ABSTRACT
Preterm birth (PTB) is an immune-inflammatory disease that needs to be resolved. This study aimed to identify the role of interleukin-27 (IL-27), an immunomodulatory factor, in PTB and its associated mechanisms. Here, we analyzed the high-throughput of samples data from the maternal-fetal interface to the peripheral circulation obtained from public databases and reported that the elevated IL-27 was involved with the onset of PTB. Further bioinformatics analyses (e.g. GeneMANIA and GSEA) revealed that IL-27 overexpression in the peripheral circulation as well as maternal-fetal interface is related to the activation of the immune-inflammatory process represented by IFN-γ signaling, etc. In addition, IL-27 and immune infiltration correlation analysis demonstrated that IL-27 mediates this immune-inflammatory imbalance, plausibly mainly through monocyte-macrophage and neutrophils. This finding was further validated by analyzing additional datasets. Overall, this is the first study to elaborate on the role of IL-27-mediated immuno-inflammation in PTB from the perspective of bioinformatics, which may provide a novel strategy for the prevention and treatment of PTB.
Introduction
Preterm birth (PTB), one of the most common complications of pregnancy, is characterized by birth before 37 weeks of gestation [Citation1]. At present, the average PTB rate worldwide is about 10.6%, which accounts for around 15 million premature babies every year [Citation2,Citation3]. Worse yet, PTB is not only the major cause of neonatal mortality (>27%), but also the reason for the morbidity increase (cerebral palsy, growth retardation, etc.) of surviving newborns, adversely affecting their long-term quality of life [Citation4–6]. Thus, presently, PTB is a focal concern in obstetrics and a severe socioeconomic problem for every country [Citation7]. Despite significant medical advances in the last decades, the diagnosis and treatment of preterm labor remain a challenging task, mainly due to the lack of knowledge on PTB pathogenesis [Citation8]. PTB has long been recognized as a multifactorial syndrome, and recently, increasing studies have shown that the immune-inflammatory imbalance is a hallmark pathogenetic feature of PTB [Citation9,Citation10]. This theory holds that the mother’s immune tolerance toward the fetus starts diminishing before term, which leads to profound proliferation of the mother’s immune cells and activation of the complement cascade, inducing the release of an array of pro-inflammatory factors. Thus, an excessive inflammatory response is generated within the overall maternal-fetal system, leading to uterine contractions, fetal membrane disruption, and other signs of labor, culminating in PTB. However, the key regulatory factors and details of these systemic biological processes in PTB remain obscure [Citation11–13].
Interleukin-27 (IL-27), a novel member of the IL-12 family, was first discovered in 2002 [Citation14]. It is a heterodimeric cytokine that comprises a unique IL-27p28 subunit (encoded by gene IL27) and Epstein-Barr virus-induced gene 3 (EBI3), the structural component shared with IL-35 [Citation15]. IL-27 is primarily synthesized by antigen-presenting cells, and it is widely expressed in a myriad of cells, including placental trophoblast cells [Citation16]. The biological effects of IL-27 are mediated through its receptor (IL-27 R), which is expressed by multiple hematopoietic and immune cells. IL-27 R entails gp130 (utilized by IL-6 and so on) and a specific subunit IL-27Rα (also known as WSX-1) [Citation17]. Although multiple studies have reported IL-27 as an essential regulator of immune response and inflammation, its precise role in the immune response is still disputable [Citation18,Citation19]. Conventionally, IL-27 has been envisaged as a potent promoter of inflammation. When first discovered, it was characterized as a promoting factor in the rapid initiation of inflammatory responses, processing the ability to stimulate the rapid expansion of naïve CD4+T and then the production of IFN-γ, which has been demonstrated by various subsequent studies [Citation14,Citation19]. J.H. Cox et al. demonstrated that IL-27 signaling plays a vital role in the development of colitis in mouse models, which promotes the differentiation of the CD4+T into Th1 and IFN-γ production, while inhibits the conversion of naïve T cells into Foxp3+ regulatory T cells (Tregs), thus contributing the inflammatory environment of the intestinal tract [Citation20]. Besides, IL-27 also enhances inflammation by activating innate immune cells such as monocytes and dendritic cells. In human peripheral blood monocyte, IL-27 induces secretion of pro-inflammatory cytokines, such as IL-6, IP-10, MIP-1, MIP-1, and TNF-α, through STAT1/3 and NF-κB signaling pathways [Citation21,Citation22]. Although the contribution of IL-27 in initiating and maintaining inflammation is well evidenced, some studies still suggested that it is an immune-inflammatory antagonist. In disorders such as Sjögren Syndrome, psoriasis, and cancers, IL-27 stimulates IL-10 production of CD4+ T cells, promotes specific T regulatory cell responses, and downregulates the function of Th1 and other immune cells that secrete inflammatory factors [Citation23–27]. Thus, we are wondering what role will IL-27 play in PTB as a pleiotropic cytokine.
In recent years, high-throughput sequencing technology has generated a massive amount of molecular information about various diseases. Based on this, bioinformatics analysis brings us the possibility to unravel the role of various RNA molecules and the underlying mechanism in diseases (e.g. cancer, diabesity) at a holistic level [Citation28–30]. For instance, from the perspective of mRNA, single-cell sequencing analysis demonstrated that the increased level of specific immune cell type signatures in the placenta is linked with preterm labor [Citation31]. In addition, 153 miRNAs in maternal peripheral blood were found to be dynamically associated with the gestational week of PTB pregnancies, and bioinformatic analysis further suggested that they were enriched in pathways regulating the immune process [Citation32]. Recently, transcriptomic analysis has shown that circRNAs act as novel biomarkers of PTB and regulate the immune-inflammatory imbalance in PTB [Citation33]. Thus, high-throughput data-based bioinformatics strategies are efficient and comprehensive research methods that have effectuated new perspectives and remarkable breakthroughs in resolving disease perplexities [Citation34–36].
IL-27 has a significant regulatory effect on immuno-inflammation, whose imbalance is the important link in PTB development. Therefore, we speculate that IL-27 might play a crucial role in the PTB pathology. Our previous study showed that IL-27 is elevated in the serum of preterm pregnant women [Citation37]. This study aims to investigate the role and mechanism of IL-27 in the PTB from the maternal circulation to the maternal-fetal interface using bioinformatics strategies. The outcomes of this analysis suggested the increased levels of IL-27 expression in PTB and its association with the activation of immune-inflammatory pathways and the altered immune cell abundance. This might provide new ideas and theoretical guidance for the diagnosis and treatment of PTB.
