ABSTRACT
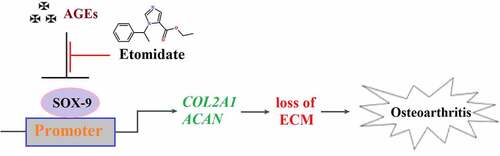
Osteoarthritis (OA) is a rheumatic disease common in the elderly. AGEs are the end products of glycation reactions and play an important role in the development of OA. Etomidate is a general anesthesia-inducing agent recently reported to exert significant anti-inflammatory effects. The present study aims to explore the protective effect of Etomidate against advanced glycation end-products (AGEs)-induced reduction of extracellular matrix gene expression in chondrocytes. In the present study, we found that AGEs significantly reduced the expression of Collagen II (COL2A1) and Aggrecan (ACAN) at the gene level. Furthermore, AGEs inhibited the expression of SRY-related high mobility group-box gene 9 (SOX-9), promoting the expression of COL2A1 and ACAN. COL2A1, ACAN, and SOX-9 in chondrocytes were significantly elevated by treatment with Etomidate alone. Consistently, Etomidate ameliorated AGEs-induced downregulation of COL2A1, ACAN, and SOX-9 in a dose-dependent manner. Importantly, we found that knockdown of SOX-9 eliminated the beneficial effects of Etomidate against AGEs-induced decrease in COL2A1 and ACAN genes. Based on these findings, we demonstrated that Etomidate could ameliorate AGEs-induced reduction of extracellular matrix gene expression in chondrocytes by upregulating SOX-9.
KEYWORDS:
Introduction
Osteoarthritis (OA) is a rheumatic disease with high morbidity in people over 60 years of age [Citation1]. It is accompanied by knee or hip joint pain and dysfunction and is currently lacking effective clinical therapies [Citation2,Citation3]. OA is considered as a disease of the entire joint, affecting all joint tissue. Thus, it is characterized not only by the degeneration of the cartilage and inflammation of the synovial membrane but also by meniscal degeneration, subchondral sclerosis, inflammation, and fibrosis of the infrapatellar fat pad [Citation4]. However, the most significant pathological characteristic of OA is the degenerative disruption of cartilage tissues. It is widely accepted that maintaining normal metabolism and preventing the destruction of cartilage homeostasis are key steps in the treatment of OA [Citation5,Citation6].
As the main cell type of cartilage tissues, chondrocytes are responsible for the synthesis of extracellular matrix (ECM) and provide sufficient mechanical support for the shear force and pressure produced from articular movements [Citation7,Citation8]. ECM is mainly composed of collagens, proteoglycan, structural aggrecans, lipids, and water. A grid structure is formed by the arranged collagens to maintain the morphology and rigidity of cartilage tissues. Collagen II is the main subtype of collagens and is encoded by collagen type II alpha-1 gene (COL2A1) [Citation9]. It is reported that the degradation of collagen II is observed in OA cartilage, and the fragments of collagen fibronectin produced from the degraded collagen II aggravate the further degradation of cartilage tissues.
Advanced glycation end-products (AGEs) are the end products of glycation reactions. It has been reported that increased accumulation of AGEs in cartilage plays an important role in the pathogenesis of OA [Citation10]. The interaction of AGEs with receptor for AGEs (RAGE) is responsible for the inflammatory response in the development of OA through the upregulation of pro-inflammatory cytokines, which further stimulates the expression of matrix metalloproteinases (MMPs) and disintegration and metalloproteinase with thrombospondin motifs (ADAMTS) [Citation11].
