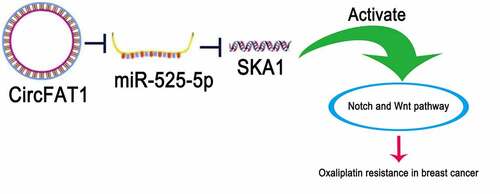
ABSTRACT
Increasing evidence has confirmed the vital roles of circular RNAs (CircRNAs) in the drug resistance of breast cancer (BC). Herein, we intended to study the effect of circular RNA FAT atypical cadherin 1 (circFAT1) on BC oxaliplatin (OX) resistance and find out the potential molecular mechanism in it. In this study, mRNA and protein levels of genes were measured by RT-qPCR and western blotting, respectively. Luciferase reporter assay confirmed the relationship between microRNA-525-5p (miR-525-5p) and circFAT1 or spindle and kinetochore-associated complex subunit 1 (SKA1). CCK-8, transwell, and flow cytometry experiments were utilized to investigate the chemosensitivity, migration, invasion, and apoptosis of BC cells. Gene Set Enrichment Analysis (GSEA) was applied to discover possible pathways related to SKA1. It was uncovered that circFAT1 was overexpressed in OX-resistant BC tissues and cells. Functional experiments showed that circFAT1 depletion reduced the level of chemoresistance-related genes. Moreover, circFAT1 knockdown remarkably facilitated apoptosis and decreased OX (half-maximal inhibitory concentration) IC50 value, migration, and invasion in OX-resistant BC cells. It was identified that miR-525-5p directly targeted circFAT1 and SKA1. Besides, rescue assays exhibited that circFAT1 promoted OX resistance in BC cells via the miR-525-5p/SKA1 regulatory network. Furthermore, GSEA and western blotting identified that SKA1 activated the Notch and Wnt pathway in OX-resistant BC cells. In conclusion, our results demonstrated that circFAT1 conferred OX resistance in BC by regulating the miR-525-5p/SKA1 via the Notch and Wnt pathway, providing a potential therapeutic target for patients with OX-resistant BC.
Background
As a major malignant neoplasm in females, breast cancer (BC) is a leading cause of mortality related to cancers for women worldwide [Citation1,Citation2]. In spite of great advances achieved in and extensive use of adjuvant chemotherapy for BC, almost 60% of the BC patients suffered from distant metastases, and the median survival time after definite diagnosis of metastases is approximately 2 years [Citation3]. Platinum complexes, including cisplatin, carboplatin, and oxaliplatin, are mainstream drugs for BC treatment [Citation4]. As a third-generation platinum compound, oxaliplatin (OX) exerts its anti-tumor effect by disrupting DNA synthesis and is even effective in cells resistant to cisplatin and carboplatin [Citation5]. Moreover, OX also showed its anticancer function in BC chemotherapy [Citation6]. Hence, to discover novel targets for treatment and improve the prognosis of BC patients, it is reasonable to explore the underlying mechanism of OX resistance in BC.
Circular RNAs (circRNAs), a kind of closed-loop RNAs, are deeply involved in the regulation of gene expression [Citation7]. Accumulated studies have identified the critical roles of circRNAs in a variety of human cancers, including BC. For instance, circACAP2 facilitates BC proliferation and metastasis by regulating COL5A1 via miR-29a/b-3p [Citation8]. Circ-HIPK3 accelerates colorectal cancer development through interacting with miR-7 [Citation9]. CircCDR1as promotes hepatocellular carcinoma tumorigenesis as a sponge for miR-1270 [Citation10]. Chemoresistance is a critical barrier for chemotherapeutics in BC. Multiple circRNAs have been evidenced as regulators in BC chemoresistance at the molecular level. To cite an instance, hsa_circ_0006528 confers resistance to paclitaxel in BC Cells through upregulating CDK8 via miR-1299 [Citation11]. Circ-UBE2D2 aggravates tamoxifen resistance in BC through interacting with miR-200a-3p [Citation12]. Besides, circRNA RNF111 induces BC resistance to paclitaxel via the miR-140-5p/E2F3 axis [Citation13]. As disclosed by previous studies, circular RNA FAT atypical cadherin 1 (circFAT1) plays an oncogenic role in human tumors, such as osteosarcoma [Citation14], colorectal cancer [Citation15], and hepatocellular carcinoma [Citation16]. Moreover, circFAT1 has been reported to increase the resistance to cisplatin, a platinum drug, in colorectal cancer [Citation17]. Therefore, it could be concluded from the above findings that circFAT1 might induce chemoresistance to platinum-based drugs during chemotherapy. However, the specific molecular function of circFAT1 in BC resistance to OX remains to be sequentially investigated.
