ABSTRACT
Migraine is a primary headache characterized by moderate or severe headache attacks, accompanied with reversible neurological and systemic symptoms. There are rare biomarkers for the disease. While emerging evidence has indicated the connection between gut microbiota and migraine, the relation between oral microbiota and migraine is barely known. Thus, the objective of the current study was to explore a possible correlation between oral microbiota and migraine. We compared the oral microbiota communities of migraine patients (26) with healthy subjects (29) via 16S rRNA gene sequencing. Alpha diversity indices were higher in migraine group compared with control group, whereas beta diversity indices also showed significant difference. A total of 23 genera were found differentially abundant between migraine and control groups. To conclude, there was a significant compositional difference in oral microbiota in migraine patients compared with healthy subjects.
Introduction
Migraine is a primary headache ranked as the second most disabling neurological disorder in the world [Citation1,Citation2]. It is characterized by moderate or severe headache attacks, accompanied with reversible neurological and systemic symptoms including photophobia, phonophobia, nausea, emesis and cutaneous allodynia [Citation3,Citation4]. The headache attack attributes to activation of the trigeminal sensory pathways which innervate pain-sensitive intracranial structures, including the eye, dura mater, large cerebral and pial blood vessels and the dural venous sinuses [Citation5], whereas cortical spreading depression is considered as the underlying pathophysiological cause of the aura phase [Citation6]. However, there are many controversies and uncertainties in studies on migraine pathogenesis [Citation3]. Moreover, there are rare biomarkers for the disease.
Imbalance of oral microbiota has been connected to a variety of oral diseases such as periodontitis, dental caries and oral mucosal diseases [Citation7], as well as other systemic diseases including bacterial endocarditis [Citation8], ischemic stroke [Citation9], cardiovascular disease [Citation10,Citation11], pancreatic cancer [Citation12], pediatric Crohn’s disease [Citation13], pneumonia [Citation14] and central nervous system (CNS) diseases including Parkinson’s disease [Citation15], Alzheimer’s disease [Citation16] and autism spectrum disorder [Citation17]. However, the relation between oral microbiota and migraine is barely known.
The present study analyzed and compared the oral microbiota composition of migraine patients with healthy subjects via 16S rRNA gene sequencing, so as to explore the relation between oral microbiota and migraine. The study will provide new insight for researchers who are interested in the pathogenesis of migraine.
Materials and methods
Study design and subject selection
Protocol of the current study was approved by the Research Ethics Committee, Renji Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China). Each participant was informed of the objective of the study and signed informed consent. Personal information of each participant was kept confidential.
We designed a cross-sectional study (1 January 2018–18 August 2019) at the outpatient clinic of the Department of Neurology, Renji Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China).
A cohort of 55 individuals was analyzed. We recruited migraine patients (n = 26) diagnosed according to the ICHD-3 (the International Classification of Headache Disorders, 3rd edition) criteria [Citation4]. Healthy participants (n = 29) matched by age and sex of the patients were enrolled as the control group. Exclusion criteria for both cases and control participants were any type of CNS disease; serious chronic illnesses including diabetes, hypertension, heart failure and cancers; any type of oral disease; and use of antibiotics or probiotics in the past 3 months. To address potential sources of bias, confounding factors [Citation18] were considered in our exclusion criteria. Meanwhile, to minimize the bias due to diet habits [Citation19], both the patient and control groups were individuals who had lived in the same city (Shanghai) within at least 3 months before sample collection.
Sample collection and DNA extraction
Saliva samples from migraine patients were collected during outpatient visits. Weight and height were measured. Clinical characteristics of migraine included age of onset, disease course, migraine days (per month), visual analog scale (VAS) scores and medication consumption. Diagnosis was made according to ICHD-3 criteria by neurologists. Subjects were informed not to eat or drink 30 min before saliva collection. Unstimulated saliva was collected with salivettes and was immediately kept on ice and stored in −80°C until use.
Total DNA extraction from the saliva samples was performed with the QIAamp DNA Mini Kit (Qiagen, Germany). DNA quality assessment was conducted via agarose gel electrophoresis.
16S rRNA gene sequencing
Polymerase chain reaction (PCR) amplification was performed using universal primers (341 F and 806 R) for the V3-V4 regions of the 16S rRNA gene. Subsequent sequencing was performed on an Illumina Miseq platform for further paired-end reads. Altogether 1,966,711 raw reads and 1,427,897 mapped reads were obtained. Sequence analyses which include operational taxonomic unit (OTU) clustering and taxonomy assignment were performed by Realbio Genomics Institute (China). Detailed protocols were followed as previously described [Citation20].
