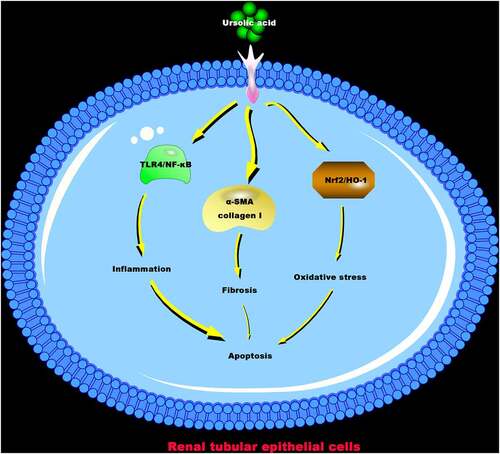
ABSTRACT
Ursolic acid (UA) has been proved to have antioxidant and anti-inflammatory effects. However, it is not clear whether it has a protective impact on kidney damage induced by crystals of calcium oxalate monohydrate (COM). This work aimed to make clear the potential mechanism of UA protecting COM-induced kidney damage. The results manifested that high- and low-dose UA reduced COM crystals in COM rats’ kidney, down-regulated urea, creatinine, and neutrophil gelatinase-associated lipocalin (NGAL) levels in rat plasma, declined kidney tissue and HK-2 cell apoptosis, inhibited Bax expression but elevated Bcl-2 expression. Additionally, UA alleviated renal fibrosis in COM rats, repressed α-SMA and collagen I protein expressions in the kidney and COM rats’ HK-2 cells, depressed COM-induced oxidative damage in vivo and in vitro via up-regulating Nrf2/HO-1 pathway, up-regulated SOD levels and reduced MDA levels, down-regulated TNF-α, IL-1β, and IL-6 levels in vivo and in vitro via suppressing activation of TLR4/NF-κB pathway. In summary, the results of this study suggest that COM-induced renal injury can be effectively improved via UA, providing powerful data support for the development of effective clinical drugs for renal injury in the future.
1. Introduction
Kidney stone, a common kidney disease, due to lifestyle and diet and other reasons, features increasing global prevalence [Citation1]. The most vital component of kidney stones is calcium oxalate monohydrate (COM) crystals. Oxalate, one-end product of glyoxylate, amino acids, and carbohydrates metabolized in the liver [Citation2], is mainly filtered and secreted through the glomeruli and renal tubules, finally excreted by the kidney. This process is mediated by the SLC26A anion exchanger expressed in basolateral membrane [Citation3]. Based on a great many studies, high-level oxalate or calcium oxalate crystals in the body can accelerate free radicals and inflammatory factors to produce in the kidney, causing kidney inflammation, oxidative stress, and even cell apoptosis [Citation4–6]. Currently, there is still a lack of natural and safe inhibitors to depress renal calcium oxalate crystallization and alleviate kidney damage. Therefore, it is urgent to seek an effective inhibitor for renal calcium oxalate crystallization.
Ursolic acid (UA), a pentacyclic triterpene compound stems from the berries, fruits, leaves, and flowers of many medicinal plants. In Asia, it has been applied as an anti-inflammatory and hypoglycemic agent for centuries [Citation7]. UA is available to suppress inflammation in various ways. Chen Z et al. reported that UA protects lipopolysaccharide-induced inflammation via inhibiting NLRP3 inflammatory nucleosomes [Citation8]. Additionally, it also plays a protective role in neuroinflammation via depressing the activation of NF-κB and Iba1 [Citation9] and improve cell damage in the body via curbing oxidative stress. Samiver R et al. reported that UA relieves UVB radiation-induced oxidative stress in human dermal fibroblasts via dampening the production of reactive oxygen species (ROS) and lipid peroxidation, changing mitochondrial membrane potential, and regulating antioxidant pathway activity [Citation10]. Additionally, UA mitigates intestinal oxidative stress in rat models of intestinal fibrosis [Citation11]. Recent studies have revealed that UA improves lipopolysaccharide-induced acute kidney injury via blocking TLR4/MYD88 pathway [Citation12]. Ma TK et al. also reported that UA lightens inflammation, oxidative stress, and apoptosis in diabetic nephropathy via regulating ARAP1/AT1R signaling pathway [Citation13], indicating that ursolic acid may act actively in treating kidney diseases. In 2010, Hsu CL et al. found that a plant containing UA is able to treat kidney stones and dysuria [Citation14]. Although previous studies have revealed that UA has ameliorative effects on various kidney injuries, it is ill-defined whether UA has a protective effect on oxalate-induced kidney injury and kidney stones.