Methods and materials
Data collection and processing
High-throughput data of maternal and fetal tissues were obtained from the NCBI Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) and the Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) databases. The selection criteria were as follow: (a) mRNA dataset of pregnant women with singleton gestation; (b) pregnant women with significant complications (e.g., preeclampsia) or comorbidities were excluded. (c) The preterm group with the signs and symptoms of spontaneous preterm labor (such as regular uterine contractions) before 37 weeks of gestation. (d) The gestational age of the term group greater than 37 weeks. Thus, the high-throughput data from a total of 152 samples of maternal peripheral blood (GSE96083), chorion (GSE73685 and SRP139931), placenta (GSE73685, GSE118442, and GSE73712), decidua (GSE73685 and GSE73712), and myometrium (GSE73685, GSE9159, and ERP116770) were selected for this study ().
Table 1. Detail information of datasets included in this study
RNA-Seq data were aligned against the human reference genome (h19) using the HISAT2 software [Citation38]. The mapped reads were assembled using StringTie and normalized using DESeq2. All the software was set to default parameters [Citation39,Citation40].
In addition, Microarray data were normalized and analyzed by the R package ‘limma’ [Citation41].
Interaction network analysis
Protein-protein interaction (PPI) network was deciphered using GeneMANIA tool in Cytoscape (version 3.6.1) software with the default settings (max resultant genes: 20; max resultant attributes: 10) [Citation42]. Based on its homo database, GeneMANIA can generate a global view of the validated interactions between the selected genes, including co-expression, co-localization, genetic, shared protein domains, pathway, and physical interactions of the genes.
Functional enrichment analysis
The GO (Gene Ontology) and the KEGG (Kyoto Encyclopedia of Gene and Genomes) pathway enrichment analyses were conducted using the R package ‘Clusterprofiler’ [Citation43]. GO terms or KEGG pathways with P-value < 0.05 were assembled and clustered according to their membership similarities. Then, the results were visualized by Cytoscape software and the ‘ggplot2’ package of R software [Citation44,Citation45].
Gene set enrichment analysis (GSEA)
For GSEA, the GSEA (version 4.0.3) software with the Hallmark and C7 gene sets obtained from the MSigDB database (http://software.broadinstitute.org/gsea/msigdb/) were employed [Citation46]. All the samples were categorized into two groups based on the IL-27 expression levels, and genes were pre-ranked from the highest to the lowest using log2 fold-change values. Gene-set permutation was applied 1000 times, and P < 0.05 was set as the filtering threshold. The normalized enrichment score (NES) was considered as the major statistic index for to evaluate the enrichment results.
Immune infiltration analysis
ImmuCellAI tool, a gene set signature-based method that can accurately assess the abundance of 24 immune cells, was employed to determine the relative abundances of immune cells [Citation47]. This tool is suitable for analyzing both RNA-Seq and microarray expression data from blood or tissue samples. Our analyses were completed using the default settings. Subsequently, the correlations between IL-27 expression and immune cell abundances were calculated using Pearson’s test and visualized using R software.
Statistical analysis
Statistical analysis was performed using the SPSS software (version 25.0, Chicago, IL, USA). The mean and standard deviation (mean ± SD) of mRNA expression data were calculated. The Student’s t-test was used to analyze the differences between groups. P < 0.05 was set as statistical significance. The significance of coefficients and line regression models was analyzed using Student’s t-test and F-test, respectively. Significant correlation of genes with IL-27 expression was determined through Pearson correlation analysis. GraphPad Prism (version 8.0, San Diego, CA) and R (version 3.6.2) software were used for the visualization of the results.
Results
IL-27 is a potent regulator of immune inflammation. This study, for the first time, employed bioinformatic analysis to discern the role and potential mechanism of IL-27 in PTB. Firstly, using a series of high-throughput data obtained from GEO and SRA databases, we identified the expression landscape of IL-27/IL-27 R and its related gene networks in pregnant women. Subsequent enrichment analysis revealed that IL-27 is involved in PTB by promoting immune-inflammatory activities. Finally, we explored and validated the relative percentages of 24 immune cell subgroups and their correlations with IL-27 expression in preterm and term pregnancy.
The mRNA expression levels of IL-27 and its receptor
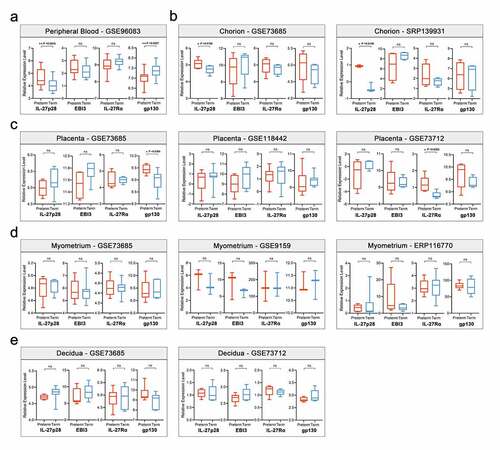
We downloaded one peripheral blood dataset, two chorion datasets, three placental datasets, two decidual datasets, and three myometrial datasets from the SRA and GEO databases, covering the major sample types for maternal-fetal studies, to detect the mRNA expression levels of IL-27 (IL-27p28 and EBI3) and its receptor (IL-27Rα and gp130) (). As shown in , IL-27/IL-27 R mRNA expression was expressed in all samples. Most notably, we observed that IL-27p28 expression was significantly elevated, but EBI3 expression remained unaltered in preterm peripheral blood and chorion samples. However, gp130 expression decreased, but IL-27Rα expression was not altered significantly in preterm peripheral blood samples. Besides, expression levels of neither of them differed between preterm and term chorions (). There appears to be a differential expression of IL-27 R in placenta tissues, however, it was not stable (). Additionally, no significant correlations were identified between IL27/IL27R and PTB in myometrial and decidual samples ().