Aggrecan is another main component of ECM and the decomposition of aggrecan induced by elevated expressions of MMPs and ADAMTS, is involved in the pathogenesis of OA. SOX-9 is an important transcriptional factor involved in the regulation of developing cartilage [Citation12], reported to be significantly downregulated in the OA cartilage tissues [Citation13]. In the development and processing of cartilage, a significant parallel correlation is observed on SOX-9 and COL2A1 [Citation14]. Bel claimed that the expression of COL2A1 in cartilage tissues was directly regulated by SOX-9 through binding with the promoter of COL2A1 [Citation15], which is further confirmed by the investigation in TNF-α treated chondrocytes reported by Wang [Citation16]. Hence, activating the function of SOX-9 may be a useful way for treating OA. Etomidate () is a general anesthesia inducer developed by Janssen Pharmaceuticals [Citation17] and approved for clinical trials by the U.S Food and Drug Administration in 1972 [Citation18]. Etomidate is a highly selective agonist of γ-aminobutyric acid type A receptors (GABBAARs) and exerts pharmacological effects by enhancing the affinity between the inhibitory neurotransmitter GABA and the GABBAARs. Recently, promising anti-inhibitory effects of Etomidate have been widely reported. Liu reported that the production of pro-inflammatory cytokines in LPS-treated rat macrophages was significantly mitigated by Etomidate. Etomidate, according to Li, protects against ischemia-reperfusion injury by lowering inflammatory markers. However, whether Etomidate has any beneficial capacity in chondrocytes and the pathogenesis of OA still needs to be elucidated. In this study, we aim to investigate the protective effect of Etomidate against AGEs-induced insults in chondrocytes and clarify the underlying mechanism.
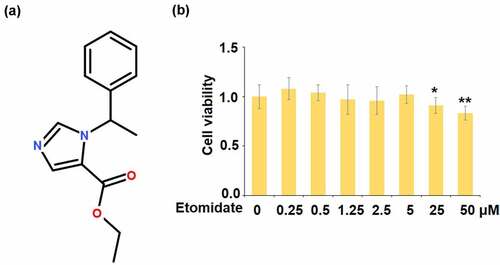
Figure 1. Effects of Etomidate in cell viability. (a). Molecular structure of Etomidate; (b). Human SW1353 chondrocytes were stimulated with Etomidate alone at 0.25, 0.5, 1.25, 2.5, 5, 25, and 50 μM for 24 hours, cell viability was measured using the MTT assay (*. **, P < 0.05, 0.01 vs. control, N = 5–6)
Materials and methods
Cell culture and transfection
SW1353 chondrocytes were obtained from ATCC (ATCC, Manassas, USA) and cultured in DMEM supplemented with 5% FBS at 37°C and 5% CO2 [Citation19]. A specific siRNA was designed and transfected into human SW1353 chondrocytes together with the transfection agent lipofectamine 3000 (Thermo, MA, USA) according to the instructions of the manufacturer, followed by verifying the efficacy of silencing SOX-9 using Western blotting assay. Cells were stimulated with 1.25, 2.5, and 5 μM Etomidate (#E6530, Sigma-Aldrich, USA) for 24 hours [Citation20]. Etomidate was dissolved in DMSO. Cells were then stimulated with 100 μg/mL AGEs (Glycosylated BSA, # bs-1158P, Beijing Biosynthesis Biotechnology, China).
Cell viability determination
Human SW1353 chondrocytes were stimulated with Etomidate alone at 0.25, 0.5, 1.25, 2.5, 5, 25, and 50 μM for 24 hours. After treatment, media was discarded from cell cultures. 50 µL of serum-free media and 50 µL of 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) solution were added into each well. After incubation for 37°C for 3 hours, 150 µL DMSO was added to dissolve the MTT formazan by wrapping the plate in foil and shaking it on an orbital shaker for 15 minutes. Absorbance was read at 590 nM to calculate cell viability.
Real-time PCR analysis
After isolating the total RNA from the treated human SW1353 chondrocytes using the TRIzol reagent (Invitrogen, California, USA), the RNAs were quantified and transcribed into cDNA utilizing the Revert Aid First-strand cDNA Synthesis Kit (Thermo, Massachusetts, USA) based on the manufacturers’ instruction. Then, the SYBR system (Invitrogen, California, USA) was used to perform the real-time PCR in a 20 μL PCR reaction system. Lastly, the 2−ΔΔCt method was applied to calculate the expression of target genes, with GAPDH as a normalization gene [Citation21]. The primer sequences are listed in .