In this study, we intended to investigate the role of circFAT1 in BC resistance to OX and its underlying mechanism. Our data demonstrated that circFAT1 enhanced BC resistance to OX via the miR-525-5p/SKA1 pathway, indicating an important therapeutical target for BC.
Materials and methods
Clinical specimens
A total of 66 BC tissues were collected from patients who underwent surgical resection surgery at Changzhou No.2 People’s Hospital Affiliated to Nanjing Medical University. The clinicopathologic characteristics of the 66 patients enrolled in this study are presented in . The chemotherapy plan (OX administration) for patients was 85 to 100 mg/m2 (2-hour intravenous infusion) every 2 weeks for 6 consecutive weeks. Patients who had recurrent disease within 6 months of completing primary chemotherapy were classified as OX-resistant (n = 36). Patients with recurrence beyond 6 months or without recurrence were classified as OX-sensitive (n = 30). The present study was approved by the Ethics Committee of Changzhou No.2 People’s Hospital Affiliated to Nanjing Medical University. All patients signed the informed consent forms.
Table 1. Clinicopathologic features of BC patients (n = 66)
Cell culture
Human breast epithelial cells (MCF-10A) provided by BeNa Culture Collection (Beijing, China) were cultured in DMEM/F12 supplemented by 10% FBS at 37°C with 5% CO2. Human BC cell lines (BT474, MCF-7, and T47D) (BeNa Culture Collection, Beijing, China) were cultivated in DMEM with 10% FBS under the same conditions.
To establish OX-resistant BC cells (BT474/OX, MCF-7/OX, T47D/OX), BC cells (BT474, MCF-7, T47D) were continuously exposed to progressively increasing OX concentrations for 3 months. OX-resistant BC cells were cultured in DMEM medium supplemented with 5 μmol/L OX and 10% FBS at 37°C with 5% CO2.
Cell transfection
Short hairpin RNA (shRNA) targeting circFAT1 (sh-circFAT1: 5ʹ-GAGAAAGATTCCCGACAGTTA-3ʹ), SKA1 (sh-SKA1: 5ʹ-ACGGAGATGAGATCATTGTAA-3ʹ) and negative control (sh-NC: 5ʹ-ACGGUACCGUAGGCGAGCCAC-3ʹ), miR-525-5p inhibitor (5ʹ-GUAACGCUACGGGCGCAACGC-3ʹ), miR-525-5p mimics (5ʹ-AAGUCCAGGGGUCCCCGGCAA-3ʹ), and corresponding negative controls (NC inhibitor: 5ʹ-ACCCCGGUAAAAGUCGGCCGG-3ʹ; NC mimics: 5ʹ-UAAUGCCCGACGAACGGGCCG-3ʹ) were acquired from GenePharma (Shanghai, China). The transfection was performed using LipofectamineTM 2000 (Thermo Fisher Scientific).
