ABSTRACT
Axis formed by integrin β3 (ITGβ3)-Ras homolog gene family, member A (RhoA), and Yes-associated protein (YAP) plays an important role in atherosclerosis. In addition, ITGβ3 overexpression was noted in high-glucose (HG) exposure podocytes. However, the ITGβ3–RhoA–YAP axis on HG-induced podocyte injury remains unclear. This study aimed to investigate whether ITGβ3 regulates podocyte injury by regulating the RhoA–YAP axis. The function and potential mechanism of ITGβ3 were observed through in vitro wound-healing assays, flow cytometry, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and western blot assay. Results showed that HG treatment increased the ability of wound closure and apoptosis; however, in spite of HG treatment, ITGβ3 inhibition mitigated the ability of wound closure and apoptosis in podocytes. By contrast, overexpression of ITGβ3 increased the wound closure and apoptosis abilities of podocytes. Under HG treatment, ITGβ3 knockdown is associated with upregulation of RhoA, total YAP1, and nucleus YAP1, whereas ITGβ3 overexpression has opposite effect. In addition, RhoA overexpression in podocytes reverses the effect of ITGβ3 overexpression on the wound closure and apoptosis abilities of podocytes, rescue the expression of YAP in ITGβ3 overexpression podocytes. Taken together, ITGβ3 overexpression promotes podocytes injury by inhibiting RhoA-YAP axis. This will provide a new clue for preventing podocyte from damage.
1. Introduction
Diabetic nephropathy (DN) has become a major health issue worldwide. Increasing DN accounts for the large proportion of advanced primary renal diseases that has emerged in recent years greatly affects the survival rate and life quality of patients, and causes a significant economic burden to both individuals and the country [Citation1,Citation2]. Hyperglycemia-induced podocytes cellular injury and dysfunction is a main cause of DN [Citation3,Citation4]. Podocytes are a vital component of the glomerular filtration membrane barrier [Citation5–7]. The loss and depletion of podocyte occurs in the early stages of DN, which is often accompanied by a degree of proteinuria [Citation8]. Hence, it is necessary to identify new therapeutic strategies to prevent damage, decrease proteinuria, and improve DN in podocytes.
Integrin β3 (ITGβ3), a member of the cell membrane surface receptors family, regulates the adhesion of cells and transmits molecular signals related to the cellular environment. Studies reported that ITGβ3 plays an important role in gene transcription, cell migration, proliferation, and apoptosis [Citation9,Citation10]. The increasing expression of ITGβ3 is associated with podocyte fusion, skeleton protein damage, podocyte shedding, and proteinuria production, which contributes to the development of DN [Citation11–13]. Inhibition of ITGβ3 by antibody or inhibitor reversed the effect of ITGβ3 overexpression on podocyte injury [Citation13,Citation14]. Our previous study also found that the expression of ITGβ3 was increases and the ability of podocyte would closure was also increased under high glucose (HG) conditions, which was alleviated by spironolactone_ – have been reported reduce proteinuria and delay DN progress [Citation8].
Yes-associated protein (YAP) is an important podocyte transcription cofactor of the downstream Hippo signaling pathway, which regulates the related genes that promote cell proliferation and inhibit apoptosis [Citation15,Citation16]. Studies [Citation9,Citation15,Citation16] found that there is a certain level of YAP in the nucleus of podocytes, which can be used as a receptor for pressure or cytoskeletal signals in the cell microenvironment that are related to podocyte apoptosis. Studies also reported that YAP is an anti-apoptotic molecule in podocytes, and podocytes-specific deletion of YAP results in proteinuria kidney diseases [Citation7,Citation17].
Ras homolog gene family, member A (RhoA), is an important protein involved in the development of various kidney diseases and it is also an important upstream activator of YAP. RhoA regulates many functions of podocytes, including cell morphology and skeleton aggregation, cell proliferation, and apoptosis [Citation18–23]. Kistler AD., Buvall L., and our previous study showed that RhoA is necessary for the normal functioning of glomerular podocytes, silencing or intervening RhoA in podocytes results in loss of tension fibers and production of proteinuria [Citation22,Citation24,Citation25]. The potential mechanism was reducing the expression and nucleation of YAP, thereby causing podocyte cytoskeletal damage and promoting podocyte apoptosis [Citation25].