Table 1. Primer sequences
Western blot assay
After lysing the treated human SW1353 chondrocytes with the cell lysis buffer (Invitrogen, California, USA), the total proteins were extracted for quantification using a BCA kit (Beyotime, Shanghai, China) and were further loaded and run on the SDS-PAGE. Subsequently, the proteins were transferred onto the PVDF membrane, followed by being blocked with 5% nonfat milk. After washing 3 times, the membrane was incubated with primary antibodies against SOX-9 (1:1000, CST, Boston, USA) and GAPDH (1:1000, CST, Boston, USA) at 4°C overnight, followed by being incubation with secondary antibody. Lastly, the blots were incubated with ECL solution and exposed to the Tonan exposure (Tonan, Shanghai, China). The blots were further quantified using the Image J software [Citation22].
Immunofluorescence staining
After washing the human SW1353 chondrocytes with PBS buffer, cells were fixed using 4% paraformaldehyde (Sigma-Aldrich, USA) and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, USA), followed by being incubation with the primary antibody against SOX-9 (ab185966 Abcam, Cambridge, UK) at room temperature for 2 hours. Subsequently, the appropriate secondary antibodies conjugated with TRITC and DAPI (a nuclear marker and the color is blue) were used to incubate with the collected cells. Lastly, the fluorescence density of each group was observed under a fluorescence microscope (Olympus, Toyko, Japan) [Citation23].
Statistical analysis
All experiments were repeated at least three times. All data were expressed as the mean ± SD and analyzed by ANOVA, followed by Turkey’s post-hoc test, using Graph Pad Prism 6.0. P less than 0.05 was considered significant [Citation24].
Results
Using an in vitro SW1353 chondrocytes model stimulated with AGEs, we examined the function of Etomidate in the expression of matrix biomarkers. We found that Etomidate mitigated AGEs-induced reduction of COL2A1 and ACAN by increasing the expression of the transcriptional factor SOX-9.
Etomidate promoted COL2A1, ACAN in human SW1353 chondrocytes
To screen the optimized concentrations of Etomidate for cell culture, human SW1353 chondrocytes were stimulated with Etomidate alone at 0.25, 0.5, 1.25, 2.5, 5, 25, and 50 μM, cell viability was measured using the MTT assay. Interestingly, we found that when the concentration of Etomidate was lower than 5 μM, it did not affect cell viability. However, exposure to 25, and 50 μM Etomidate resulted in lower cell viability of human SW1353 chondrocytes (). Therefore, 1.25, 2.5, and 5 μM Etomidate were used in the subsequent experiments.
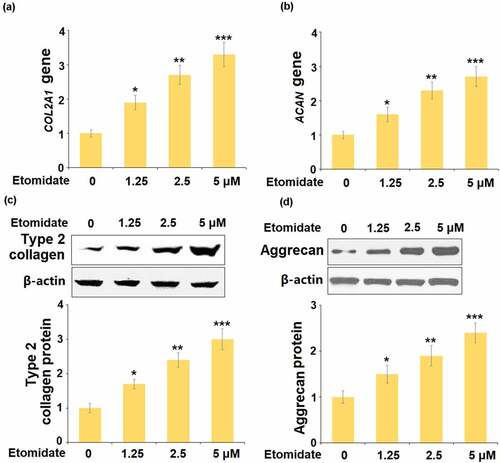
To assess the roles of Etomidate on the components of the ECM, cells were stimulated with 1.25, 2.5, and 5 μM Etomidate for 24 hours. The levels of COL2A1 and ACAN were significantly elevated by the presence of Etomidate ( and ). Consistently, we found that treatment with Etomidate increased the protein expressions of type 2 collagen () and aggrecan () in a dose-dependent manner.