RT-qPCR assay
Total RNAs were extracted from tissue specimens and cells using Trizol reagent (Invitrogen, USA). Then, a Reverse Transcription kit (Takara) was used to generate cDNAs (for circFAT1 and SKA1) under the following conditions: 42°C for 15 minutes followed by 3 cycles at 85°C for 5 seconds. Regarding miR-525-5p, TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher Scientific) was utilized to generate cDNA under certain reaction conditions: 50°C for 5 minutes and 80°C for 2 minutes. Thereafter, qPCR assay was conducted with SYBR Premix ExTaq II kit (Takara, Dalian, China) and ABI 7500 PRISM 750 (Applied Biosystems) [Citation18]. All the primers used are as follows: circFAT1 forward (F): 5ʹ-AACAGAAGAGAACTGGGGCG-3ʹ and reverse (R): 5ʹ-GATCAGGGTGCCAATGGTGA-3ʹ; SKA1 F: 5ʹ-TGAGGTTGGAGTCTGTGTGTT-3ʹ and R: 5ʹ-GATCTGACGAGGCCATCCTT-3ʹ; GAPDH F: 5ʹ-TATTGTTGCCATCAATGACCC-3ʹ and R: 5ʹ-ACTCCACGACGTACTCAGC-3ʹ; miR-525-5p F: 5ʹ-GTCGTATCCAGTGCGTGTCGTG-3ʹ and R: 5ʹ-GCGAGCACAGAATTAATACGACTCAC-3ʹ; U6 F: 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and R: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ.
Drug sensitivity assay
The sensitivity of BC cells to OX was measured using a CCK-8 assay kit (Dojindo Molecular Technologies, Inc.). In short, BT474/OX or MCF-7/OX cells (5 × 104 cells/well) were seeded into 96-well plates and exposed to OX in a concentration gradient from 0 to 20 μmol/L for 48 h. Then, the cells were cultured for another 2 hours after the 10 μL CCK-8 reagent (Beyotime, China) was added to the plates. The absorbance was detected using a microplate reader (BioTek China) at 450 nm.
Transwell
For invasion assay, BT474/OX or MCF-7/OX cells were seeded in the upper chamber with Matrigel-coated membrane (8 μm pore size) (BD Bioscience, USA). Serum-free medium was added in the upper chamber; for the lower chamber, medium with 10% FBS was supplemented. Subsequently, cells underneath the membrane were fixed with 75% methanol and stained with 0.1% crystal violet 24 hours later. For migration assay, membrane without Matrigel was employed. Five random fields per chamber were selected to count the number of the migrated and invaded cells using a microscope (Olympus, Japan) [Citation19].
Flow cytometry
Annexin V-PE Apoptosis Detection Kit (BD Pharmingen, USA) was used to assess the cell apoptosis according to the manufacturer’s protocol [Citation20]. In brief, 1 × 106 BT474/OX or MCF-7/OX cells were washed with PBS three times before resuspension with binding buffer. Then, 5 μl PE Annexin V and 5 μl 7-AAD were added to stain the cells on ice for 30 minutes. Thereafter, 400 μl binding buffer was added into each tube. The cells stained were measured by flow cytometry (FACSCalibur, Germany).
Luciferase reporter assay
Briefly, to build pGL3-circFAT1 (or pGL3-SKA1) wild type (WT)/mutant type (MUT) luciferase reporter vectors, the RNA sequence of circFAT1 and SKA1 3ʹ-UTR containing potential binding sites to miR-525-5p was inserted into pGL3 (Promega, USA). Then, BT474/OX or MCF-7/OX cells (1 × 104) were put into 24-well plates, cultivated, and co-transfected with pGL3 WT/MUT vectors and miR-525-5p mimics/NC mimics, respectively. At last, Dual-Luciferase Reporter Assay System (Promega, USA) was utilized to detect the luciferase activity 48 hours after transfection [Citation21].
Western blotting
Extraction of total protein from BT474/OX or MCF-7/OX cells was conducted with RIPA buffer (Sigma-Aldrich). Then, total protein quantitation was performed by Bradford method. Then, protein samples were subjected to SDS-PAGE and transferred onto PVDF membranes (Roche, Switzerland). After blocked with 5% skim milk, the PVDF membranes were cultivated with primary antibodies against MRP1 (1:1000; ab260038; Abcam), MDR1 (1:1000; #13,978; Cell signaling technology), LRP1 (1:1000; ab92544; Abcam), Notch2 (1:1000; ab245325; Abcam), GSK-3β (1:1000; #12,456; Cell signaling technology), β-catenin (1:1000; ab68183; Abcam), or β-actin (1:1000; ab8227; Abcam) at 4°C overnight and incubated with secondary antibody for 2 hours at room temperature. The protein bands were visualized with an enhanced chemiluminescent substrate kit (Millipore). The proteins were quantified using Quantity One software (Bio-Rad Laboratories, USA).
