event [Citation27–29]. We further investigated if intracellular ROS generated during apoptosis of DU145 and PC3 cells using the fluorescent probes DCFH-DA. Treatment with PEU (2.5 and 10 μM) induced a remarkable burst of ROS production in DU145 and PC3 cells as compared with untreated cells. Therefore, we speculated that ROS-induced apoptosis was involved in the antitumor effect of PEU on DU145 and PC3 cells. It was also confirmed that increased intracellular generation of ROS is closely related to LDH release and the occurrence of the inflammatory response [Citation30]. As anticipated, PEU (2.5 and 10 μM) stimulation for 48 h remarkably triggered the production of LDH and pro-inflammatory cytokines (IL-1β and IL-6) in DU145 and PC3 cells, whereas the content of anti-inflammatory cytokines IL-10 was attenuated, which was in line with the results of intracellular ROS production in this study.
The KEAP1/NRF2/ARE pathway is currently recognized as one most important cell defense systems to counteract oxidative injury and inflammation [Citation31], thus its dysregulation is implicated in many human illnesses, particularly cancers [Citation32,Citation33]. Under unstressed conditions, Nrf2 is sequestered by Keap1 and anchored in the cytoplasm in the resting state [Citation34]. While, under oxidative stress conditions, Nrf2 is activated and dissociated from Keap1 binding, then translocated into nuclei where it binds to the antioxidant response element (ARE) and promotes the expression of downstream antioxidative proteins, including NQO1 and HO1 [Citation35,Citation36]. Besides attenuation of oxidative stress, Nrf2 activation is also able to prevent inflammation and the inactivation of Nrf2 will result in increased oxidative stress and subsequently amplification of cytokine production [Citation37]. Previous studies have suggested that Nrf2 has double edge in the development and progression of cancer. Activation of Keap1/NrF2/ARE signaling pathway can prevent the development of cancer during early stages [Citation38]. However, Nrf2 overexpression greatly reduces the sensitivity of tumor cells to radiotherapy and chemotherapy when tumor forms [Citation39]. A growing body of clinical research has manifested that high Nrf2 expression is observed in human prostate cancer tissues than benign prostatic hyperplasia [Citation40,Citation41]. As such, targeting the overexpression of Nrf2 provide an indispensable strategy for the prevention and therapy of prostate cancer. In the present study, supplement with PEU (2.5 and 10 μM) for 48 h pronouncedly enhanced the expression of Keap1 protein, and suppressed the Nrf2, HO-1 and NQO1 protein expression of DU145 and PC3 cells. This result kept in line with that recent report by Chen et al., who declared that the high glucose promotes prostate cancer cells apoptosis via Nrf2/ARE signaling pathway [Citation42].
5. Conclusion
In this study, we demonstrated for the first time that PEU promoted the apoptosis of DU145 and PC3 prostate cancer cells by targeting the Keap1/Nrf2/ARE signaling pathway. Our findings suggested that PEU may be a promising candidate as chemotherapeutic drug for the treatment of androgen-independent prostate cancer.
Research highlights
Puerarin (PEU) inhibited the growth of DU145 and PC-3 prostate cancer cells
PEU induced the apoptosis of DU145 and PC-3 prostate cancer cells
PEU modulated the amount of inflammatory cytokines (IL-1β, IL-6, and IL-10)
PEU increased Keap1 protein expression in DU145 and PC3 cells
PEU decreased Nrf2, HO-1 and NQO1 protein expression in DU145 and PC3 cells
Author contribution
Luo Q and Zhang RG conceived and designed the study. Li JJ, Yan L, Xiong C, Xu P and Luo Q performed the experiments. Xiong C and Xu P analyzed the data. Li JJ and Luo Q wrote and reviewed the final manuscript. All authors read and approved the manuscript.
Supplemental Material
Download ()Disclosure statement
The authors have no commercial or other associations that might pose a conflict of interest.
Data availability statement
The datasets used or/and analyzed during the current study are available from the corresponding author on reasonable request.
Funding This study was supported by General program of Chongqing Natural Science Foundation (No. cstc2019jcyj-msxmX0843); Science and health joint medical research project of Nan'an District, Chongqing (No.2019-01) and High-tech Field Expansion Project of Sichuan Academy of Agricultural Sciences (No.2018GXTZ-001).Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27(1):2–12.
- Cai F, Zhang Y, Li J, et al. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci Rep. 2020;40(3):BSR20192826.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71.
- Yang B, Zhang D, Qian J, et al. Chelerythrine suppresses proliferation and metastasis of human prostate cancer cells via modulating MMP/TIMP/NF-κB system. Mol Cell Biochem. 2020; 10.1007/s11010-020-03845-0 (1–2): 199–208. DOI: 10.1007/s11010-020-03845-0.
- Uemura H, Ishiguro H, Nakaigawa N, et al. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Mol Cancer Ther. 2003;2(11):1139–1147.
- Li J, Luo J, Gu D, et al. Adenovirus-mediated Angiotensin II type 2 receptor overexpression inhibits tumor growth of prostate cancer in vivo. J Cancer. 2016;7(2):184–191.
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45.
- Petrylak DP. The current role of chemotherapy in metastatic hormone-refractory prostate cancer. Urology. 2005;65(5):3–8.