Figure 2. Etomidate promoted the expression of COL2A1 and ACAN. Cells were stimulated with 1.25, 2.5, and 5 μM Etomidate for 24 hours. (a). mRNA of COL2A1; (b). mRNA of ACAN; (c). Protein expression of type 2 collagen; (d). Protein expression of Aggrecan (*. **, ***, P < 0.05, 0.01, 0.005 vs. control, N = 5–6)
Etomidate upregulated SOX-9
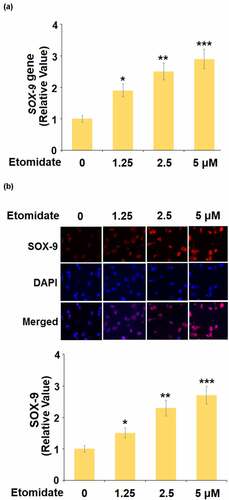
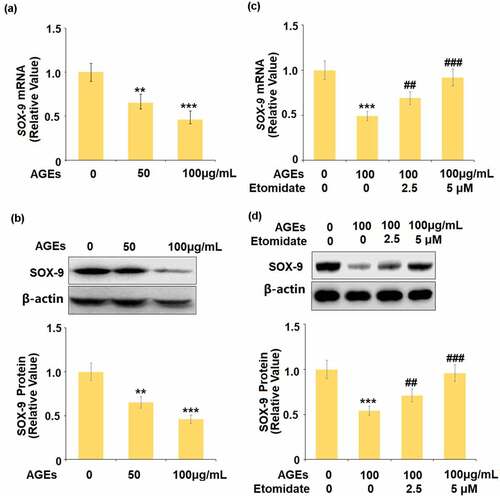
To evaluate the potential mechanism underlying the effect of Etomidate on COL2A1 and ACAN, the expression of SOX-9 was detected following stimulation with 1.25, 2.5, 5 μM Etomidate for 24 hours. As shown in , as the concentration of Etomidate increased from 1.25 to 5 μM, the expression of SOX-9 was dramatically promoted by treatment with Etomidate.
Etomidate ameliorated AGEs-induced decrease in the expression of COL2A1 and ACAN
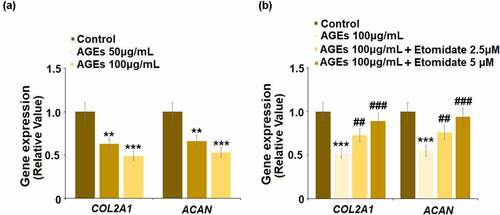
To evaluate the protective effect of Etomidate on ECM degradation induced by AGEs, cells were stimulated with AGEs (50, and 100 μg/mL) for 24 hours. As shown in , COL2A1 and ACAN were significantly inhibited by stimulation with AGEs, indicating that the in-vitro model was successfully established. Subsequently, cells were stimulated with AGEs (100 μg/mL) with Etomidate. Compared to the control group, COL2A1 and ACAN were reduced by AGEs, which were upregulated by treatment with Etomidate, suggesting a beneficial effect of Etomidate on ECM degradation induced by AGEs.
Figure 4. Etomidate ameliorated AGEs-induced decrease in COL2A1 and ACAN. (a). Cells were stimulated with AGEs (50, and 100 μg/mL) for 24 hours. Gene levels of COL2A1 and ACAN were measured; (b). Cells were stimulated with AGEs (100 μg/mL) with 2.5, and 5 μM Etomidate for 24 hours. Gene levels of COL2A1 and ACAN were measured (**, ***, P < 0.01, 0.005 vs. control group; ##, ###, P < 0.01, 0.005 vs. AGEs treatment group, N = 6)
To further confirm the beneficial effects of Etomidate on the expression of COL2A1 and ACAN, human C28/I2 chondrocytes cell lines were used. We found that exposure to AGEs reduced the expression of both COL2A1 and ACAN in human C28/I2 chondrocytes, which were rescued by Etomidate in a dose-dependent manner (Supplementary Figure S1).
Etomidate ameliorated AGEs-induced reduction of SOX-9
We firstly evaluated the effects of Etomidate on SOX-9 in chondrocytes. The gene and protein expression levels of SOX-9 were significantly suppressed by the stimulation with AGEs ( and ). In the presence of Etomidate, the downregulated gene and protein expression levels of SOX-9 caused by AGEs were remarkably alleviated by Etomidate.