IL-27p28-correlated genes involved in PTB
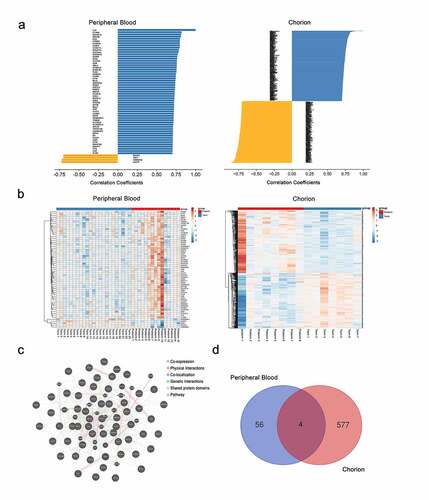
The genes correlated to IL-27p28 were selected by estimating the correlation coefficient between the expression of all genes and IL-27p28 (Pearson’s r > 0.7 and P-value < 0.05). In peripheral blood, a total of 56 and 4 genes were identified to be positively and negatively correlated to IL-27p28, respectively. In chorion, 309 and 272 genes were identified to be positively and negatively correlated to IL-27p28, respectively ()). The hierarchical clustering results showed a clear distinction in gene expression levels between preterm and term groups, indicating their vital role in PTB development ()). PPI network of these genes was constructed to reveal the potential interactions between them. In the peripheral blood and chorion samples, 79.4% and 59.4% of IL-27p28-related genes were co-expressed, 14.66% and 24.35% genes showed physical interactions, 2.94% and 7.18% showed co-localization, 2.59% and 2.9% showed genetic interactions, and 0.55% and 0% shared protein domains, respectively ()) (The network of genes in the chorion is not shown). As per the outcomes, IL10RB, SLC22A4, RIPK3, and TIFA were found to be associated with IL-27p28 in both peripheral blood and chorion () and Supplementary Figure 1).
Figure 2. The characteristics of IL-27p28-correlated genes. (a) Pearson’s correlation coefficients of IL-27p28-correlated genes in maternal peripheral blood and chorion tissues. (b) Heatmap of expressions of IL-27p28-correlated genes in preterm and term pregnancies. (c) PPI network of IL-27p28-correlated genes in maternal peripheral blood. (d) Venn analysis of IL-27p28-correlated genes in maternal peripheral blood and chorion tissues
Functional annotations of IL-27p28-correlated genes
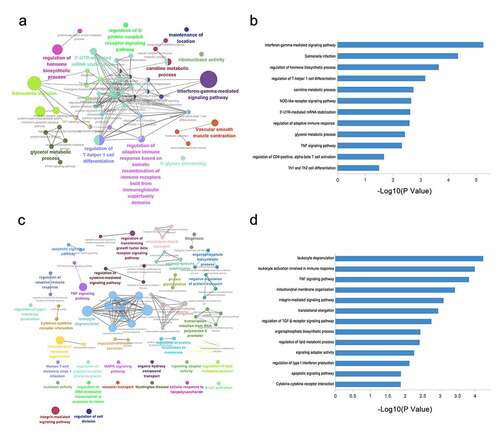
To elucidate the underlying mechanisms for IL-27p28 contribution in PTB progression, the above-mentioned correlated genes were subjected to GO and KEGG pathway analyses. The results revealed that these genes were involved in the IFN-γ-mediated signaling pathway, regulation of hormone biosynthetic process, and regulation of T-helper 1 cell differentiation, and so on in peripheral blood (). In the chorion, the related genes were enriched in leukocyte degranulation, TNF signaling pathway, and regulation of TGF-β receptor signaling pathway, and so on (). These findings indicate that IL-27p28 might play a crucial role in PTB by regulating immune-inflammatory processes through its associated gene networks.
IL-27p28 promote the immune-inflammatory processes in PTB
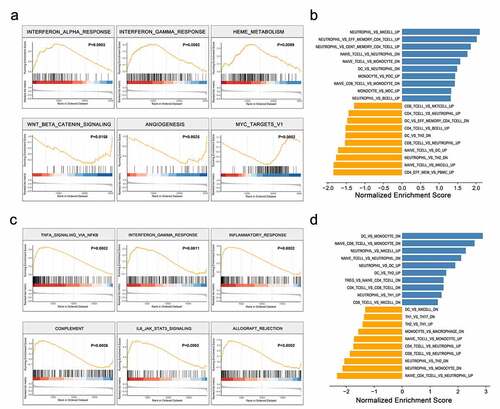
To gain a better understanding of the biological pathways involved in the PTB pathogenesis as a result of IL-27p28 overexpression, GSEA analyses were performed. In peripheral blood, enrichment plots of GSEA showed an apparent overlap between IFN-α/γ response-dependent and IL-27p28-upregulated gene sets, suggesting that IL-27p28 may activate IFN-α and IFN-γ signaling (). Interestingly, the top six gene sets that were positively enriched in IL-27p28 overexpressing chorion samples were TNFA signaling via NF-κB, IFN-γ response, inflammatory response, complement, IL-6-JAK-STAT3, and allograft rejection (). These findings indicate activation of inflammation as the potential function of IL-27p28 in PTB. Besides, we also observed significant changes in relative levels of multiple immune cells as a result of IL-27 overexpression ().
Correlation analysis between IL-27p28 and immune cells in PTB
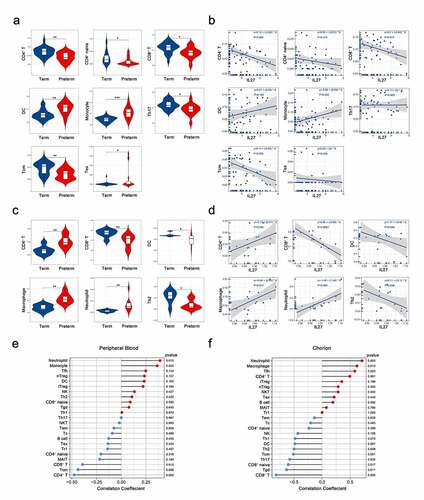
Based on the potential function of IL-27p28 as a PTB pathological factor, mediated via increased immuno-inflammation, we determined the immune cell profiles in preterm and term groups and analyzed their associations with IL-27p28 levels (Supplementary Figure 2). In peripheral blood, the relative abundances of a total of eight immune cells were significantly altered in the preterm group. In particular, CD4+T, CD4+naïve T, CD8+T, central memory T (Tcm), and Th17 cells were decreased, and dendritic cells (DC), monocyte, and Exhausted T cells (Tex) increased ()). Linear regression analysis indicated a negative correlation between IL-27p28 and CD4+T, CD8+T, and Tcm, and a positive correlation between monocyte and IL-27p28 ()). In the chorion, CD4+T, macrophage, and neutrophil abundance increased, and that of CD8+T, DC, and Th2 decreased in the preterm group (). Out of these immune cells, CD8+T was linearly and negatively correlated with IL-27p28 expression, while macrophages and neutrophils were linearly and positively correlated with IL-27p28 expression (). Pearson correlation analysis validated the significant correlation between IL-27p28 and the above-mentioned immune cells in peripheral blood and chorion, respectively (). Thus, the role of IL-27p28 in the PTB pathogenesis may be inextricably linked to the alterations in these immune cells.