Statistical analysis
Data analyses were subjected to SPSS 21.0 software (IBM, NY, USA) and the data were displayed as mean ± SD. Comparisons between two groups were done by Student’s t-test, while one-way analysis of variance was employed for multiple-group comparisons. P < 0.05 was regarded with statistical significance.
Results
Here, we intended to investigate the role of circFAT1 in regulating chemoresistance of BC to OX. We performed a series of in vitro assays and found that circFAT1 promoted OX resistance in BC through activating the Notch and Wnt signaling pathway via the miR-525-5p/SKA1 axis. Hence, our data for the first time investigated the biological function of circFAT1 in regulating chemoresistance of BC to OX.
Upregulated circFAT1 is discovered in OX-resistant BC tissues and cells
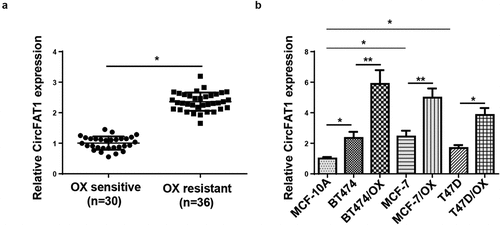
To explore the expression of circFAT1 in OX-resistant BC, RT-qPCR was carried out to detect circFAT1 abundance in OX-resistant BC tissues and cells. It was shown that circFAT1 level was increased in OX-resistant BC tissues and cell lines (BT474/OX, MCF-7/OX, and T47D/OX), relative to OX-sensitive BC tissues and parental BC cell lines (BT474, MCF-7, and T47D) (). These results indicated that circFAT1 expression was upregulated in OX-resistant BC.
CircFAT1 knockdown decreases the resistance of BC cells to OX
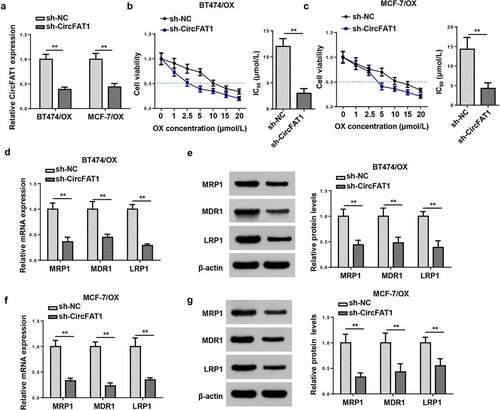
Since upregulated circFAT1 level was discovered in OX-resistant BC tissues and cells, we assumed a promoting role of circFAT1 in OX resistance in BC. Hence, cellular experiments were further conducted to detect the biological function of circFAT1 OX-resistant BC cells. RT-qPCR showed that circFAT1 expression level was significantly decreased in OX-resistant BC cells (BT474/OX and MCF-7/OX) after circFAT1 knockdown (). The IC50 value for OX was decreased in BT474/OX and MCF-7/OX cells after circFAT1 inhibition, relative to the control group ()). Furthermore, circFAT1 depletion downregulated the mRNA and protein levels of chemoresistance-related genes (MRP1, MDR1, and LRP1) in BT474/OX and MCF-7/OX cells ()). In conclusion, it was demonstrated that circFAT1 silencing suppressed the resistance of BC cells to OX in vitro.
Figure 2. CircFAT1 knockdown decreases resistance of BC cells to OX
CircFAT1 inhibition promotes apoptosis and repressed metastatic capabilities of OX-resistant BC cells
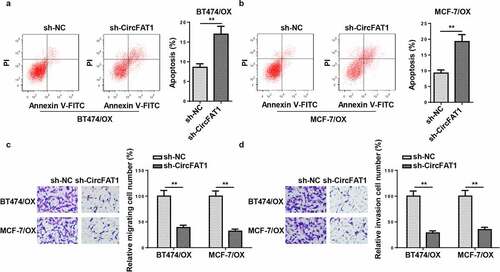
To further investigate the effects of circFAT1 on OX-resistant BC cell migration, invasion, and apoptosis, loss-of-function assays were carried out. Flow cytometry disclosed that circFAT1 silencing significantly accelerated the apoptosis of BT474/OX and MCF-7/OX cells (). Moreover, circFAT1 repression reduced the number of migrated and invaded cells, as indicated by Transwell assays (. In general, the results demonstrated that circFAT1 knockdown promoted cell apoptosis and suppressed cell migration and invasion in OX-resistant BC.