In addition, steady unidirectional shear stress exert anti-inflammatory and atheroprotective effects by regulating ITGβ3-RhoA-YAP pathway [Citation23]. However, whether ITGβ3 regulates podocyte injury progress by regulating RhoA-YAP axis remains unknown. In this study, we investigate the effect of overexpression and interference of ITGB3 expression on the ability of podocytes in terms of wound closure and apoptosis in the process of podocyte injury, as well as the expression of RhoA and YAP. This will provide a new clue to prevent damage, decrease proteinuria, and improve DN in podocytes.
2. Materials and methods
2.1 Cell culture and HG exposure
The mouse podocyte cell line MPC5 was purchased from the American Type Culture Collection (Manassas, VA, USA). In addition, MPC5 was cultured according to the manufacturer’s instructions. Briefly, cells were cultured in a Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 100 U/ml IFN-γ (Cyagen, Guangzhou, China) and incubated with 5% CO2 at 33°C for proliferation. Cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco) without IFN-γ for 14 days with 5% CO2 at 37°C, which induces differentiation and maturation of podocytes. After differentiation, MPC5 cells were serum-starved for 24 h, and then treated with a normal glucose concentration (NG, 5.3 mM) and HG concentration (HG, 20 mM) for 48 h for further analysis.
2.2 Cell transfection
Transfection of MPC5 cells was conducted using Lipofectamine 3000 Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s manuals. In brief, when cells reached an 80–90% confluence, transfection was performed. siRNAs or plasmids and Lipofectamine 3000 Reagent were added to the Opti-MEM medium to prepare the siRNA- or plasmid DNA-lipid complexes at room temperature for 15 min. Then the siRNA- or DNA-lipid complexes was added into MPC5 cells. The transfection efficacy was examined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analyses separately after 48 h transfection. siRNAs were synthesized by GenePharma (Shanghai, China), and the sequences were as follows: ITGβ3 siRNA1 (si-ITGβ3-1), sense: 5′-CCUGUUACAAUAUGAAGAAUG-3′, antisense: 5′-UUCUUCAUAUUGUAACAGGGG-3′; ITGβ3 siRNA2 (si-ITGβ3-2), sense: 5′-CAAUGAAGAAGUGAAGAAACA-3′, antisense: 5′-UUUCUUCACUUCUUCAUUGAA-3′; siRNA negative control (si-NC): sense: 5′-UCUCCGAACGUGUCACGUTT-3′, antisense: 5′-ACGUGACACGUUCGGAGAATT-3′. The ITGβ3 was cloned into p HBAd-MCMV-GFP, which was provided by Hanbio (Guangzhou, China), RhoA was clone into pcDNA3.1, which was provided by General Biol (Anhui, China).
2.3 RNA extraction and RT-qPCR analysis
The total RNA was extracted from MPC5 cells with TRIzol (Invitrogen) and was converted into complementary DNA (cDNA) using the PrimeScript™ RT Regent kit (Takara, Tokyo, Japan). RT-qPCR analysis was carried out with a Bio-Rad CFX96 Touch q-PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using Power SYBR Green PCR Master Mix (Roche, Basel, Switzerland) with cDNA and primer pairs (Invitrogen). The mRNA expression was calculated by the 2−ΔΔCt method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. Primer sequences were as follows: ITGβ3 forward, 5′-TTGGCTACAAACACGTGCTG-3′ and reverse, 5′-CTGCATGATGGCATCAAAGC-3′; RhoA forward, 5′-CAGAAAAGTGGACCCCAGAA-3′ and reverse, 5′-GCAGCTCTCGTAGCCATTTC-3′; YAP forward, 5′-GCAACTCCAACCAGCAGCAACA-3′ and reverse, 5′-CGCAGCCTCTCCTTCTCCATCTG-3′; GAPDH forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3′.