Based on this, we hypothesized that UA had a therapeutic effect on oxalate-induced renal tubular epithelial cell injury. In order to prove this hypothesis, the present study investigated the effects of UA on oxalate-induced fibrosis, oxidative stress, inflammation, and apoptosis of renal tubular epithelial cells in vivo and in vitro.
2. Methods
2.1. Animal model establishment
In total 8-week-old SFP-grade Sprague-Dawley (SD) rats from the Animal Laboratory of the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, were by randomly allocated into Control group, COM group, low-dose UA group (L-UA) and high-dose UA group (H-UA), six rats each. Except for Control group, all groups received 1% ethylene glycol and 2% 2 mL of ammonium chloride via gavage once a day. Meanwhile, the L-UA and H-UA groups were daily given 20 mg/kg and 40 mg/kg of UA via gavage, Control group receiving the same volume of distilled water via gavage each day. After 4 weeks, the rats were sacrificed through intraperitoneal injection of sodium pentobarbital (40 mg/kg) and their blood was taken from aorta. After being set aside for 2 h at room temperature, the blood received centrifugation at 4°C for 10 min to collect serum and store at −80°C for follow-up analysis. After death, some rat kidneys were quickly collected and fixed in 4% paraformaldehyde, and the remaining kidney tissue was stored at −80°C for subsequent analysis. All steps of animal experiments strictly followed the research ethics regulations of the First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology.
2.2. Hematoxylin-Eosin (HE) and Masson staining
HE and Masson staining were constructed as before [Citation15]. The renal tissue fixed in 4% paraformaldehyde for another 24 h, was later fixed in paraffin to cut into continuous coronal sections (4 µm), and deparaffinized in xylene. The sections were hydrated in gradient ethanol. Hematoxylin and Eosin (H&E) staining was applied to observe the tissue structure. Based on the kit instructions, the sections were stained with Masson’s trichrome stain (Solarbio), digital microscope cameras (DP12 SZX7, Olympus Corporation, Japan) applied to take images.
2.3. TdT-mediated dUTP-biotin nick end-labeling (TUNEL) staining
TUNEL staining was carried out as same as before [Citation16]. TUNEL positive cells (green) and total cells (blue) were counted in five by random selected fields of each slide under fluorescence microscopes (Zeiss AxioVision, Germany) at 400 × magnification, and the apoptosis rate analyzed via Image Pro Plus 6.0 (Media Cybernetics, US.).
2.4. Immunohistochemistry
Immunohistochemical analysis was performed based on previous studies [Citation17]. The sections were deparaffinized at 60°C for 1 h, washed twice in xylene for 10 min, and later rehydrated in descending alcohol series. After the antigen was recovered in microwave for 12 min, the tissue was combined with primary antibodies Bax (ab173026; 1:1000; Abcam), Bcl-2 (ab182858; 1:1000; Abcam), α-SMA (A2547, 1:1000, MilliporeSigma), collagen I (ab34710, 1:1000, Abcam), MYD88 (4283, 1:1000, Cell Signaling Technology), TLR4 (sc-293,072, 1:1000, Santa Cruz Biotechnology), Nrf2 (ab62352, 1:1000, Abcam), HO-1 (ab13248, 1:1000, Abcam) to incubate together. After 16 h, the tissue with goat anti-rabbit immunoglobulin G (IgG) secondary antibody conjugated with horseradish peroxidase was incubated for 1 h (1:2,000, catalog number TA130024, OriGene Technologies, Inc.), and later stained with diaminobenzidine for 5 min at room temperature, digital microscope cameras applied to take images.
2.5. Cell culture
Renal tubular epithelium (HK-2 cells) bought from American Type Cell Collection (CRL-2190, ATCC, France), were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12, 1:1) with 10% fetal calf serum (FCS), 2% antibiotic-antifungal drugs, at 37°C with 5% CO2, stored in a 25 cm2 flask (Gibico, US) for cell monolayer.
2.6. UA toxicity testing
Cytotoxicity was tested via 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay [Citation18]. The cells transferred to 96-well plates (1 × 105/well) were exposed to serum- and pyruvate-free medium. Aiming to obtain optimal UA concentration, HK-2 cells were exposed to 0, 2.5, 5, 7.5, and 10 μM UA. After 24 h, followed by MTT cell proliferation and cytotoxicity assay kit (Nanjing Jiancheng Biotechnology Co., Ltd.) to evaluate cell viability via measuring the absorbance at 570 nm.