Statistical analysis
Statistical analyses were performed using the SPSS ver. 21.0 and R software ver. 3.1.0. Student’s t-test was performed for quantitative data and Pearson’s Chi-square test was performed for categorical data. Alpha diversity and beta diversity indices were calculated with the Qiime program based on the rarefied OTU counts [Citation20]. Distinguishment of the oral microbiota specific to migraine was identified with the linear discriminant analysis (LDA) effect size (LEfSe) method. Differential abundance analyses of migraine and control groups were performed with a generalized linear model with a negative binomial distribution. Prediction of the abundances of functional categories in the Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog was conducted using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt). P < 0.05 was considered as statistically significant. Detailed analysis methods were followed as previously described [Citation20].
Results
To explore a possible correlation between oral microbiota and migraine, we compared the oral microbiota communities of migraine patients with healthy subjects via 16S rRNA gene sequencing and found significant compositional differences of oral microbiota in migraine patients compared with healthy subjects. Meanwhile, nitrate-, nitrite- and nitric oxide-reducing bacteria may be correlated with migraine. These results provide implication for the potential relation between oral microbiota and migraine pathogenesis.
Clinical characteristics
Basic characteristics of migraine and control groups are shown in . Age, gender and body mass index (BMI) showed no difference between the two groups. The age range of the participants included was from 25 to 58 years old. Migraine patients had average age of onset of 24.5 ± 9.3 years, disease course of 15.8 ± 8.0 years, migraine days (per month) of 10.3 ± 10.0 and VAS scores of 7.4 ± 1.6. Additionally, two patients (7.7%) were diagnosed with migraine with typical aura, five patients (19.2%) were diagnosed with medication-overuse headache and five patients (19.2%) were taking preventive medications (flunarizine, trazodone or deanxit).
Table 1. Characteristics of the study subjects
Species diversity between migraine and control groups
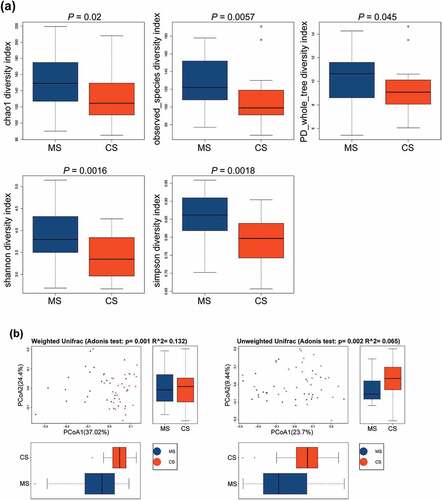
Based on OTU levels, alpha diversity indices were analyzed to quantify both ‘species’ richness and diversity [Citation15]. Chao1, observed species, phylogenetic diversity (PD) whole tree, and Shannon and Simpson indices were significantly higher in migraine group than those in control group ()), indicating that the richness and diversity of the oral microbiota in migraine patients were significantly higher than those in healthy subjects. Meanwhile, beta diversity indices also showed significant differences in weighted and unweighted UniFrac between migraine and control groups ()), indicating that the composition community [Citation15] of oral microbial in migraine group was different from that in control group.
Figure 1. The alpha diversity and beta diversity indices of the oral microbiota in migraine and control groups
Taxa alteration between migraine and control groups
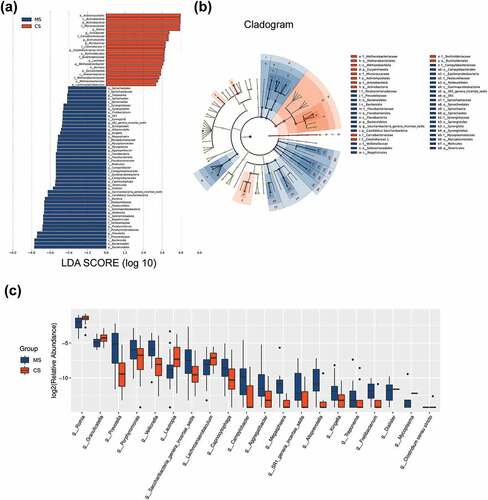
The data set of this study was composed of 6858 OTUs, covering 208 genera, 228 families, 243 classes, 240 orders and 260 phyla (), among which the core microbiota (microbiota covering 100% of the samples) were listed in Table A1. A total of 77 OTUs (data not shown) and 23 genera (Table A2, P < 0.05) were found to be distinctively abundant between migraine and control groups. To further identify vital taxonomic differences between migraine and control groups, we conducted LEfSe analysis and found significant abundance differences in oral microbiota between the two groups (, LDA scores (log10) >2, P < 0.05). The relative abundances of genera Rothia, Turicibacter, Granulicatella, Micrococcus, Clostridium sensu stricto, Lautropia, Methanobrevibacter and Lachnoanaerobaculum were higher in control group, while the relative abundances of genera Treponema, Fretibacterium, SR1 genera incertae sedis, Alloprevotella, Kingella, Megasphaera, Mycoplasma, Aggregatibacter, Campylobacter, Capnocytophaga, Dialister, Saccharibacteria genera incertae sedis, Veillonella, Porphyromonas and Prevotella were higher in migraine group (), LDA score (log10) >2, P < 0.05). These results suggested that the changed abundances of genera were probably associated with migraine.