2.4 Western blot analysis
The nucleus protein was extracted followed the kit instructions (Beyotime Biotechnology, Shanghai, China). The total protein was extracted using a radioimmunoprecipitation assay (RIPA, Beyotime Biotechnology) buffer that contained a protease inhibitor (Beyotime Biotechnology). The supernatants were collected through centrifugation, and an Enhanced BCA Protein Assay Kit (Beyotime Biotechnology) was used to calculate the protein. Subsequently, 30 μg protein in each sample were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred into polyvinylidene fluoride (PVDF). Then, the PVDF was incubated in 5% fat-free milk for 1 h at room temperature (Thermo, Waltham, MA, USA) and in primary antibody at 4°C overnight. The antibodies were as follows: ITGβ3 (#ab182773, abcam, Cambridge, MA, USA), GAPDH (#60004-1-lg, Proteintech, Wuhan, China), YAP (#4912, Cell Signaling Technology, Danvers, MA, USA), RhoA (#ab187027, abcam), and Histone H3 (#4499s, Cell Signaling Technology). After that, the membrane was incubated with secondary antibody (Proteintech) for 1 h at room temperature. Finally, ECL Plus Substrate (Thermo) and Tanon-5200CE (Biotanon, Shanghai, China) were used to observe the target protein bands.
2.5 Flow cytometry analysis
MPC5 cells were collected in phosphate-buffered saline (PBS), washed with a fluorescence-activated cell sorter (FACS) buffer and stained with annexin V-APC and 7-AAD (BD Biosciences, San Jose, CA, USA) in the dark for 30 min at 4°C. Subsequently, cells were washed once with FACS buffer and were resuspended. Then, 104 cells were obtained using a FACScan Flow Cytometer (BD Biosciences, San Jose, CA, USA). The analysis was performed using FlowJo Software 7.6 (TreeStar, Ashland, OR, USA). Apoptotic cells were expressed as a percentage of the total cells.
2.6 Wound healing analysis in vitro assay
After transfection, podocytes MPC5 (1 × 105/ml) were grown on six-well dishes overnight and then treated with and without HG. Each well was scratched with a sterile 200-µl pipette tip, washed with PBS, and placed into a fresh medium. Images were acquired by a phase-contrast Leica SP5-FCS microscope (Leica Microsystems, Wetzlar, Germany) at 0, 24, and 48 h after scratching. The wound closure was measured using Image J (National Institutes of Health, Bethesda, MD, USA).
2.7 Statistical analysis
Results are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) with student’s t-test or analysis of variance followed by Bonferroni post-hoc test. P < 0.05 was determined as the significant level.
3. Results
ITGβ3 expression, cell apoptosis, and the ability of wound closure was increased after HG treatment in podocytes. The silence of RhoA also promoted podocyte apoptosis by reducing the expression and nucleation of YAP. Steady unidirectional shear stress exerts an anti-inflammatory and atheroprotective effect through the regulation of the ITG3-RhoA-YAP pathway. However, whether ITGβ3 regulates podocytes injury progression by regulating the RhoA-YAP axis remains unknown. In this study, we investigated the effect of overexpression and interference of ITGB3 on the podocytes’ ability of wound closure and apoptosis as well as the expression of RhoA and YAP. These findings are expected to provide a new insight toward the prevention of podocyte damage, decreased proteinuria, and improved DN.
3.1 Test the efficiency of overexpressed ITGβ3 vector and ITGβ3 siRNAs
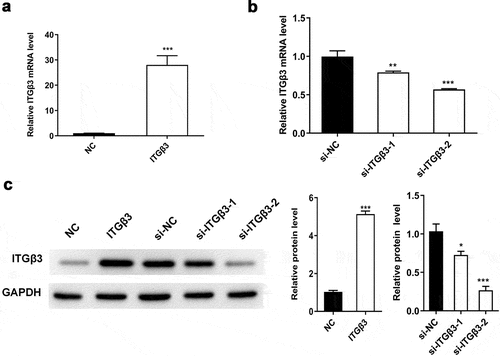
At the beginning, we tested the efficiency of overexpressed ITGβ3 vector and ITGβ3 siRNAs on the expression of ITGβ3 through RT-qPCR and western blot analysis after 48 h transfection in mouse podocyte cell line MPC5. Compared with negative control of overexpressed ITGβ3 vector (NC) group, the expression of ITGβ3 in the ITGβ3 group was significantly upregulated (). Meanwhile, si-ITGβ3-1 and si-ITGβ3-2 significantly decreased the expression of ITGβ3, in which si-ITGβ3-2 appeared to be more efficient in inhibiting ITGβ3 expression than si-ITGβ3-1 (). Western blot analysis also confirmed the RT-qPCR results (). Therefore, si-ITGβ3-2 was used for the following experiments to inhibit ITGβ3 expression. The above results verified the overexpressed ITGβ3 vector and ITGβ3 siRNAs was constructed successfully.