Figure 3. CircFAT1 inhibition promotes apoptosis and repressed metastatic capabilities of OX-resistant BC cells
CircFAT1 directly targets miR-525-5p to enhance metastasis and attenuate apoptosis in OX-resistant BC cells
Next, miR-525-5p was predicted as a possible target of circFAT1 through StarBase website (http://starbase.sysu.edu.cn/) (). Luciferase reporter assay revealed that miR-525-5p overexpression led to a significant reduction in luciferase activity of circFAT1-WT rather than circFAT1-MUT in BT474/OX and MCF-7/OX cells (). RT-qPCR revealed that miR-525-5p level was downregulated in BT474/OX and MCF-7/OX cells, relative to their parental cells (). Furthermore, BT474/OX and MCF-7/OX cells exhibited an increase in miR-525-5p abundance after circFAT1 blocking (). According to Pearson’s correlation analysis, there was a negative correlation between circFAT1 and miR-525-5p expressions in OX-resistant BC tissues (). Furthermore, it was revealed that circFAT1 knockdown promoted OX-resistant BC cell apoptosis and impaired OX-resistant BC cell metastasis, which was reversed by miR-525-5p downregulation ()). Therefore, the data suggested that circFAT1 negatively modulated miR-525-5p to confer OX resistance in OX-resistant BC cells.
Figure 4. CircFAT1 directly targets miR-525-5p to enhance metastasis and attenuate apoptosis in OX-resistant BC cells
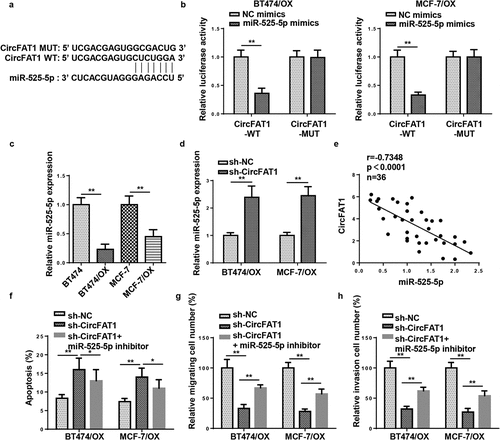
CircFAT1 accelerates metastasis and reduces apoptosis in OX-resistant BC cells via miR-525-5p/SKA1 axis
StarBase website predicted SKA1 as a downstream target for miR-525-5p (). According to the analysis performed with TCGA database, SKA1 exhibited an increased expression in breast invasive carcinoma (BRCA) tissues (). Thereafter, luciferase reporter assay confirmed that SKA1 was directly targeted by miR-525-5p in BC cells (). Rescue experiments demonstrated that miR-525-5p inhibition restrained apoptosis but exacerbated metastatic capabilities of OX-resistant BC cells; however, such phenomena were abrogated by SKA1 blocking ()). It was also revealed by RT-qPCR that circFAT1 inhibition decreased SKA1 abundance, while miR-525-5p knockdown markedly increased SKA1 level ( H and I). Besides, SKA1 expression was inversely correlated to miR-525-5p level in OX-resistant BC tissues as shown by Pearson’s correlation analysis (). To sum up, circFAT1 positively modulated SKA1 level via miR-525-5p to aggravate OX resistance in OX-resistant BC cells, suggesting the circFAT1/miR-525-5p/SKA1 regulating network.