Figure 5. Correlation between IL-27p28 expression and immune sub-cells in PTB. The different immune cells in (a) maternal peripheral blood and (c) chorion tissues. The linear regression analysis for the immune cells and IL-27p28 in (b) maternal peripheral blood and (d) chorion tissues. (e, f) Pearson’s correlation coefficients between the immune cells and IL-27p28. (Th: T helper; Tfh: T follicular helper; Treg: regulatory T; Tgd: γδ T; Tem: effector memory T; Tcm: central memory T; Tc: cytotoxic T; Tex: exhausted T; Tr1: Type 1 regulatory T; MAIT: mucosal-associated invariant T; NK: Natural killer; NK T: Natural killer T. * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001.)
Validation of the IL-27p28 expression and its potential role in PTB
To confirm the IL-27p28 expression level in the peripheral blood of PTB and its correlation with CD4+T, CD8+T, monocyte, and neutrophil cells, we performed validation studies based on additional peripheral blood transcriptome data (SRP107901 and SRP144363, preterm group = 19, term group = 16). The results were in line with those observed above. The IL-27p28 overexpressed in the peripheral blood of the preterm group while the EBI3 expression level did not differ significantly between the two groups. In addition, expression levels of previously screened IL-27p28- related genes (IL10RB, SLC22A4, RIPK3, and TIFA) also increased, validating the activation of the IL-27p28 biological network in PTB ()). Analysis of the immune cell expression profile and its association with IL-27 expression indicates a favorable correlation between IL-27 and immune cells, especially CD4+T, CD8+T, monocyte, and neutrophil cells ()). As shown in ), IL-27p28 has a strong positive linear relationship with neutrophils and monocytes, and a significant negative linear relationship with CD4+ T and CD8+ T cells.
Figure 6. Validation of IL-27p28 expression level and its role in PTB. (a) The mRNA expression of IL-27 (IL-27p28 and EBI3), IL-27 receptor (IL-27Rα and gp130) and associated genes in the peripheral blood of preterm and term pregnancies. (b) The correlations between IL-27p28 expression level and immune cells in PTB. (c) The linear regression analysis for the immune cells and IL-27p28. (d) The mRNA expression levels of IL-27 (IL-27p28 and EBI3), IL-27 receptor (IL-27Rα and gp130) and related genes in the peripheral blood monocyte of preterm and term pregnancies. (* indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001)
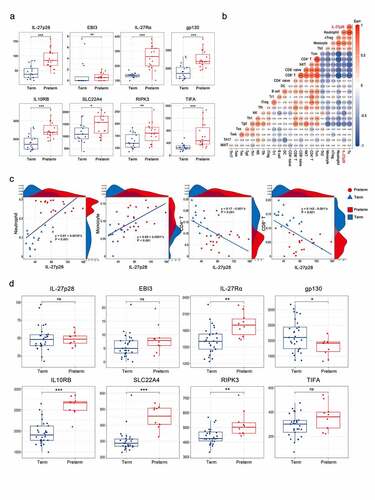
Furthermore, considering the close correlation between IL-27p28 and these immune cells, sequencing data from peripheral blood monocytes (GSE96077, preterm group = 10, term group = 29) were selected for in-depth exploration. Interestingly, in monocytes, IL-27p28 and EBI3 expression levels did not differ between the two groups, but the receptor IL-27Rα expression was significantly increased in the preterm group. In addition, the levels of IL-27-related genes also increased in the preterm group ()). These results may indicate that the increased IL-27p28 level in peripheral blood may activate the associated biomolecular network by acting on immune cells and thus regulate the immune response.
Discussion
Although PTB is recognized as an immune-inflammatory disease, its specific mechanism remains ambiguous. In this study, for the first time, we reported the expression landscape of IL-27/IL-27 R and their potential functions in PTB from the bioinformatic perspective. The outcomes of the analysis indicate that the IL-27p28 subunit is significantly correlated to PTB, and it might promote immuno-inflammation in the PTB. In addition, we also identified the altered immune cells and their underlying associations with IL-27p28 in PTB. These findings may unveil new prospects for studying the immune mechanism of PTB.
Previous studies have demonstrated that IL-27/IL-27 R is expressed in various tissues (for instance, decidua and placenta) from early to late pregnancy in normal pregnant humans and mice [Citation48,Citation49]. These findings were in line with our study, as IL-27/IL-27 R expression was detected in peripheral blood, chorion, decidua, myometrium, and placenta of normal pregnancies (). Depending upon the conditions, the IL-27/IL-27R exerts either pro-inflammatory or anti-inflammatory effects to maintain the immune homeostasis in pregnancy [Citation50–52]. Thus, altered levels of IL-27/IL-27R are of great importance in the development of gestational complications. As reported in previous studies, decreased level of IL-27 in the decidua during early pregnancy attenuates its promoting effect on the secretion of anti-inflammatory IL-10 by CD4+Treg cells and its inhibiting effect on the production of pro-inflammatory IL-17 by CD4+Th17 cells, which is an essential mechanism of recurrent miscarriage [Citation49]. Similarly, increased levels of IL-27 in peripheral blood during late pregnancy might activate a systemic inflammatory state culminating in preeclampsia [Citation53,Citation54]. Our study revealed that increased levels of IL-27 in peripheral blood and chorion were strongly associated with PTB (). Peripheral blood reflects systemic changes in the mother, while the chorion, a component of the fetal membrane that directly encases the fetus and amniotic fluid, has its outer surface attached to the uterine wall and is an important site for maternal-fetal signal transmission and immune response [Citation55,Citation56]. Thus, we believe that IL-27 is involved in the mechanism of PTB from the peripheral circulation to the maternal-fetal interface. Interestingly, we observed that IL-27p28, the specific subunit of IL-27, was significantly associated with PTB, rather than EBI3, a subunit shared with other members of the IL-12 family. This phenomenon can be explained by the transcription of p28 and EBI3 are activated by different signals thus independent of each other, and subsequently, these two subunits together are secreted after combined as the IL-27 heterodimer in the endoplasmic reticulum [Citation17].