Figure 1. The efficiency of overexpressed ITGβ3 vector and ITGβ3 siRNAs was evaluated in mouse podocyte cell line MPC5. (a) ITGβ3 expression in NC and ITGβ3 group was tested by RT-qPCR, (b) ITGβ3 expression in si-NC, si-ITGβ3-1 and si-ITGβ3-2 group was detected by RT-qPCR. (c) ITGβ3 protein expression was detected by Western blot analysis. Data are shown as mean ± standard deviation, N = 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001
3.2 Effects of ITGβ3 inhibition on podocytes under HG treatment
In our previous study, ITGβ3 expression was upregulated and the ability of wound closure in vitro and cell apoptosis was promoted under HG situation [Citation8]. Therefore, we had silenced ITGβ3 expression in podocytes, and then investigated the ability of wound closure in vitro, changes in apoptosis, and RhoA and YAP expression after exposure to HG conditions. The in vitro wound healing assay showed that the wound closure ability of podocytes was accelerated after exposure to HG for 24 and 48 h, which was consistent with our previous study [Citation8]. However, ITGβ3 knockdown decreased wound closure ability of podocytes under HG conditions (). During flow cytometry analysis, we observed that the apoptosis of podocytes increased after exposure to HG, whereas the apoptosis was decreased after ITGβ3 expression was silenced under the HG treatment (). These results indicated that HG exposure promotes apotosis and the in vitro wound closure ability in podocytes, whereas the inhibition of ITGβ3 decreases HG-induced the in vitro wound closure ability and apoptosis.
Figure 2. Effects of ITGβ3 inhibition on mouse podocyte cell line MPC5 with NG or HG treatment. (a) Wound healing analysis in vitro assay was used to evaluate the ability of wound closure in MPC5 cells with NG or HG treatment after transfection with si-NC or si-ITGβ3. (b) Apoptosis analysis was detected by Flow cytometry in MPC5 cells with NG or HG treatment after transfection with si-NC or si-ITGβ3. (c) The ITGβ3, RhoA, and YAP mRNA expressions was analyzed by RT-qPCR in MPC5 cells with NG or HG treatment after transfection with si-NC or si-ITGβ3. (d) The ITGβ3, RhoA, total YAP, nuclear YAP protein expression was analyzed by western blot analysis in MPC5 cells with NG or HG treatment after transfection with si-NC or si-ITGβ3. Data are shown as mean ± standard deviation, N = 3. *, p < 0.05
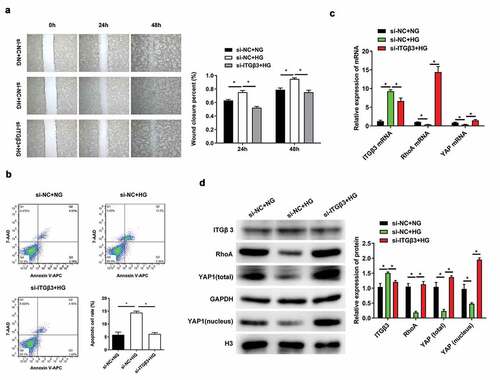
We subsequently investigated whether changes in ITGβ3 expression affect the expression of RhoA and YAP. As RT-qPCR results presented in , ITGβ3 expression was significantly increased in HG-treated podocyes along with the downregulation of RhoA and YAP compared with the NG-treated podocyes after transfection with si-NC. However, RhoA and YAP were upregulated in podocytes under HG conditions when ITGβ3 was inhibited. The same expression trend were also confirmed by western blot analysis (). ITGβ3 expression were upregulated, whereas RhoA, total YAP, and intranuclear YAP expression were downregulated when podocytes transfected with si-NC and were exposed to the HG environment. ITGβ3 knockdown upregulated the expression of RhoA, total YAP, and intranuclear YAP under HG conditions (). The experimental results show that HG promoted the upregulation of ITGβ3 expression in podocytes, along with decreased RhoA, total YAP, and intranuclear YAP expression. However, interfered with the expression of ITGβ3 reversed RhoA, total YAP, and intranuclear YAP expression under HG treatment.