- Xu YM, Wijeratne EMK, Babyak AL, et al. Withanolides from Aeroponically grown physalis peruviana and their selective cytotoxicity to prostate cancer and renal carcinoma cells. J Nat Prod. 2017;80(7):1981–1991.
- Zhang X, Chen LX, Ouyang L, et al. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012;45(5):466–476.
- Ye G, Kan S, Chen J, et al. Puerarin in inducing apoptosis of bladder cancer cells through inhibiting SIRT1/p53 pathway. Oncol Lett. 2019;17(1):195–200.
- Liu X, Zhao W, Wang W, et al. Puerarin suppresses LPSinduced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed Pharmacother. 2017;92:429–436.
- Zhang XL, Wang BB, Mo JS. Puerarin 6″-O-xyloside possesses significant antitumor activities on colon cancer through inducing apoptosis. Oncol Lett. 2018;16(5):5557–5564.
- Huang P, Du SX. Puerarin enhances the anti-tumor effect of cisplatin on drug-resistant A549 cancer in vivo and in vitro through activation of the Wnt signaling pathway. Cancer Manag Res. 2020;12:6279–6289.
- Jia L, Hu Y, Yang G, et al. Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp Ther Med. 2019;18(1):543–549.
- Wang J, Yang ZR, Guo XF, et al. Synergistic effects of puerarin combined with 5-fluorouracil on esophageal cancer. Mol Med Rep. 2014;10(5):2535–2541.
- Jiang K, Chen H, Tang K, et al. Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15(1):167–174.
- Guo XF, Yang ZR, Wang J, et al. Synergistic antitumor effect of puerarin combined with 5-fluorouracil on gastric carcinoma. Mol Med Rep. 2015;11(4):2562–2568.
- Yu Z, Li W. Induction of apoptosis by puerarin in colon cancer HT-29 cells. Cancer Lett. 2006;238(1):53–60.
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661.
- Arias-González I, García-Carrancá AM, Cornejo-Garrido J, et al. Cytotoxic effect of Kalanchoe flammea and induction of intrinsic mitochondrial apoptotic signaling in prostate cancer cells. J Ethnopharmacol. 2018;222:133–147.
- Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13(3):751–778.
- Shen W, Guan Y, Wang J, et al. A polysaccharide from pumpkin induces apoptosis of HepG2 cells by activation of mitochondrial pathway. Tumour Biol. 2016;37(4):5239–5245.
- Zeng CC, Lai SH, Yao JH, et al. The induction of apoptosis in HepG-2 cells by ruthenium(II) complexes through an intrinsic ROS-mediated mitochondrial dysfunction pathway. Eur J Med Chem. 2016;122:118–126.
- Wu P, Meng X, Zheng H, et al. Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of Apoptosis on bladder cancer. Molecules. 2018;23(10):2592.
- Wongtongtair S, Chanvorachote P, Hutamekalin P, et al. Barakol-induced apoptosis in P19 cells through generation of reactive oxygen species and activation of caspase-9. J Ethnopharmacol. 2011;137(2):971–978.
- Moharram S, Zhou A, Wiebe LI, et al. Design and synthesis of 3ʹ- and 5ʹ-O-(3-benzenesulfonylfuroxan-4-yl)-2ʹ-deoxyuridines: biological evaluation as hybrid nitric oxide donor-nucleoside anticancer agents. J Med Chem. 2004;47(7):1840–1846.
- Fukuzawa K, Kogure K, Morita M, et al. Enhancement of nitric oxide and superoxide generations by alpha-tocopheryl succinate and its apoptotic and anticancer effects. Biochemistry (Mosc). 2004;69(1):50–57.
- Li P, Li Z. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response. Exp Ther Med. 2015;9(5):1768–1772.
- Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8(1–2):99–106.
- Kansanen E, Kuosmanen SM, Leinonen H, et al. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1(1):45–49.
- O’Connell MA, Hayes JD. The Keap1/Nrf2 pathway in health and disease: from the bench to the clinic. Biochem Soc Trans. 2015;43(4):687–689.
- Li AL, Shen T, Wang T, et al. Novel diterpenoid-type activators of the Keap1/Nrf2/ARE signaling pathway and their regulation of redox homeostasis. Free Radic Biol Med. 2019;141:21–33.
- Chen M, Xi Y, Chen K, et al. Upregulation Sestrin2 protects against hydrogen peroxide-induced oxidative damage bovine mammary epithelial cells via a Keap1-Nrf2/ARE pathway. J Cell Physiol.
- Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64(4):972–1003.
- Hassanein EHM, Sayed AM, Hussein OE, et al. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid Med Cell Longev. 2020;2020:1675957.
- Zhang DD. The Nrf2-Keap1-ARE signaling pathway: the regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13(11):1623–1626.
- Satoh H, Moriguchi T, Taguchi K, et al. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31(10):1833–1843.
- Hao B, Miao Z, Yuan Y. Clinical significance of Nrf2 expression in benign prostatic hyperplasia and prostate cancer tissues. Int J Clin Exp Pathol. 2016;9(1):118–123.
- Bellezza I, Scarpelli P, Pizzo SV, et al. ROS-independent Nrf2 activation in prostate cancer. Oncotarget. 2017;8(40):67506–67518.
- Chen JY, Wang FB, Xu H, et al. High glucose promotes prostate cancer cells apoptosis via Nrf2/ARE signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(3 Suppl):192–200.