The potential function of a single gene can be revealed by analyzing the genes associated with its expression, an approach known as ‘guilt of association’ [Citation57–59]. We constructed the networks of IL-27p28-related genes in the peripheral blood and at the chorion, respectively, to explore its possible effects in PTB (). The functional network of peripheral blood and chorion had four overlapping genes (RIPK3, TIFA, SLC22A4, and IL-10RB) (Supplementary Figure 1). RIPK3, a crucial regulator of inflammation and cell death with serine/threonine kinase activity, is expressed in a variety of human tissues and interacts with the TNF receptor family and toll-like receptors (TLRs) to control their downstream signalings, such as activation of the NF-κB pathway and inflammasome [Citation60–63]. Moreover, the activation of these downstream signals has been corroborated as a vital part of the PTB pathogenesis. Similarly, as an inflammatory signaling adaptor, TIFA also activates the NF-κB pathway and NLRP3 inflammasome, mediating the adaptive and innate immunity. Strikingly, TIFA induces the HMGB1 secretion from hepatocytes in vitro, while the increase of HMGB1 in the amniotic cavity is one of the predisposing factors for PTB [Citation64–66]. In slight contrast to the former two, SLC22A4 encodes an organic cation transporter and plasma integral membrane protein that transports drugs and L-carnitine to the placenta during the gestational period [Citation67,Citation68]. However, as per the previous studies, SLC22A4 gene polymorphism is associated with an increased risk of inflammatory bowel disease and rheumatoid arthritis, and immune imbalance in these diseases is similar to that of PTB [Citation69,Citation70]. Nevertheless, we observed that IL-10RB is upregulated in PTB, suggesting a bidirectional regulatory effect of IL-27p28 in PTB. IL-10RB, encoding the IL-10 receptor β subunit, which is necessary for IL10-induced signal transduction [Citation71]. IL-10 has significant anti-inflammatory activity, reducing pro-inflammatory cytokines (such as IL-1, IL-6, IL-12, and TNF-α) and blocking antigen presentation. Previous reports stated that its abnormal expression plays a crucial role in the pathology of gestational complications, such as PTB, miscarriage, and preeclampsia [Citation72,Citation73]. In a word, these above genes, which are strongly associated with IL-27p28 in peripheral blood and chorion, are engaged in the immune-inflammatory activity. They reflected the biological function of IL-27p28 in PTB to some extent, which was confirmed by the function enrichment and GSEA analysis in this study.
It is worth noting that the IL-27p28-related gene networks were involved in the immune-inflammatory imbalance of PTB in both the peripheral circulation and the chorion. However, their specific functions seem to be different. Our results showed that the top pathways associated with IL-27p28 are the ‘IFN-γ signaling pathway’ and ‘salmonella infection’ in the peripheral circulation, while are ‘leukocyte activation’ and ‘leukocyte degranulation’ in the chorion (). Peripheral circulation is a direct window to observe the pathophysiology of the perinatal disease, which mediates a myriad of systemic biological activities [Citation74–76]. It is widely known that with increasing gestational age, IFN-γ level also increases in the peripheral blood, and it peaks at labor [Citation77]. Moreover, the abnormal elevation of IFN-γ in the circulation may result in miscarriage or PTB by stimulating uterine contraction or damaging blood vessels [Citation78–80]. IFN-γ is a classic downstream molecule of IL-27 since IL-27 activates immune cells, such as NK cells and T cells, to secret IFN-γ, which causes pro-inflammatory effects in various diseases [Citation19]. Therefore, we speculate that the IL-27-IFN-γ axis in the peripheral circulation may potentially promote PTB. Indeed, the maternal-fetal interface serves as the primary site for interaction between fetal antigens and the maternal immune system, and it is also the original site of the signals that trigger the labor. In such a microenvironment, IL-27 seems to exhibit stronger immune-inflammatory activation than in peripheral circulation. The GSEA results indicated that in addition to the classic IFN-γ, TNF, and IL-6 inflammatory signals, complement and allograft rejection activities are also related to the increase of IL-27 in PTB (). As we know, allograft rejection is a vital component of labor initiation. During pregnancy, the maternal immune system develops a tolerance to the fetus (as a semi-allograft) to maintain the progress of the pregnancy. As the labor time nears, antigen presentation increases and immune cells is activated in large numbers, resulting in excessive inflammation and rejection response [Citation81,Citation82]. Previous studies have demonstrated that IL-27 promotes allograft rejection by activating the STAT pathway and up-regulating IFN-γ in the skin transplantation mouse model. Based on this, we speculated that increased levels of IL-27 at the maternal-fetal interface might induce PTB by prematurely activating the maternal rejection response to the fetus [Citation83]. Moreover, as an important part of innate immunity, the complement cascade has recently been considered as a promising direction for PTB study since its activation involved in labor, especially the deposition of complement components C1q and C5b-9 at the maternal-fetal interface. Meanwhile, IL-27 has also been reported to be participated in influencing the complement cascade pathway in the retina and macrophages [Citation84–87]. Thus, unraveling the regulatory function of IL-27 in the complement cascade activation in PTB could be highly valuable as far as its role in PTB is concerned. Overall, IL-27 might have different immune-inflammatory regulatory effects on the circulation and the local region, acting as a crucial modulator of the biological processes discussed above to induce PTB, respectively.