Figure 5. CircFAT1 accelerates metastasis and reduces apoptosis in OX-resistant BC cells via miR-525-5p/SKA1 axis
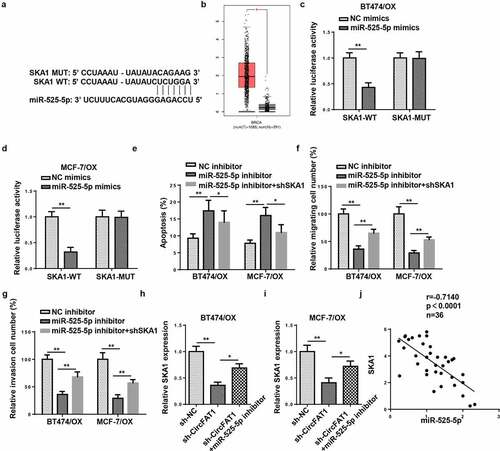
SKA1 knockdown hinders Notch and Wnt signaling in BC cells resistant to OX
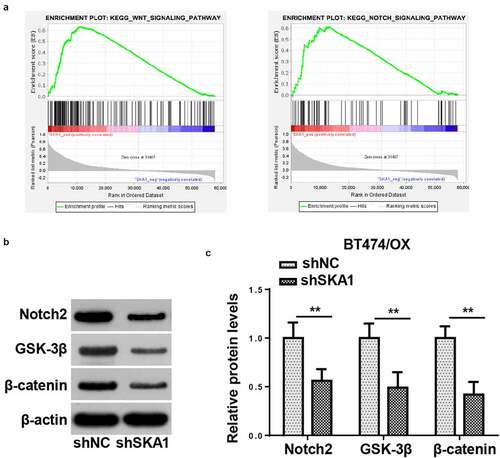
SKA1 has been proven to accelerate tumorigenesis through the activation of several signaling pathways [Citation22]. Herein, Gene Set Enrichment Analysis (GSEA) indicated that Notch and Wnt signaling pathways were both positively associated with SKA1 expression in BC (). To further validate the GSEA results, protein expressions of Notch2, GSK-3β, and β-catenin were detected in BT474/OX cells by western blotting. It was shown that SKA1 silencing suppressed Notch2, GSK-3β, and β-catenin expressions in BT474/OX cells, indicating SKA1 might activate the Notch and/or Wnt signaling pathways ()).
Figure 6. SKA1 knockdown hinders notch and Wnt signaling in BC cells resistant to OX
Discussion
Conventional treatments have no curative impact on advanced breast cancer; moreover, treatment of advanced breast cancer is complex and even controversial since there are still no universally accepted therapies [Citation23]. Oxaliplatin is a third-generation platinum analog, which has been widely used in clinical treatment for breast cancer treatment [Citation4]. Besides, unlike other platinum compounds such as cisplatin and carboplatin, oxaliplatin is of no nephrotoxicity and minimal myelosuppression [Citation3]. As drug resistance has been regarded as a major problem for BC oncotherapy in clinical practice [Citation24], it is important to explore the underlying molecular mechanism of OX resistance in BC. In the current study, we explored the role of circFAT1 in resistance of BC cells to OX in vitro and discovered its molecular mechanism in regulating OX resistance in BC.
As reported in previous studies, circFAT1 has been investigated in several human cancer types for its diagnostic and prognostic values. To cite an instance, Wang et al. reported that circFAT1 regulated EZH2 level as a sponge for miR-600, thus accelerating colorectal cancer progression [Citation25]. Additionally, it was demonstrated by Wei et al. that circFAT1 facilitated the development of hepatocellular carcinoma via the miR-30a-5p/REEP3 axis [Citation16]. Furthermore, Liu et al. uncovered that circFAT1 promoted osteosarcoma cell proliferation and metastasis via the miR-375/YAP1 axis [Citation14]. Our results revealed that circFAT1 level was increased in OX-resistant BC tissues and cells, and circFAT1 blocking led to a decline in chemoresistance-related genes, including MRP1, MDR1, and LRP1, in OX-resistant BC cells. Further functional experiments exhibited that circFAT1 knockdown manifestly reduced IC50, promoted apoptosis and reduced metastasis in OX-resistant BC cells. To sum up, it was strongly demonstrated that circFAT1 aggravated the resistance of BC cells to OX.