3.3 Effects of ITGβ3 overexpression on podocytes
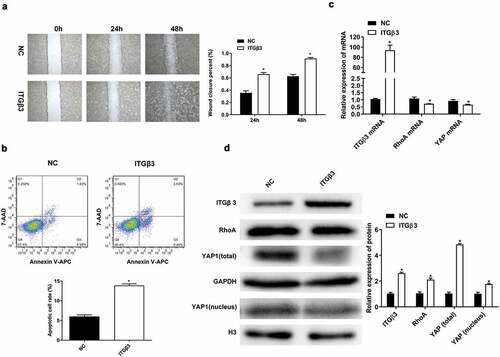
We also conducted a series of experiments using ITGβ3 overexpression to investigate the wound closure ability of podocytes in vitro, changes in apoptosis, and RhoA and YAP expressions. The wound closure rate of podocytes cells MPC5 was accelerated at 24 and 48 h in the ITGβ3 overexpression group compared to the NC group (), which suggests that ITGβ3 overexpression promotes the ability of podocyte wound closure in vitro. Podocyte apoptosis also increased because of the overexpression of ITGβ3 when compared to NC group (). The result indicated that the overexpression of ITGβ3 contributes to the ability of wound closure and apoptosis in podocytes. In addition, compared with the NC group, the expressions of RhoA and YAP were downregulated when ITGβ3 was overexpression (). These results are also confirmed by western blot analysis shown in , the overexpression of ITGβ3 is associated with the downregulation of RhoA, total YAP, and intranuclear YAP expression. The above results indicate that overexpression of ITGβ3 also decreases the expressions of RhoA, total YAP, and intranuclear YAP in podocytes.
Figure 3. Effects of ITGβ3 overexpression on mouse podocyte cell line MPC5. (a) Wound healing analysis in vitro assay was used to detect the ability of wound closure in MPC5 cells after transfection with NC or ITGβ3. (b) Flow cytometry was used to test apoptosis in MPC5 cells after transfection with NC or ITGβ3. (c) RT-qPCR analysis was used to evaluate ITGβ3, RhoA, and YAP mRNA expressions in MPC5 cells after transfection with NC or ITGβ3. (d) Western blot analysis was used to evaluated ITGβ3, RhoA, total YAP, and nuclear YAP protein expression in MPC5 cells after transfection with NC or ITGβ3. Data are shown as mean ± standard deviation, N = 3. *, p < 0.05
3.4 ITGβ3 regulates the RhoA-YAP pathway to participate in podocyte injury progression
To further confirm whether ITGβ3 regulates the RhoA-YAP pathway in podocytes damage, the RhoA overexpression vector was synthesized, and cotransfection with ITGβ3 overexpression vector into MPC5 cells was performed. The efficiency of overexpressed RhoA vector was confirmed by RT-qPCR and western blotting. The results demonstrated that RhoA expression was significantly increased when ITGβ3 was overexpressed in MPC5 cells compared to that in the case of only transfection with the ITGβ3 overexpression vector ( and ). Based on the results of and ; and , the overexpression of ITGβ3 in MPC5 cells significantly decreases the expression of RhoA, and the expression of RhoA is increased after transfection with RhoA overexpression vector in ITGβ3 overexpression MPC5 cells, these results revealed that the RhoA overexpression vector was constructed successfully. Then, the ability of wound closure was measured in MPC5 cells through an in vitro wound healing analysis. As shown in , the RhoA overexpression attenuated the effect of ITGβ3 overexpression on the ability of wound closure. In addition, RhoA overexpression partially reversed apoptosis in ITGβ3 overexpression-induced podocytes apoptosis through flow cytometry analysis (). Furthermore, the RhoA overexpression upregulated YAP expressions when compared with those of the ITGβ3 overexpression group (). This change was also observed in western blotting analysis. RhoA overexpression reversed the inhibition effect of ITGβ3 overexpression on the expression of total YAP and intranuclear YAP in MPC5 cells (). The above results indicate that ITGβ3 overexpression promotes podocytes injury by inhibiting the RhoA-YAP pathway.