Figure 2. Relative abundances of the oral microbiota in migraine and control groups
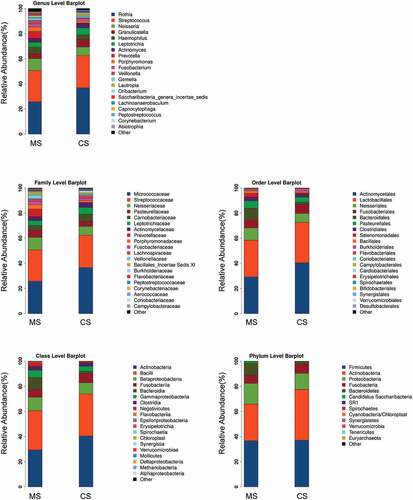
Figure 3. Taxonomic differences of the oral microbiota in migraine and control groups
Functional predicition
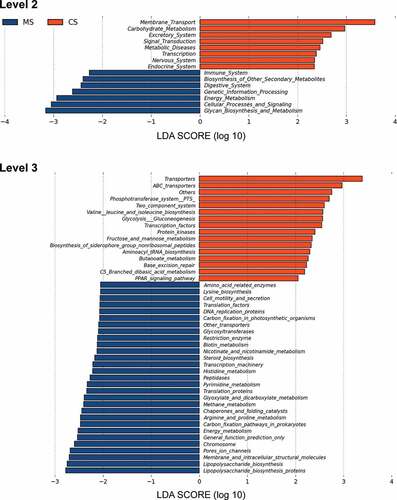
We further predicted the abundances of functional categories in the KEGG ortholog (KO) using PICRUSt based on closed-reference OTU. As a result, 73 KOs were identified to be differentially abundant in the oral microbiota between migraine and control groups (Supplementary Figure S1, FDR, P < 0.05). To mention microbial gene functions in the level 2 KEGG pathways, pathways involved in membrane transport, carbohydrate metabolism, excretory system, signal transduction, metabolic diseases, transcription, nervous system and endocrine system were higher in the oral microbiota of control group, while the microbial gene functions involving in immune system, biosynthesis of other secondary metabolites, digestive system, genetic information processing, energy metabolism, cellular processes and signaling and glycan biosynthesis and metabolism were higher in the oral microbiota of migraine group (, P < 0.05).
Figure 4. Functional predictions for the oral microbiota of migraine and control groups
Discussion
The current study provided evidence of compositional differences in oral microbiota between migraine patients and healthy subjects.
Oral microbiota and central nervous system
Oral microbial dysbiosis has been reported in CNS diseases including Parkinson’s disease [Citation15], Alzheimer’s disease [Citation16] and autism spectrum disorder [Citation17]. In the past decade, great efforts have been made to illustrate the important role of the gut microbiota in brain function and diseases via the concept of gut microbiota–brain axis [Citation21,Citation22]. Bidirectional communications between the microbiota and brain were suggested to achieve via pathways such as the hypothalamic–pituitary–adrenal (HPA) axis, the neurotransmitter pathways and the immune system and also via recognition of bacterial and host metabolites [Citation23]. While most studies concerned gut microbiota, the impact of oral microbiota on the brain is much less studied.