Premature activation of the maternal and fetal immune system and recruitment of circulating immune cells to the maternal-fetal interface to form an inflammatory cascade is a vital component of PTB [Citation13,Citation88]. Consistently, in this study also, we identified the significant changes in innate and adaptive immune cells in the circulation and at the maternal-fetal interface in PTB. More importantly, we observed that out of these immune cells, elevated monocyte-macrophage and neutrophils (innate leukocytes) were significantly and positively correlated with IL-27 levels, while the altered levels of CD4+T and CD8+T cells (adaptive leukocytes) showed an intriguing association with IL-27 levels (). The outcomes of this study in context to innate leukocytes and IL-27 are in line with the study by Peshkova et al. that suggested IL-27 signaling as the key regulator of the pathological stimuli-induced expansion of myeloid cells (such as monocyte-macrophages and neutrophils) in abdominal aortic aneurysm [Citation89]. Circulating monocytes are recruited to the maternal-fetal interface and then differentiate into macrophages, which promotes cervical maturation and labor initiation by secretion of pro-inflammatory mediators, such as IL-1, IL-6, TNF, and MMPs and NO [Citation12,Citation90]. During inflammatory responses, IL-27 has been proven to enhance pro-inflammatory cytokine secretion and the NLRP3 activation inflammasome via upregulating TLR4 expression and signaling in monocytes [Citation91,Citation92]. Interestingly, monocyte-macrophages can be activated by pathogenic stimulation and produce IL-27, which in turn enhances the response of these cells to stimulation and increases secretion of inflammatory cytokines from these cells, forming an increasingly amplified inflammatory response [Citation93]. Similarly, neutrophils are terminally differentiated innate leukocytes that can rapidly migrate from the circulation to target sites when acted upon by low levels of chemokines. In PTB, the neutrophils in the maternal circulation infiltrate the chorion-decidua, increasing the levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) to induce labor and causing damage of the fetal membrane through their major extracellular killing function – degranulation (a main biological activity of the IL-27-related gene network involved in the chorion) [Citation94]. Due to its pleiotropic nature, the effects of IL-27 on neutrophils remain controversial. However, previous studies have shown that IL-27 directly acts upon hematopoietic stem cells, resulting in enhanced expansion of neutrophils and significant upregulation of the production of pro-inflammatory cytokines, such as IL-1β and TNF-α by neutrophils [Citation95]. In addition to the innate leukocytes mentioned above, adaptive leukocytes, specifically T cells, are also involved in labor initiation. The outcomes of this study demonstrated an attractive phenomenon that CD4+T cells decreased in peripheral blood while increased in the chorion. That is possibly due to its characteristics of migrating from peripheral blood to the maternal-fetal interface resulting in promoting a pro-inflammatory microenvironment before labor. Moreover, this recruitment appears to be significantly associated with the level of IL-27p28 (p = 0.002) (). Coincidentally, E. Gwyer Findlay et al. have shown that IL-27 receptor signaling modulates CD4+T cell chemotactic responses during infection [Citation96]. Thus, IL-27 might have a similar regulatory effect on CD4+T cells during pregnancy or PTB. Besides, we observed that the relative abundance of CD8+T cell was significantly lower in preterm than in term pregnancies; also, it was strongly and negatively correlated with IL-27 levels (). CD8+T cells, commonly known as cytotoxic T lymphocytes (CTL), play a crucial role in the body’s immune defense by secreting cytokines (mainly TNF-α and IFN-γ) and releasing cytotoxic granules that kill the target cells. However, during pregnancy, CD8+ T cells appear to be in a specific tolerance phenotype, expressing extremely low levels of the cytolytic molecules of perforin and granular enzyme B, and high levels of co-repressing molecules, thus contributing to the maintenance of pregnancy [Citation97–99]. Notably, reduced CD8+T cell levels were observed in miscarriage (a condition similar to PTB), and depleted CD8+T cells could abolish the pregnancy protective effect of progesterone treatment [Citation100,Citation101]. However, studies on its specific mechanisms are still scarce. So, it might be interesting to further explore whether IL-27 has a potential effect on these special CD8+T cells during pregnancy, thereby disrupting maternal-fetal immune tolerance and leading to PTB. All the above evidence suggests the great potential of IL-27 in the immune activity of PTB.
Certainly, this study has limitations. Firstly, the IL-27/IL-27R expression profiles of different samples were derived from different data sets. Thus, these profiles might be slightly different from their actual status in various samples within the same body at the same time. Secondly, the specific relationships between IL-27 and the above-mentioned immune cells in PTB are still worth further exploring. Thirdly, we revealed the expression and effects of IL-27 in PTB from a macro level for the first time using the bioinformatics strategies, but these results need to be further investigated and validated by analytical approaches. Thus, in future studies, we will deeply dissect the specific role of IL-27 in the complex biological system of PTB from multiple perspectives through systematic molecular experiments.
Conclusion
In summary, based on bioinformatics strategies, we identified that an increase in IL-27 in the peripheral circulation and at the maternal-fetal interface is associated with the onset of PTB. This phenomenon involves the activation of immune-inflammatory processes represented by IFN-γ signaling. Furthermore, we observed and validated that IL-27 mainly mediates monocyte-macrophages and neutrophils during these processes. These results deserve further exploration through molecular experiments. This study provides a novel strategy for interpreting the immune-inflammatory mechanism of PTB.
Authors’ contributions
HQ and NY designed the experiments and supervised the study. YR performed the experiments, analyzed and interpreted the data. YR wrote the manuscript. YM and DH helped to perform the statistical analysis. ZL, JH, and YZ contributed to the acquisition of high-throughput data. HZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Ethics statement
All the datasets analyzed in this study were obtained from the GEO and SRA databases (publicly available and open-access), which were approved by the ethics committee of their submitting institutions. Therefore, the Medical Research Ethics Committee of The First Affiliated Hospital of Chongqing Medical University ruled that no formal ethics approval was required in this particular case.
Supplemental Material
Download ()Acknowledgements
The authors thank E Gong of the Department of Obstetrics, The First Affiliated Hospital of Chongqing Medical University, for administrative support. Special thanks to Dr. Bunny for ongoing support and encouragement.
The authors also would like to appreciate the support from “111 program” of Ministry of Education P.R.C and State Administration of Foreign Experts Affairs P.R.C.
Disclosure statement
The authors declare that they have no competing interests.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Vogel JP, Chawanpaiboon S, Moller AB, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12.
- Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46.
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388:3027–3035.
- Antony KM, Levison J, Suter MA, et al. Qualitative assessment of knowledge transfer regarding preterm birth in Malawi following the implementation of targeted health messages over 3 years. Int J Women’s Health. 2019;11:75–95.
- Glover AV, Manuck TA. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: a review. Semin Fetal Neonatal Med. 2018;23:126–132.
- The L. The unfinished agenda of preterm births. Lancet. 2016;388:2323.
- Hall ES, Greenberg JM. Estimating community-level costs of preterm birth. Public Health. 2016;141:222–228.
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535.
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765.
- Di Renzo GC, Tosto V, Giardina I. The biological basis and prevention of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:13–22.
- Peterson LS, Stelzer IA, Tsai AS, et al. Multiomic immune clockworks of pregnancy. Semin Immunopathol. 2020;42:397–412.
- Cappelletti M, Della Bella S, Ferrazzi E, et al. Inflammation and preterm birth. J Leukoc Biol. 2016;99:67–78.
- Gomez-Lopez N, StLouis D, Lehr MA, et al. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11:571–581.
- Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790.
- Chen X, Deng R, Chi W, et al. IL-27 signaling deficiency develops Th17-enhanced Th2-dominant inflammation in murine allergic conjunctivitis model. Allergy. 2019;74:910–921.
- Yao G, Qi J, Liang J, et al. Mesenchymal stem cell transplantation alleviates experimental Sjögren’s syndrome through IFN-β/IL-27 signaling axis. Theranostics. 2019;9:8253–8265.
- Wang Q, Liu J. Regulation and immune function of IL-27. Adv Exp Med Biol. 2016;941:191–211.
- Fabbi M, Carbotti G, Ferrini S. Dual roles of IL-27 in cancer biology and immunotherapy. Mediators Inflamm. 2017;2017:3958069.
- Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443.
- Cox JH, Kljavin NM, Ramamoorthi N, et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–123.
- Guzzo C, Che Mat NF, Gee K. Interleukin-27 induces a STAT1/3- and NF-kappaB-dependent proinflammatory cytokine profile in human monocytes. J Biol Chem. 2010;285:24404–24411.