Figure 5. Etomidate ameliorated AGEs-induced reduction of SOX-9. (a-b). Cells were stimulated with AGEs (50, and 100 μg/mL) for 24 hours. mRNA and protein levels of SOX-9; (c-d). Cells were stimulated with AGEs (100 μg/mL) with 2.5, and 5 μM Etomidate for 24 hours. Expression of SOX-9 were measured (**, ***, P < 0.01, 0.005 vs. vehicle group; ##, ###, P < 0.01, 0.005 vs. AGEs treatment group, N = 5)
Knockdown of SOX-9 abolished the effects of Etomidate against AGEs-induced reduction of COL2A1 and ACAN genes
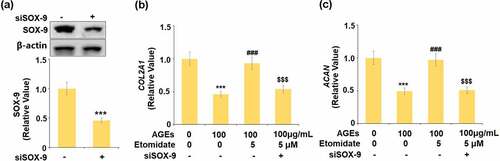
To further confirm that Etomidate protected the degradation of ECM through upregulating SOX-9, cells were transfected with SOX-9 siRNA. The expression of SOX-9 was significantly downregulated in the siRNA transfected cells, indicating that the SOX-9 silenced chondrocytes were successfully established (). COL2A1 and ACAN were remarkably reduced in AGEs-challenged cells and pronouncedly elevated by treatment with Etomidate. However, the knockdown of SOX-9 abolished the effects of Etomidate against AGEs-induced reduction of COL2A1 and ACAN. These data indicate that Etomidate might protect the reduction of ECM genes through upregulating SOX-9.
Figure 6. Knockdown of SOX-9 abolished the beneficial effects of Etomidate against AGEs-induced reduction of COL2A1 and ACAN genes. Cells were transfected with siRNA SOX-9, followed by stimulation with AGEs (100 μg/mL) with 5 μM Etomidate for 24 hours. (a). Successful knockdown of SOX-9; (b). Gene level of COL2A1; (c). Gene level of ACAN (***, P < 0.005 vs. vehicle group; ###, P < 0.005 vs. AGEs treatment group; $$$, P < 0.005 vs. AGEs+ Etomidate group, N = 5–6)
Discussion
The development of OA is closely related to factors such as aging, heredity, development, metabolism, and trauma, with aging being one of the most important variables. Excessive development and buildup of advanced glycation end products (AGEs) are commonly generated as people get older [Citation25,Citation26].
AGEs are the stable covalent compounds derived from the non-enzymatic glycation between proteins or lipids and glucose or other reductive monosaccharides, which is an inescapable and irreversible process in our body. It is reported that compared to other tissues, AGEs are highly expressed on cartilage tissues and are closely associated with the pathogenesis of OA [Citation27]. The collagen injury and development of OA symptoms can be directly induced by upregulating AGEs, which are reported to be alleviated by the inhibitor of AGEs formation or crosslinking [Citation28,Citation29]. Although the pathological mechanism underlying the inducement of AGEs for OA, is unknown, it has been proven that stimulation of AGEs causes glycosylation of articular cartilage collagen and a decrease in aggrecan synthesis which further contributes to the increase of matrix brittleness, and a decrease in resistance to pressure or shear force [Citation30,Citation31]. In the present study, AGEs were used to simulate the in vitro injury model on chondrocytes, which was verified by the downregulation of COL2A1 and ACAN, indicating that the ECM degradation might be induced by the stimulation with AGEs.
After Etomidate treatment, COL2A1 and ACAN were dramatically elevated, showing that Etomidate could help to restore the degraded ECM caused by AGEs. In our future work, more elements will be taken into consideration to better understand the protective effect of Etomidate against ECM degradation, including measuring the expression of MMPs and detecting the production of inflammatory factors, which are reported to be involved in the pathological changes in chondrocytes induced by AGEs [Citation31]. Furthermore, the therapeutic effect of Etomidate against OA will be further confirmed in animal models.