2.7. Cell grouping
The cells were allocated into four groups: Control group (HK-2 cells not exposed to any reagent); COM group (HK-2 cells exposed to 200 mg/L COM crystals and 2 mmol/L oxalate); L-UA and H-UA groups (2.5 μM and 5 μM UA added to medium, respectively). After 30 min-incubation, HK-2 cells were exposed to 200 mg/L COM crystals and 2 mmol/L oxalate. After 24 h, the medium and cells were for subsequent testing.
2.8. Enzyme-linked immunosorbent assay (ELISA)
In reference to the instructions, malondialdehyde (MDA), superoxide dismutase (SOD), interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 levels in rat kidney tissue and HK-2 cells, Na+/K+ ATPase activity in HK-2 cells, as well as creatinine, urea and NGAL levels in rat serum were detected, all ELISA kits were purchased from Nanjing Jiancheng Biotechnology Company.
2.9. Flow cytometry
The cells were digested with trypsin, analyzed via flow cytometry and APC Annexin V/propidium iodide (PI) apoptosis detection kit (BioLegend, US), washed with phosphate buffered saline (PBS), suspended in AV binding buffer (BioLegend), and adjusted to 1 × 105 cells/mL, and later stained with AV/PI Detection Reagent for 30 min in the dark. Cell apoptosis was detected via FACSCalibur flow cytometer (BD).
2.10. Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was applied according to previous methods [Citation19]. Total RNA isolated from cells via TRIzol reagent (Invitrogen) received reverse transcription via M-MLV reverse transcriptase (Invitrogen) to synthesize complementary DNA (cDNA). The RT-qPCR analysis was performed on ABI Prism 7500 fast real-time PCR system (Applied Biosystems, US) via Power SYBR Green Master Mix (Applied Biosystems). Relative expression was evaluated via 2−ΔΔCt method, GAPDH as an internal reference. The primer sequence is in .
Table 1. qRT-PCR primer sequences
2.11. Western blot
Cells were lysed via RIPA lysis buffer (Beyotime, China), and total protein concentration was measured via Pierce BCA protein analysis kit (Pierce, US). After elution in sample loading buffer (Beyotime) and separation via SDS-PAGE, the samples were transferred to PVDF films at a constant current of 200 mA. The films blocked with 5% skim milk in Tris-buffered saline Tween (TBST) at room temperature, and the primary antibodies GAPDH (2118, Cell Signaling Technology, 1:1000), p-NF-κB (3033, 1: 1000), Cell Signaling Technology), NF-κB (8242, 1:1000, Cell Signaling Technology), TLR4 (sc-293,072, 1:1000, Santa Cruz Biotechnology), Nrf2 (ab62352, 1:1000, Abcam), HO-1 (Ab13248, 1:1000, Abcam), α-SMA (A2547, 1:1000, MilliporeSigma), collagen I (ab34710, 1:1000, Abcam) were incubated. After being washed in triplicate with TBST, they were cultured with secondary antibody (goat anti-rabbit IgG, ab6721, 1:5000) in an incubator for 1.5 h at room temperature. Later being washed again with TBST 3 times, the films were treated with ECL Plus substrate bought from Life Technologies Corporation (Gaithersburg, US) to detect protein signals. The image acquisition and analysis system of Lab Works version 4.5 software (SIL Technologies, Inc., IL, US) were applied to detect and quantify the immunoreactive signal of protein bands.
2.12. Data analysis
The experimental findings were shown as mean ± standard deviation (SD), via SPSS 22 software for data analysis, including Student’s t-test and one-way analysis of variance (ANOVA). Via Tukey’s test to perform multiple variance correction on samples, the difference among experimental groups is considered significant when P < 0.05. All experiments were biologically duplicated.