- Beizavi Z, Zohouri M, Asadipour M, et al. IL-27, a pleiotropic cytokine for fine-tuning the immune response in cancer. Int Rev Immunol. 2020;1–11. DOI:10.1080/08830185.2020.1840565
- Qi J, Zhang Z, Tang X, et al. IL-27 regulated CD4(+)IL-10(+) T cells in experimental sjögren syndrome. Front Immunol. 2020;11:1699.
- Chen W, Gong Y, Zhang X, et al. Decreased expression of IL-27 in moderate-to-severe psoriasis and its anti-inflammation role in imiquimod-induced psoriasis-like mouse model. J Dermatol Sci. 2017;85:115–123.
- Cui B, Lu S, Lai L, et al. Protective function of interleukin 27 in colitis-associated cancer via suppression of inflammatory cytokines in intestinal epithelial cells. Oncoimmunology. 2017;6:e1268309.
- Wang L, Cao J, Li C, et al. IL-27/IL-27 receptor signaling provides protection in clostridium difficile-induced colitis. J Infect Dis. 2018;217:198–207.
- Guo Y, Cao W, Zhu Y. Immunoregulatory functions of the IL-12 family of cytokines in antiviral systems. Viruses. 2019;11. DOI:10.3390/v11090772
- Udhaya Kumar S, Thirumal Kumar D, Bithia R, et al. Analysis of differentially expressed genes and molecular pathways in familial hypercholesterolemia involved in atherosclerosis: a systematic and bioinformatics approach. Front Genet. 2020;11. DOI:10.3389/fgene.2020.00734
- Rajan B, Abunada T, Younes S, et al. Involvement of essential signaling cascades and analysis of gene networks in diabesity. Genes (Basel). 2020;11:1256.
- Fu D, Zhang B, Yang L, et al. Development of an immune-related risk signature for predicting prognosis in lung squamous cell carcinoma. Front Genet. 2020;11. DOI:10.3389/fgene.2020.00978
- Pique-Regi R, Romero R, Tarca AL, et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife. 2019;8. DOI:10.7554/eLife.52004
- Menon R, Debnath C, Lai A, et al. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. 2019;160:249–275.
- Ran Y, Yin N, Huang D, et al. Identification and characterization of circular RNA as a novel regulator and biomarker in preterm birth. Front Bioeng Biotechnol. 2020;8:566984.
- Kumar SU, Kumar DT, Siva R, et al. Integrative bioinformatics approaches to map potential novel genes and pathways involved in ovarian cancer. Front Bioeng Biotechnol. 2019;7. DOI:10.3389/fbioe.2019.00391
- Udhaya Kumar S, Thirumal Kumar D, Siva R, et al. Dysregulation of signaling pathways due to differentially expressed genes from the b-cell transcriptomes of systemic lupus erythematosus patients – a bioinformatics approach. Front Bioeng Biotechnol. 2020;8. DOI:10.3389/fbioe.2020.00276
- Yan H, Zheng G, Qu J, et al. Identification of key candidate genes and pathways in multiple myeloma by integrated bioinformatics analysis. J Cell Physiol. 2019;234:23785–23797.
- Yin N, Wang H, Zhang H, et al. IL-27 induces a pro-inflammatory response in human fetal membranes mediating preterm birth. Int Immunopharmacol. 2017;50:361–369.
- Kim D, Paggi JM, Park C, et al. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–915.
- Pertea M, Pertea GM, Antonescu CM, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–295.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
- Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
- Franz M, Rodriguez H, Lopes C, et al. GeneMANIA update 2018. Nucleic Acids Res. 2018;46:W60–w4.
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–287.
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504.
- Ito K, Murphy D. Application of ggplot2 to pharmacometric graphics. CPT. 2013;2:e79.
- Liberzon A, Birger C, Thorvaldsdóttir H, et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425.
- Miao YR, Zhang Q, Lei Q, et al. ImmuCellAI: a unique method for comprehensive T-cell subsets abundance prediction and its application in cancer immunotherapy. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2020;7:1902880.
- Coulomb-L’Herminé A, Larousserie F, Pflanz S, et al. Expression of interleukin-27 by human trophoblast cells. Placenta. 2007;28:1133–1140.
- Wang WJ, Liu FJ, Qu HM, et al. Regulation of the expression of Th17 cells and regulatory T cells by IL-27 in patients with unexplained early recurrent miscarriage. J Reprod Immunol. 2013;99:39–45.
- Hu X, Zhu Q, Wang Y, et al. Newly characterized decidual Tim-3+ Treg cells are abundant during early pregnancy and driven by IL-27 coordinately with Gal-9 from trophoblasts. Hum Reprod. 2020;35:2454–2466.
- Xu F, Yi J, Wang Z, et al. IL-27 regulates the adherence, proliferation, and migration of MSCs and enhances their regulatory effects on Th1 and Th2 subset generations. Immunol Res. 2017;65:903–912.
- Tagoma A, Haller-Kikkatalo K, Roos K, et al. Interleukin-7, T helper 1, and regulatory T-cell activity-related cytokines are increased during the second trimester of healthy pregnancy compared to non-pregnant women. Am J Reproduct Immunol. 2019;82:e13188.
- Yin N, Zhang H, Luo X, et al. IL-27 activates human trophoblasts to express IP-10 and IL-6: implications in the immunopathophysiology of preeclampsia. Mediators Inflamm. 2014;2014:926875.
- Jahantigh D, Mousavi M, Forghani F, et al. Association between maternal circulating IL-27 levels and preeclampsia. Cytokine. 2018;102:163–167.
- Verbruggen SW, Oyen ML, Phillips AT, et al. Function and failure of the fetal membrane: modelling the mechanics of the chorion and amnion. PloS One. 2017;12:e0171588.
- Menon R. Human fetal membranes at term: dead tissue or signalers of parturition? Placenta. 2016;44:1–5.
- Meng J, Wekesa JS, Shi GL, et al. Protein function prediction based on data fusion and functional interrelationship. Math Biosci. 2016;274:25–32.
- Mishra S, Shah MI, Udhaya Kumar S, et al. Network analysis of transcriptomics data for the prediction and prioritization of membrane-associated biomarkers for idiopathic pulmonary fibrosis (IPF) by bioinformatics approach. Adv Protein Chem Struct Biol. Amsterdam, Netherlands: Elsevier; 2021; 123:241-73.
- Udhaya Kumar S, Saleem A, Thirumal Kumar D, et al. A systemic approach to explore the mechanisms of drug resistance and altered signaling cascades in extensively drug-resistant tuberculosis. Advances in protein chemistry and structural biology. Academic Press; 2021.