Figure 4. ITGβ3 overexpression inhibits the RhoA-YAP pathway in order to promote podocyte injury progression. (a). Wound healing analysis in vitro assay was used to determine the ability of wound closure in MPC5 cells after transfection with NC, ITGβ3, or ITGβ3+ RhoA. (b). Flow cytometry was used to test apoptosis in MPC5 cells after transfection with NC, ITGβ3 or ITGβ3+ RhoA. (c). RT-qPCR was used to evaluate ITGβ3, RhoA, and YAP mRNA expressions in MPC5 cells after transfection with NC, ITGβ3 or ITGβ3+ RhoA. (d). Western blotting was used to determine ITGβ3, RhoA, total YAP, and nuclear YAP in MPC5 cells after transfection with NC, ITGβ3 or ITGβ3+ RhoA. Data are shown as mean ± standard deviation, N = 3. *, p < 0.05; NC: negative control of ITGβ3 overexpression vector; ITGβ3: integrin β3; ITGβ3+ RhoA: the group of cotransfection with integrin β3 overexpression vector and Ras homolog gene family, member A overexpression vector
In addition, a schematic was drawn to unveil our findings in the present study. As shown in , HG induced the expression of ITGβ3 overexpression, which consequently inhibited the expression of RhoA-YAP1 axis, which resulted in the promotion of the ability of wound closure and apoptosis.
Figure 5. A schematic to unveil our work in this study. HG-induced ITGβ3 overexpression inhibited the expression of RhoA-YAP1 axis, resulting in the promotion of the ability of wound closure in vitro and podocyte apoptosis
4. Discussion
DN has become a serious complication in diabetics with the increasing trend of diabetes diagnoses in recent years [Citation1,Citation26]. Podocytes are a vital component of the glomerular filtration membrane barrier, and previous studies indicate that the damage of podocytes is one of the important pathological mechanisms of DN [Citation5,Citation6,Citation27]. Our previous study revealed that the ITGβ3 expression was upregulated, and the ability of wound closure and cell apoptosis was promoted in podocytes in HG conditions [Citation8]. A previous study also demonstrated the silence of RhoA-promoted podocyte apoptosis via reduction of the expression and nucleation of YAP [Citation25]. Furthermore, steady unidirectional shear stress exerted anti-inflammatory and atheroprotective effects by regulating the ITGβ3-RhoA-YAP pathway [Citation23]. Whether ITGβ3 regulates podocytes injury progression by regulating the RhoA-YAP axis is the main purpose of the present study.
In this study, we found that the ability of wound closure in vitro and cell apoptosis was promoted and that the expression of RhoA, total YAP, and intranuclear YAP were inhibited in podocytes under HG exposure; the silence of the expression of ITGβ3 in MPC5 podocytes attenuated the effect of HG on the ability of wound closure and cell apoptosis, increased the expression of RhoA, total YAP, and intranuclear YAP in HG-treated MPC5 podocytes. In addition, transfection with ITGβ3 overexpression vector promoted the ability of wound closure in vitro and cell apoptosis along with the inhibition of the expression of RhoA, total YAP, and intranuclear YAP. However, RhoA overexpression reversed the effects of ITGβ3 overexpression on the ability of wound closure and cell apoptosis as well as rescued the expression of total YAP and intranuclear YAP. These results together indicated that the ITGβ3 overexpression regulated podocyte injury by inhibiting the RhoA–YAP pathway.