Accumulating evidence has demonstrated the critical role of circRNA/miRNA/mRNA regulatory network in regulating chemoresistance in BC [Citation26–28]. To investigate the downstream mechanism of circFAT1 in OX-resistant BC cells, the target genes of circFAT1 were screened. Through bioinformatics analysis and dual-luciferase assay, miR-525-5p was predicted and verified to target circFAT1 and SKA1. MiR-525-5p has been confirmed as an anti-tumor gene in various malignant tumors, such as cervical cancer [Citation29], ovarian cancer [Citation30], and bladder cancer [Citation31]. SKA1 has been reported to act as an oncogenic role in various human cancers, including bladder cancer [Citation32], hepatocellular carcinoma [Citation33], and esophageal squamous cell carcinoma [Citation34]. Additionally, it was also demonstrated that SKA1 expedites cisplatin resistance in non-small cell lung cancer, indicating its promoting role in the development of chemoresistance [Citation35]. In our study, functional assays revealed that circFAT1 enhanced chemoresistance of BC cells to OX via miR-525-5p/SKA1 axis.
Multiple studies have proven the important regulatory functions of Notch and Wnt pathways in BC progression. To cite an instance, Chen et al. revealed the promoting role of Wnt signaling in cell proliferation and migration abilities in BC [Citation36]. Xia et al. discovered that Notch signaling promoted BC tumor growth and metastasis [Citation37]. In addition, Yan et al. demonstrated that HIF-2alpha increased chemoresistance in BC cells by activating Wnt and Notch pathways [Citation38]. Herein, a positive relation between SKA1 expression and the Notch and Wnt signaling pathways was identified by GSEA. It was further confirmed by western blotting that SKA1 blocking suppressed Notch2, GSK-3β, and β-catenin levels, indicating the inactivation of the Notch and Wnt signaling pathway. Hence, we demonstrated that SKA1 might mediate OX resistance in BC via the Notch and Wnt pathways.
Conclusions
Our results illustrated the crucial role of circFAT1 on OX-resistant BC cells, that is, circFAT1/miR-525-5p/SKA1 axis enhanced OX resistance in BC via the activation of Notch and Wnt pathway. However, in vivo experiments are required to further validate these findings in follow-up studies.
Ethics approval and consent to participate
The study received approval from Changzhou No.2 People’s Hospital Affiliated to Nanjing Medical University. Written informed consent was obtained from the participants.
Authors’ contributions
BW and YY contributed to the study conception and design. Material preparation, data collection and analysis were performed by XL, LC and XW. The first draft of the manuscript was written by YY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Anderson BO, et al. Early detection of breast cancer in countries with limited resources. Breast J. 2003;9(Suppl s2):S51–9.
- DeSantis C, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62.
- Garufi C, et al. Single-agent oxaliplatin in pretreated advanced breast cancer patients: a phase II study. Ann Oncol. 2001;12(2):179–182.
- Garutti M, et al. Platinum salts in patients with breast cancer: a focus on predictive factors. Int J Mol Sci. 2019;20(14):14.
- Raymond E, et al. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1(3):227–235.
- Lim S, et al. Inhibition of Chk1 by miR-320c increases oxaliplatin responsiveness in triple-negative breast cancer. Oncogenesis. 2020;9(10):91.
- Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42–51.
- Zhao B, Song X, Guan H. CircACAP2 promotes breast cancer proliferation and metastasis by targeting miR-29a/b-3p-COL5A1 axis. Life Sci. 2020;244:117179.
- Zeng K, et al. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018;9(4):417.
- Su Y, et al. CircRNA Cdr1as functions as a competitive endogenous RNA to promote hepatocellular carcinoma progression. Aging (Albany NY). 2019;11(19):8183–8203.
- Liu G, et al. Circ_0006528 contributes to paclitaxel resistance of breast cancer cells by regulating miR-1299/CDK8 Axis. Onco Targets Ther. 2020;13:9497–9511.
- Hu K, et al. Exosomes mediated transfer of circ_UBE2D2 enhances the resistance of breast cancer to tamoxifen by binding to miR-200a-3p. Med Sci Monit. 2020;26:e922253.
- Zang H, et al. Circ-RNF111 contributes to paclitaxel resistance in breast cancer by elevating E2F3 expression via miR-140-5p. Thorac Cancer. 2020;11(7):1891–1903.