- Orozco S, Oberst A. RIPK3 in cell death and inflammation: the good, the bad, and the ugly. Immunol Rev. 2017;277:102–112.
- Moriwaki K, Chan FK. The Inflammatory Signal Adaptor RIPK3: functions Beyond Necroptosis. Int Rev Cell Mol Biol. 2017;328:253–275.
- Shlomovitz I, Zargrian S, Gerlic M. Mechanisms of RIPK3-induced inflammation. Immunol Cell Biol. 2017;95:166–172.
- Najjar M, Saleh D, Zelic M, et al. RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by toll-like receptor 4. Immunity. 2016;45:46–59.
- Shen W, Chang A, Wang J, et al. TIFA, an inflammatory signaling adaptor, is tumor suppressive for liver cancer. Oncogenesis. 2015;4:e173.
- Ding N, Zhang Y, Loughran PA, et al. TIFA upregulation after hypoxia-reoxygenation is TLR4- and MyD88-dependent and associated with HMGB1 upregulation and release. Free Radic Biol Med. 2013;63:361–367.
- Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reproduct Immunol. 2016;75:3–7.
- Karahoda R, Ceckova M, Staud F. The inhibitory effect of antiretroviral drugs on the L-carnitine uptake in human placenta. Toxicol Appl Pharmacol. 2019;368:18–25.
- Jinno N, Furugen A, Kurosawa Y, et al. Effects of single and repetitive valproic acid administration on the gene expression of placental transporters in pregnant rats: an analysis by gestational period. Reprod Toxicol. 2020;96:47–56.
- Lee YH, Bae SC, Kim JH, et al. Meta-analysis of SLC22A4 and RUNX1 polymorphisms: associations with rheumatoid arthritis susceptibility. Z Rheumatol. 2015;74:351–358.
- McCann MJ, Johnston S, Reilly K, et al. The effect of turmeric (Curcuma longa) extract on the functionality of the solute carrier protein 22 A4 (SLC22A4) and interleukin-10 (IL-10) variants associated with inflammatory bowel disease. Nutrients. 2014;6(10):4178–4190.
- Mukhopadhyay S, Heinz E, Porreca I, et al. Loss of IL-10 signaling in macrophages limits bacterial killing driven by prostaglandin E2. J Exp Med. 2020; 217(2):e20180649.
- Mobini M, Mortazavi M, Nadi S, et al. Significant roles played by interleukin-10 in outcome of pregnancy. Iran J Basic Med Sci. 2016;19:119–124.
- Cheng SB, Sharma S. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reproduct Immunol. 2015;73:487–500.
- Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106:1567s–74s.
- Van Boeckel SR, Davidson DJ, Norman JE, et al. Cell-free fetal DNA and spontaneous preterm birth. Reproduction. 2018;155:R137–r45.
- Chuffa LGA, Lupi LA, Cucielo MS, et al. Melatonin promotes uterine and placental health: potential molecular mechanisms. Int J Mol Sci. 2019;21. DOI:10.3390/ijms21010300
- Vassiliadis S, Ranella A, Papadimitriou L, et al. Serum levels of pro- and anti-inflammatory cytokines in non-pregnant women, during pregnancy, labour and abortion. Mediators Inflamm. 1998;7:69–72.
- Makhseed M, Raghupathy R, El-Shazly S, et al. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reproduct Immunol. 2003;49:308–318.
- Curry AE, Vogel I, Drews C, et al. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2007;86:1103–1110.
- Frascoli M, Coniglio L, Witt R, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-γ and TNF-α. Sci Transl Med. 2018;10. DOI:10.1126/scitranslmed.aan2263
- PrabhuDas M, Bonney E, Caron K, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334.
- Yang F, Zheng Q, Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front Immunol. 2019;10:2317.
- Zhao S, Liang T, Zhang C, et al. IL-27 Rα(+) cells promoted allorejection via enhancing STAT1/3/5 phosphorylation. J Cell Mol Med. 2020;24:10756–10767.
- Amadi-Obi A, Yu CR, Dambuza I, et al. Interleukin 27 induces the expression of complement factor H (CFH) in the retina. PloS One. 2012;7:e45801.
- Hu X, Goswami S, Qiu J, et al. Profiles of long non-coding RNAs and mRNA expression in human macrophages regulated by interleukin-27. Int J Mol Sci. 2019;20. DOI:10.3390/ijms20246207
- Galindo-Sevilla N, Reyes-Arroyo F, Mancilla-Ramírez J. The role of complement in preterm birth and prematurity. J Perinat Med. 2019;47:793–803.
- Girardi G. Complement activation, a threat to pregnancy. Semin Immunopathol. 2018;40:103–111.
- Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411.
- Peshkova IO, Aghayev T, Fatkhullina AR, et al. IL-27 receptor-regulated stress myelopoiesis drives abdominal aortic aneurysm development. Nat Commun. 2019;10:5046.
- Tang MX, Hu XH, Liu ZZ, et al. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol. 2015;112:73–80.
- Guzzo C, Ayer A, Basta S, et al. IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J Immunol. 2012;188:864–873.
- Gregersen I, Sandanger Ø, Askevold ET, et al. Interleukin 27 is increased in carotid atherosclerosis and promotes NLRP3 inflammasome activation. PloS One. 2017;12:e0188387.
- Petes C, Wynick C, Guzzo C, et al. IL-27 enhances LPS-induced IL-1β in human monocytes and murine macrophages. J Leukoc Biol. 2017;102:83–94.
- Tong M, Abrahams VM. Neutrophils in preterm birth: friend or foe? Placenta. 2020;102:17–20.
- Povroznik JM, Robinson CM. IL-27 regulation of innate immunity and control of microbial growth. Future Sci OA. 2020;6:Fso588.
- Gwyer Findlay E, Villegas-Mendez A, de Souza JB, et al. IL-27 receptor signaling regulates CD4+ T cell chemotactic responses during infection. J Immunol. 2013;190:4553–4561.
- Wang S, Sun F, Li M, et al. The appropriate frequency and function of decidual Tim-3(+)CTLA-4(+)CD8(+) T cells are important in maintaining normal pregnancy. Cell Death Dis. 2019;10:407.
- Wang SC, Li YH, Piao HL, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6:e1738.
- Papúchová H, Meer TB, Li Q, et al. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front Immunol. 2019;10:2730.
- Blois SM, Joachim R, Kandil J, et al. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol. 2004;172:5893–5899.
- Liu S, Diao L, Huang C, et al. The role of decidual immune cells on human pregnancy. J Reprod Immunol. 2017;124:44–53.