SOX-9 is widely expressed in precursor cells of cartilage and mature chondrocytes. It is reported that it directly regulates the transcription process of COL2Α1 by targeting specific binding sites located in the intron of COL2Α1 [Citation32]. SOX-9 not only impacts the formation and differentiation of cartilage but also promotes the differentiation of medullary mesenchymal stem cells into chondrocytes. Lee [Citation33] claimed that SOX-9, along with SOX-5 and SOX-6, participate in the repairment of chondrocytes by promoting the differentiation from medullary mesenchymal stem cells into chondrocytes. It is reported that the expression of ECM components was significantly elevated by transfecting the SOX-9 gene into the chondrocytes [Citation34]. In addition, SOX-9 is the upstream gene of Col18α1 and Col12α1 and regulates the expression of these proteins [Citation35,Citation36]. In the present study, we reported that SOX-9 was significantly suppressed by the stimulation with AGEs, revealing a potential pathological mechanism underlying the effects of AGEs on chondrocytes, which will be further verified in the animal model in our future work. Through the treatment with Etomidate, the downregulated SOX-9 was significantly reversed, indicating that the protective effects of Etomidate on degraded ECM components may be related to the upregulation of SOX-9. We further established the SOX-9 knockdown chondrocytes to verify the hypothesis. In our future work, the regulatory effect of Etomidate on SOX-9 will be further proved in the animal model to provide sufficient evidence for the application of Etomidate in the clinical treatment of OA.
Although the pathological mechanism of OA is still complex, it is well known that the inflammatory response plays a critical role in the progression of OA [Citation37]. Pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), could activate many inflammatory signaling pathways including nuclear factor – κB (NF-κB) to promote an inflammatory cascade. It is well known that RAGE, a member of the immunoglobulin superfamily, is expressed in human chondrocytes [Citation38]. When AGEs bind to RAGE, they activate MAP kinase signaling, resulting in upregulation of NF-κB activity, which promotes the inflammatory cascades [Citation39]. Furthermore, activated NF-κB can enhance the expressions of MMPs and ADAMTS, leading to cartilage degradation [Citation40,Citation41]. Previous studies have demonstrated the anti-inflammatory properties of Etomidate by inhibiting the NF-κB activity and suppressing the expression of pro-inflammatory cytokines [Citation10]. Consistently, our results indicate that the presence of Etomidate not only inhibits AGEs-induced expressions of IL-1β and TNF-α, but also the expressions of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 (Data not shown). Our future investigations will provide a complete picture of the underlying mechanism through which Etomidate exerts its protective attributes in OA.
There are several limitations of the current study. Firstly, the major findings here are based on in vitro cell culture studies. It should be noted that the pathological mechanism of OA is complicated and various risk factors have been involved [Citation42]. In vivo studies with appropriate animal models are necessary to further confirm the protective effect of etomidate in future. Secondly, it has been recently reported that administration of etomidate at a high dose will have an increased risk for adverse effects in the cardiovascular, respiratory, and central nervous systems [Citation43].
Conclusion
In conclusion, our data revealed that Etomidate might rescue the AGEs-induced downregulation of COL2A1 and ACAN to prevent reduction of extracellular matrix genes in chondrocytes by upregulating SOX-9.
Supplemental Material
Download ()Acknowledgements
This study was funded by “Tianjin Hospital”.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data available statement
Data are available on the reasonable request to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet. 2012;379:1331–1340.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheumatism. 2008;58:26–35.
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126.
- Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979.
- Wang M, Shen J, Jin H, et al. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann NY Acad Sci. 2011;1240:61–69.
- Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15:375.
- Pacifici M, Koyama E, Iwamoto M, et al. Development of articular cartilage: what do we know about it and how may it occur? Connect Tissue Res. 2000;41:175–184.
- Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78.
- Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35.
- Saudek DM, Kay J. Advanced glycation endproducts and osteoarthritis. Curr Rheumatol Rep. 2003;5:33–40.
- Li NG, Shi ZH, Tang YP, et al. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem. 2011;18:977–1001.
- Bi W, Deng JM, Zhang Z, et al. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89.
- Lee JS, Im GI. SOX trio decrease in the articular cartilage with the advancement of osteoarthritis. Connect Tissue Res. 2011;52:496–502.
- Lefebvre V, Huang W, Harley VR, et al. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346.