3. Results
3.1. UA reduced COM-induced pathological damage to the kidney
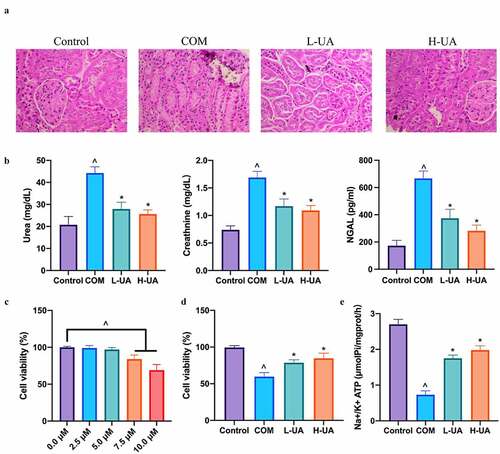
Aiming to probe into the protective impact of UA on COM rats’ kidney, the pathological morphology of their kidneys was examined via HE staining. There was no crystal deposition in Control, L-UA, and H-UA groups, but with obvious deposition in COM group (). Additionally, we examined the influence of UA on renal function indicators. Compared with Control group, plasma urea, creatinine, and NGAL levels in COM rats increased signally (), whereas high- and low-dose UA signally reversed them. Later, the study further examined the protective impact of UA on COM-induced HK-2 cell damage via in vitro experiments. We first examined the toxic effect of UA on HK-2 cells exposed to different UA concentrations to check cell viability. UA at 2.5 and 5 μM was not toxic to HK-2 cells, while at 7.5 and 10 μM decreased cell viability in a dose-dependent manner (). HK-2 cells pretreated with 2.5 and 5 μM UA were exposed to COM for 24 h, finding that () the cell viability in COM group was signally lower than that in Control group, whereas visually lower than that in L-UA and H-UA groups in a dose-dependent manner. Additionally, Na+/K+ ATPase level in COM group was signally lower than that in Control group, whereas observably lower than those in L-UA and H-UA groups significantly (). ()
Figure 1. UA reduced COM-induced pathological damage to the kidney
3.2. UA attenuated COM-induced HK-2 cell apoptosis
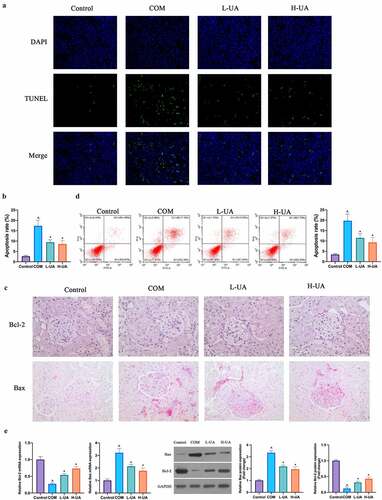
Next, the study exploited the impact of UA on COM-induced HK-2 cell apoptosis. TUNEL staining findings revealed that the number of TUNEL-positive cells in COM group was signally elevated with contrast to that in Control group, and high- and low-dose UA could visually reduce the number (). Later, the impact of UA on Bax and Bcl-2 apoptosis proteins in COM rats were examined via immunohistochemistry. Compared with Control group, Bax expression in COM group was apparently elevated whereas Bcl-2 expression was distinctly reduced (). High- and low-dose UA reversed Bax and Bcl-2 expressions in COM rats, which was further verified in vitro. Flow cytometry findings revealed that after 24-h exposure to COM, HK-2 cell apoptotic rate was up-regulated visually (), but after pretreatment with low- and high-dose UA, the apoptotic rate in COM group was observably reduced. Additionally, RT-qPCR and western blot findings revealed that, in comparison with Control group, Bcl-2 expression in COM group was evidently reduced and Bax expression was markedly elevated (). Low- and high-dose UA pretreatment obviously alleviated this phenomenon. ()
Figure 2. UA reduced COM-induced HK-2 cell apoptosis
3.3. UA reduced COM-induced renal fibrosis
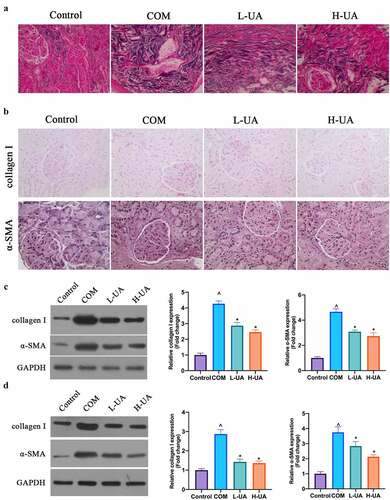
The degree of renal fibrosis in each group was examined via Masson staining. The degree in Control group was very low, with almost no blue collagen fibers stained by Masson’s staining (), the degree in COM group was high, with blue collagen fibers in great number, and the degree in L-UA and H-UA groups was manifest lower by contrast with that in COM group, with greatly reduced blue collagen fibers. Later, α-SAM and collagen I expressions in rats’ kidney tissues was examined via immunohistochemistry and western blot. α-SMA and Collagen I protein expressions in COM group was overtly up-regulated by contrast with that in Control group, while L-UA and H-UA treatment visually reduced them in COM rats (). Additionally, this finding was further verified in HK-2 cells (). ()