Normal podocytes express low levels of ITGβ3; however, increasing ITGβ3 was expressed in podocytes under certain pathological circumstances, including diabetes. Similarly, the expression level of ITGβ3 increased, accompanied by podocyte fusion, skeleton protein damage, podocyte shedding, and proteinuria. Increased ITGβ3 expression was observed in focal segmental glomerulosclerosis (FSGS) and diabetic patients, as well as in LPS-induced nephropathy rat models [Citation11–13]. An in vitro study found that increased ITGβ3 is associated with enhanced podocytes motility [Citation8]. Increased urokinase receptor (uPAR) was observed in the HG-exposed podocytes [Citation28]. Previous studies found that ITGβ3 interacts with uPAR in podocytes and produces a complex, which results in a structural modification of ITGβ3 and enhances its activation [Citation29,Citation30]. Inhabiting the expression of ITGβ3 was confirmed to be linked to an improvement in podocytes foot and anti-proteinuric effects in an in vivo study using a mice model [Citation31]. These results suggest that ITGβ3 plays an important role in the development of kidney disease. Our study observed that ITGβ3 inhibition contributes to depressing the ability of wound closure of podocytes in vitro during HG-induced injury, and a high expression of ITGβ3 promotes the ability of wound closure of podocytes in vitro; these findings are consistent with previous findings. As HG-induced podocytes injury is a main pathogenesis during the progression of DN, our findings added extra evidence that ITGβ3 is associated with DN and is likely to be a promising target for DN treatment.
RhoA is a Rho GTPases and has been considered to be a main regulator of the cytoskeletal architecture function [Citation32,Citation33]. The baseline activity of RhoA plays an important role in the normal function of glomerular podocytes. A previous study indicated that RhoA stabilizes the foot process structure of podocytes, thereby preventing proteinuria and related renal disease; however, inhibition of RhoA increases the motility of podocytes and results in proteinuria [Citation22,Citation34]. Consistent with previous findings, we found that RhoA was decreased in HG-treated podocytes, the ability of wound closure in vitro was increased at the same time. However, silence of ITGβ3 expression promoted the expression of RhoA, and attenuate the effect of HG treatment on the ability of wound closure in vitro. This result suggests that ITGβ3 regulates podocyte injury by inhibiting the expression of RhoA.
YAP is an important podocyte transcription cofactor of the downstream Hippo signaling pathway, which regulates the related genes that promote cell proliferation and inhibit apoptosis [Citation15,Citation16]. Cdc42/Nwasp/F-actin is an important signaling pathway that regulates cytoskeletal polymerization, and YAP mediates podocyte damage and apoptosis caused by the loss of Cdc42/Nwasp/stress fiber [Citation35–38]. Silencing YAP causes significant damage and the apoptosis of podocytes, which suggests that YAP overexpression protects podocytes from damage [Citation35,Citation39]. In addition, RhoA deficiency and the loss reduces the expression and nucleation of YAP, thereby causing podocyte cytoskeletal damage and promoting podocyte apoptosis [Citation7,Citation17,Citation25]. Furthermore, steady unidirectional shear stress exerted anti-inflammatory and atheroprotective effects be regulating the ITGβ3–RhoA–YAP pathway [Citation23]. In this study, we observed that ITGβ3 overexpression is associated with the downregulation of RhoA, YAP, total YAP, and nuclear YAP, which increases the apoptosis and the ability of wound closure of podocytes. However, these can be reversed by the overexpression of RhoA, which mitigates the harm to podocytes. To the best of our knowledge, our study was the first time to suggest that ITGβ3 overexpression increased the damage to the podocytes by regulating the RhoA–YAP pathway, which provides novel clue for DN treatment.
However, more studies are needed to state the way of how ITGβ3 regulates RhoA-YAP pathway, and ITGβ3-RhoA-YAP axis also need verified in vivo animal model.
5. Conclusion
Taken together, we found that ITGβ3 inhibition contributes to the protection of podocytes from damage. In addition, ITGβ3 overexpression inhibits RhoA-YAP activation, thereby promoting the HG-induced apoptosis and the ability of wound closure of podocytes in vitro. In other words, ITGβ3 overexpression promotes podocytes injury by inhibiting the RhoA–YAP pathway. These findings add new evidence for the important role of ITGβ3 in regulating podocyte injury in DN patients and highlights new insights into the molecular pathogenesis of DN.
Highlights
1. High glucose treatment promotes ITGβ3 expression.
2. High glucose treatment promotes podocyte damage.
3. ITGβ3 inhibition protects podocytes from high glucose-induced injury.
4. ITGβ3 overexpression suppresses RhoA–YAP axis contributes to podocyte injury.
Author contributions
XL conceived and designed the study as well as critically revised the manuscript. ZL performed the experiments, analyzed the data, and drafted the manuscript. ZL, JM, LZ, XL, SL, JX, ZF, TL, and HZ participated in the study design, study implementation, and manuscript revision. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27:S79.