Nonetheless, emerging studies have implicated that oral microbiota may reach and affect the brain [Citation24]. Oral bacteria including Treponema [Citation25] and C. pneumoniae [Citation26,Citation27] were detected in postmortem brains of Alzheimer’s disease patients. Meanwhile, higher levels of antibodies against F. nucleatum, P. intermedia [Citation28], A. actinomycetemcomitans, P. gingivalis and T. forsythia [Citation29] were detected in Alzheimer’s disease patients than in healthy subjects. This suggested that some oral bacteria might invade the brain through the brain–blood barrier. Furthermore, endotoxin produced by oral bacteria has been implicated in chronic inflammation and neurodegeneration, especially in Alzheimer’s disease patients [Citation16]. It has been speculated that oral microbiota could reach the brain through direct (via the olfactory tract and olfactory nerve) and indirect (via blood, blood–brain barrier, perivascular spaces and circumventricular organs) mechanisms [Citation17,Citation30]. Meanwhile, oral microbiota are also supposed to affect the brain through inflammation and metabolic alterations [Citation17].
Oral microbiota and migraine
Previous studies have proved that migraine could be impacted by the gut–brain axis, where the composition of the gut microbiota plays a major role. Though not clearly explained, the underlying mechanism is speculated to be the gut microbiota profile interacting with other multiple factors including inflammatory mediators, neuropeptides, stress hormones and nutritional substances [Citation31]. However, the relation between oral microbiota and migraine is barely known.
Recently, one relevant study detected significantly higher abundances of nitric oxide (NO)-reducing bacteria (genera Streptococcus and Pseudomonas) in oral samples with self-reported migraine status in the American Gut Project cohort [Citation32]. However, in the current study, abundances of Streptococcus and Pseudomonas were found to be of no difference between migraine patients and control groups. Indeed, high abundances of Prevotella and Veillonella and low abundance of Rothia were detected in migraine patients in the current study. Interestingly, low abundances of Prevotella and Veillonella and high abundance of Rothia were found to be correlated with greater increases in plasma nitrite in response to nitrate supplementation [Citation33], indicating that these bacteria contribute to NO homeostasis. Moreover, arginine (source of NO) metabolism in the level 3 KEGG pathways was higher in the oral microbiota of migraine group. Taking into consideration that NO is a classic migraine trigger [Citation34] and involves in nociceptive transmission [Citation3] and increased NO stress in migraine patients [Citation35,Citation36], we would propose a potential connection between migraine and these oral bacteria.
Among the 15 genera which were of higher abundance in the migraine group, higher abundances of Prevotella and Veillonella were also related with oral diseases such as gingivitis, recurrent aphthous ulcer and dental caries [Citation37–39]. However, participants with any type of oral disease were excluded from our study, as listed in the exclusion criteria of our study design. Although clinical oral examination was not conducted, any participant with self-reported oral diseases such as periodontal diseases and dental caries, as well as oral symptoms such as bleeding or swollen gums and oral ulcer, was excluded from our study. Thus, we do not consider the compositional difference of oral microbiota in migraine patients as a result of oral condition.
Limitations
There are limitations to the current study. First, the sample size of the current study was limited. Second, bias may exist due to the inclusion and exclusion criteria and medication consumption of some patients. Third, as a cross-sectional study, the results were not convincing enough to tell the causal relationship between migraine and oral microbiota dysbiosis. To sum up, it requires larger longitudinal studies focusing on different types of primary headaches to confirm our results.
Conclusion
The present study is the first to show evidence of compositional differences in oral microbiota in a Chinese cohort of migraine patients compared with healthy subjects. Further and more detailed investigations are expected to verify whether oral dysbacteriosis exists in migraine patients, making oral microbiota a potential biomarker for migraine. Meanwhile, regarding the causal relationship between migraine and oral microbiota dysbiosis, it is worth exploring whether oral microbiota involves in migraine pathogenesis and how oral microbiota could change subsequent to the disease.
Research highlights
Compositional differences in oral microbiota in migraine patients
Nitrate-, nitrite- and nitric oxide-reducing bacteria may be correlated with migraine
Evidence for migraine epidemiology, pathology and oral microbiology
Notes on contributors
Weiqing Jiang Conceptualization (lead); Data curation (equal); Formal analysis (equal); Writing‐original draft (lead). Tingting Wang: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Chen Liu: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (supporting). Mingzhu Deng: Data curation (equal); Formal analysis (equal); Writing‐original draft (supporting). Xiao Ren: Data curation (supporting); Formal analysis (supporting); Writing‐original draft (supporting). Fei Wang: Data curation (supporting); Formal analysis (supporting); Writing‐original draft (supporting). Yaqing Zhang: Methodology (supporting); Writing‐review & editing (supporting). Xueying Yu: Methodology (supporting); Writing‐review & editing (supporting). Lingling Yao: Conceptualization (lead); Formal analysis (lead); Funding acquisition (lead); Writing‐original draft (lead). Yonggang Wang: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Supervision (lead); Writing‐review & editing (lead).