- Bell DM, Leung KK, Wheatley SC, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178.
- Wang L, Mi B, Zhang Y, et al. Alendronate promotes the gene expression of extracellular matrix mediated by SP-1/SOX-9. Hum Exp Toxicol. 2021;;40(7):1173–1182.
- Hulsman N, Hollmann MW, Preckel B. Newer propofol, ketamine, and etomidate derivatives and delivery systems relevant to anesthesia practice. Best Pract Res Clin Anaesth. 2018;32:213–221.
- Morgan M, Lumley J, Whitwam JG. Etomidate, a new water-soluble non-barbiturate intravenous induction agent. Lancet. 1975;1:955–956.
- Schmucker AC, Wright JB, Cole MD, et al. Distal interleukin-1β (IL-1β) response element of human matrix metalloproteinase-13 (MMP-13) binds activator protein 1 (AP-1) transcription factors and regulates gene expression. J Biol Chem. 2011;287:1189–1197.
- Liu M, Zhang Y, Xiong JY, et al. Etomidate mitigates lipopolysaccharide-induced CD14 and TREM-1 expression, NF-κB activation, and pro-inflammatory cytokine production in rat macrophages. Inflammation. 2016;39:327–335.
- Brohan J, Goudra BG. The role of GABA receptor agonists in anesthesia and sedation. CNS Drugs. 2017;31:845–856.
- Nong AN, Li QF, Huang ZJ, et al. MicroRNA miR-126 attenuates brain injury in septic rats via NF-κB signaling pathway. Bioengineered. 2021;12:2639–2648.
- Wang SC, Li P, Jiang G, et al. Long non-coding RNA LOC285194 inhibits proliferation and migration but promoted apoptosis in vascular smooth muscle cells via targeting miR-211/PUMA and TGF-β1/S100A4 signal. Bioengineered. 2020;11:718–728.
- Tong L, Tang CX, Cai CJ, et al. Upregulation of the microRNA rno-miR-146b-5p may be involved in the development of intestinal injury through inhibition of Kruppel-like factor 4 in intestinal sepsis. Bioengineered. 2020;11:1334–1349.
- Schmidt AM, Yan SD, Stern DM. The dark side of glucose. Nat Med. 1995;1:1002–1004.
- Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1991;196:17–29.
- Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–21602.
- DeGroot J, Verzijl N, Wenting-van Wijk MJ, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–1215.
- Steenvoorden MM, Huizinga TW, Verzijl N, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263.
- Hipkiss AR. Energy metabolism, altered proteins, sirtuins and ageing: converging mechanisms? Biogerontology. 2008;9:49–55.
- Nah SS, Choi IY, Yoo B, et al. Advanced glycation end products increases matrix metalloproteinase-1, −3, and −13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581:1928–1932.
- Im GI, Kim HJ, Lee JH. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and −9) genes. Biomaterials. 2011;32:4385–4392.
- Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–2024.
- Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med (Berl). 2013;91:625–636.
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–212.
- De Crombrugghe B, Lefebvre V, Behringer RR, et al. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394.
- Shi JM, Cao FY, Chang YJ, et al. Long non-coding RNA MCM3AP-AS1 protects chondrocytes ATDC5 and CHON-001 from IL-1β-induced inflammation via regulating miR-138-5p/SIRT1. Bioengineered. 2021;12:1445–1456.
- Wang H, Jiang Z, Pang Z, et al. Engeletin protects against TNF-α-induced apoptosis and reactive oxygen species generation in chondrocytes and alleviates osteoarthritis in vivo. J Inflamm Res. 2021;14:745–760.
- Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712.
- Clancy R, Rediske J, Koehne C, et al. Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9:294–299.
- Mengshol JA, Vincenti MP, Coon CI, et al. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811.
- Sacitharan PK. Ageing and osteoarthritis. Subcell Biochem. 2019;91:123–159.
- Cherfan AJ, Arabi YM, Al-Dorzi HM, et al. Advantages and disadvantages of etomidate use for intubation of patients with sepsis. Pharmacotherapy. 2012;32(5):475–482.