- Collins AJ, Foley RN, Chavers B, et al. US renal data system 2013 annual data report. Am J Kidney Dis. 2014;63(1):A7.
- Soldatos G, Cooper M. Diabetic nephropathy: important pathophysiologic mechanisms. Diabetes Res Clin Pract. 2008;82:S75–S79.
- Remuzzi G, Schieppati A, Ruggenenti P. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346(15):1145–1151.
- Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014;38(4):252–260.
- Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74(1):22–36.
- Campbell KN, Wong JS, Gupta R, et al. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem. 2013;288(24):17057–17062.
- Li Z, Zhang L, Shi W, et al. Spironolactone inhibits podocyte motility via decreasing integrin β1 and increasing integrin β3 in podocytes under high-glucose conditions. Mol Med Rep. 2015;12(5):6849–6854.
- Wu X, Wang J, Jiang H, et al. Wnt3a activates β1-integrin and regulates migration and adhesion of vascular smooth muscle cells. Mol Med Rep. 2014;9(4):1159–1164.
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, et al. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–867.
- Kitsiou PV, Tzinia AK, Stetler-Stevenson WG, et al. Glucose-induced changes in integrins and matrix-related functions in cultured human glomerular epithelial cells. Am J Physiol Renal Physiol. 2003;284(4):F671–679.
- Dai C, Stolz DB, Bastacky SI, et al. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17(8):2164–2175.
- Wei C, Möller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63.
- Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17(8):952–960.
- Huang J, Wu S, Barrera J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434.
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505.
- Schwartzman M, Reginensi A, Wong JS, et al. Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J Am Soc Nephrol. 2016;27(1):216–226.
- Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307.
- Scott RP, Hawley SP, Ruston J, et al. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol. 2012;23(7):1149–1154.
- Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233.
- Marshall CB, Krofft RD, Pippin JW, et al. CDK inhibitor p21 is prosurvival in adriamycin-induced podocyte injury, in vitro and in vivo. Am J Physiol Renal Physiol. 2010;298(5):F1140–1151.
- Kistler AD, Altintas MM, Reiser J. Podocyte GTPases regulate kidney filter dynamics. Kidney Int. 2012;81(11):1053–1055.
- Wang L, Luo JY, Li B, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540(7634):579–582.
- Buvall L, Rashmi P, Lopez-Rivera E, et al. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat Commun. 2013;4(1):2863.
- Huang Z, Zhang L, Chen Y, et al. RhoA deficiency disrupts podocyte cytoskeleton and induces podocyte apoptosis by inhibiting YAP/dendrin signal. BMC Nephrol. 2016;17(1):66.
- Ritz E, Rychlík I, Locatelli F, et al. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34(5):795–808.
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54(6):1626–1634.
- Zhang L, Li R, Shi W, et al. NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. Br J Pharmacol. 2013;170(2):426–439.
- Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348.
- White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51(10):3083–3089.
- Zhang B, Xie S, Shi W, et al. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol Dial Transplant. 2012;27(5):1746–1755.
- Asanuma K, Yanagida-Asanuma E, Faul C, et al. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8(5):485–491.
- Yanagida-Asanuma E, Asanuma K, Kim K, et al. Synaptopodin protects against proteinuria by disrupting Cdc42: iRSp53: mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171(2):415–427.
- Mundel P, Reiser J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 2010;77(7):571–580.
- Sinha S, Yang W. Cellular signaling for activation of Rho GTPase Cdc42. Cell Signal. 2008;20(11):1927–1934.
- Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183.
- Reginensi A, Scott RP, Gregorieff A, et al. Yap-and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9(3):e1003380.
- Zhang Y, Xia H, Ge X, et al. CD44 acts through RhoA to regulate YAP signaling. Cell Signal. 2014;26(11):2504–2513.
- Carlier M-F, Ducruix A, Pantaloni D. Signalling to actin: the Cdc42-N-WASP-Arp2/3 connection. Chem Biol. 1999;6(9):R235–R240.