Ethics statement
Protocol of the current study was approved by the Research Ethics Committee, Renji Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China).
Supplemental Material
Download ()Acknowledgments
The authors would like to thank Dr Qi-nan Chen for the support with sample storage. This work was supported by the Natural Science Foundation of China [grant number 31770800, 91849104, 81870274].
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the [NCBI Sequence Read Archive] repository at [https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA680860].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease study 2015. Lancet. 2016;388(10053): 1545–1602. .
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11): 877–897. .
- Dodick DW. Migraine. Lancet. 2018;391(10127):1315–1330.
- International Headache Society 2013–2018. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia Int J Headache. 2018;38(1). DOI:10.1177/0333102417738202
- Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci off J Soc Neurosci. 2015;35(17):6619–6629.
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75(1):365–391.
- Gao L, Xu T, Huang G, et al. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488–500.
- Berbari EF, Cockerill FR, Steckelberg JM. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin proc. 1997;72(6):532–542.
- Joshipura KJ, Hung H-C, Rimm EB, et al. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34(1):47–52.
- Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11 Suppl):2089–2100.
- Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis. 2011;17(5):450–461.
- Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61(4):582–588.
- Docktor MJ, Paster BJ, Abramowicz S, et al. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflammatory Bowel Dis. 2012;18(5):935–942.
- Awano S, Ansai T, Takata Y, et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87(4):334–339.
- Pereira PAB, Aho VTE, Paulin L, et al. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:61–67.
- Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J Alzheimers dis JAD. 2015;43(3):725–738.
- Olsen I, Hicks SD. Oral microbiota and autism spectrum disorder (ASD). J Oral Microbiol. 2020;12(1):1702806.
- Claesson MJ, Clooney AG, O’Toole PW. A clinician’s guide to microbiome analysis. Nat Rev Gastroenterol Hepatol. 2017;14(10):585–595.
- Verma D, Garg PK, Dubey AK. Insights into the human oral microbiome. Arch Microbiol. 2018;200(4):525–540.
- Qian Y, Yang X, Xu S, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018;70:194–202.
- Borre YE, O’Keeffe GW, Clarke G, et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–518.
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742.
- Lin D, Hutchison KE, Portillo S, et al. Association between the oral microbiome and brain resting state connectivity in smokers. Neuroimage. 2019;200:121–131.
- Bui FQ, Almeida-da-silva CLC, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35.
- Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 2002;17(2):113–118.
- Hammond CJ, Hallock LR, Howanski RJ, et al. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer’s disease brain. BMC Neurosci. 2010;11(1):121.
- Roulis E, Bachmann NL, Myers GSA, et al. Comparative genomic analysis of human Chlamydia pneumoniae isolates from respiratory, brain and cardiac tissues. Genomics. 2015;106(6):373–383.
- Sparks Stein P, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers dementia j Alzheimers Assoc. 2012;8(3):196–203.
- Kamer AR, Craig RG, Pirraglia E, et al. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216(1–2):92–97.
- Ranjan R, Abhinay A, Mishra M. Can oral microbial infections be a risk factor for neurodegeneration? A review of the literature. Neurol India. 2018;66(2):344–351.
- Arzani M, Jahromi SR, Ghorbani Z, et al. Gut-brain axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21(1):15.
- Gonzalez A, Hyde E, Sangwan N, et al. Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the American Gut Project Cohort. mSystems. 2016;1(5):e00105–00116.
- Vanhatalo A, Blackwell JR, L’Heureux JE, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 2018;124:21–30.
- Pradhan AA, Bertels Z, Akerman S. Targeted nitric oxide synthase inhibitors for migraine. Neurotherapeutics j Am Soc Exp NeuroTher. 2018;15(2):391–401.
- Gruber HJ, Bernecker C, Lechner A, et al. Increased nitric oxide stress is associated with migraine. Cephalalgia Int J Headache. 2010;30(4):486–492.
- Shimomura T, Murakami F, Kotani K, et al. Platelet nitric oxide metabolites in migraine. Cephalalgia Int J Headache. 1999;19(4):218–222.
- Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46(4):1407–1417.
- Huang S, Yang F, Zeng X, et al. Preliminary characterization of the oral microbiota of Chinese adults with and without gingivitis. BMC Oral Health. 2011;11(1):33.
- Marchini L, Campos MS, Silva AM, et al. Bacterial diversity in aphthous ulcers. Oral Microbiol Immunol. 2007;22(4):225–231.