- Liu G, et al. CircFAT1 sponges miR-375 to promote the expression of Yes-associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17(1):170.
- Hu B, et al. CircFAT1 Suppresses Colorectal Cancer Development Through Regulating miR-520b/UHRF1 Axis or miR-302c-3p/UHRF1 Axis. Cancer Biother Radiopharm. 2021;36(1):45–57.
- Wei H, et al. CircFAT1 promotes hepatocellular carcinoma progression via miR-30a-5p/REEP3 pathway. J Cell Mol Med. 2020;24(24):14561–14570.
- Zhang W, et al. Downregulation of Circ_0071589 suppresses cisplatin resistance in colorectal cancer by Regulating the MiR-526b-3p/KLF12 Axis. Cancer Manag Res. 2021;13:2717–2731.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
- Han B, et al. Down-regulation of lncRNA DNAJC3-AS1 inhibits colon cancer via regulating miR-214-3p/LIVIN axis. Bioengineered. 2020;11(1):524–535.
- Zhang L, et al. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17(15):1886–1900.
- Yang Z, et al. Long intergenic noncoding RNA00265 promotes proliferation of gastric cancer via the microRNA-144-3p/Chromobox 4 axis. Bioengineered. 2021;12(1):1012–1025.
- Wang X, et al. SKA1 promotes malignant phenotype and progression of glioma via multiple signaling pathways. Cancer Cell Int. 2019;19(1):324.
- Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339(14):974–984.
- Ji X, et al. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019;114:108800.
- Yong W, et al. Hsa_circ_0071589 promotes carcinogenesis via the miR-600/EZH2 axis in colorectal cancer. Biomed Pharmacother. 2018;102:1188–1194.
- Yang W, et al. Circ-ABCB10 contributes to paclitaxel resistance in breast cancer through Let-7a-5p/DUSP7 Axis. Cancer Manag Res. 2020;12:2327–2337.
- Dou D, et al. CircUBE2D2 (hsa_circ_0005728) promotes cell proliferation, metastasis and chemoresistance in triple-negative breast cancer by regulating miR-512-3p/CDCA3 axis. Cancer Cell Int. 2020;20(1):454.
- Liang Y, et al. circKDM4C suppresses tumor progression and attenuates doxorubicin resistance by regulating miR-548p/PBLD axis in breast cancer. Oncogene. 2019;38(42):6850–6866.
- Chen M, Liu LX. MiR-525-5p repressed metastasis and anoikis resistance in cervical cancer via blocking UBE2C/ZEB1/2 Signal Axis. Dig Dis Sci. 2020;65(8):2442–2451.
- Wang L, et al. Role of lncRNAHCP5/microRNA-525-5p/PRC1 crosstalk in the malignant behaviors of ovarian cancer cells. Exp Cell Res. 2020;394(1):112129.
- Yang Z, et al. Androgen receptor suppresses prostate cancer metastasis but promotes bladder cancer metastasis via differentially altering miRNA525-5p/SLPI-mediated vasculogenic mimicry formation. Cancer Lett. 2020;473:118–129.
- Tian F, et al. Downregulation of SKA1 gene expression inhibits cell growth in human bladder cancer. Cancer Biother Radiopharm. 2015;30(7):271–277.
- Xiao J, Yu H, Ma Z. LINC00339 promotes growth and invasiveness of hepatocellular carcinoma by the miR-1182/SKA1 pathway. Onco Targets Ther. 2019;12:4481–4488.
- Hu D, et al. SKA1 overexpression is associated with the prognosis of esophageal squamous cell carcinoma and regulates cell proliferation and migration. Int J Mol Med. 2019;44(5):1971–1978.
- Shen L, et al. SKA1 regulates the metastasis and cisplatin resistance of non-small cell lung cancer. Oncol Rep. 2016;35(5):2561–2568.
- Chen Z, et al. RCC2 promotes breast cancer progression through regulation of Wnt signaling and inducing EMT. J Cancer. 2019;10(27):6837–6847.
- Xia S, et al. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm J. 2017;25(4):638–643.
- Yan Y, et al. HIF-2alpha promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res. 2018;37(1):256.