Figure 3. UA reduced COM-induced renal fibrosis
3.4. UA protected the kidney from COM-induced oxidative damage
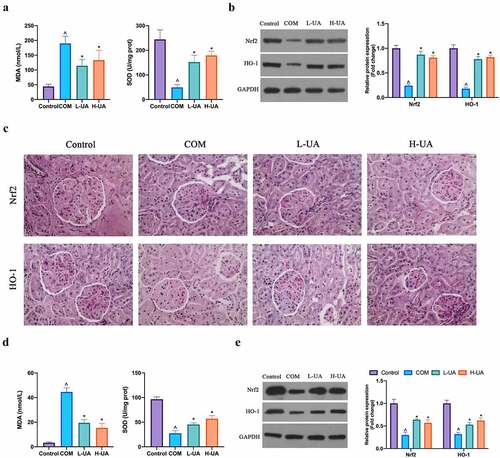
Next, the study probed into the effect of UA on COM-induced renal oxidative stress. COM increased MDA level in COM rats and decreased SOD level (), while high- and low-dose UA obviously reduced MDA level in COM rats and up-regulated SOD level. Additionally, Nrf2/HO-1 axis is an essential pathway for anti-oxidative stress in the body. The study found that COM apparently reduced Nrf/HO-1 protein expression in COM rats, but high- and low-dose UA could restore it (). In in vitro experiments, after COM exposure, MDA level in HK-2 cells elevated visually, whereas SOD level decreased signally. After pretreatment with high- and low-dose UA, MDA level was evidently reduced and SOD level was restored (), finding that COM exposure inhibited Nrf2/HO-1 protein expression in HK-2 cells, while in COM group pretreated with high- and low-dose UA, Nrf2/HO-1 protein expression was manifest elevated (). ()
Figure 4. UA protected the kidney from COM-induced oxidative damage
3.5. UA protected the kidney from COM-induced renal inflammatory damage
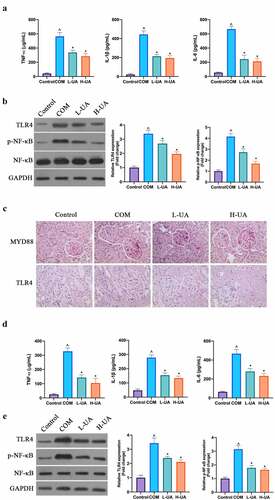
The study explored the effect of UA on COM-induced renal inflammation. COM signally elevated TNF-α, IL-1β, and IL-6 levels in rats’ kidney tissues, whereas high- and low-dose UA visually reduced them (). TLR4/NF-κB axis has been found to promote various inflammatory factors expressions in the body, finding that COM signally elevated MYD88, TLR4 protein, and phosphorylated NF-κB protein expressions in rat kidney tissue, whereas high- and low-dose UA could apparently decrease them (, c). Additionally, in in vitro experiments the study found that COM exposure signally elevated TNF-α, IL-1β, and IL-6 levels in HK-2 cells, while UA pretreatment distinctly reduced them after COM exposure (). After COM exposure, TLR4 and phosphorylated NF-κB protein expressions in HK-2 cells elevated markedly, whereas after UA pretreatment, they in COM group were signally inhibited (). ()
Figure 5. UA protected the kidney from COM-induced renal inflammation damage
4. Discussion
The study found that UA protects the kidney from COM-induced damage via inhibiting renal fibrosis, apoptosis, inflammation, and oxidative stress in in vivo and in vitro models.
In this study, it was the first time to demonstrate that UA treatment could reduce COM-induced renal crystal aggregation, indicating the large potential of UA on amelioration of kidney stones and urinary tract obstruction. Many studies have manifested that plant extracts are capable of reducing kidney stones, such as dietary polyphenolic caffeic acid [Citation20], astragalus polysaccharide [Citation21], and gambogus extract [Citation22]. However, the clinical efficacy of plenty of plant extracts in all kinds of diseases is limited by bioavailability and solubility, including UA. At present, scientists improve the bioavailability and solubility of UA by means of chemical modification [Citation23]. Therefore, UA can be applied as a potential clinical drug for the treatment of kidney stones in the future. Although the study has demonstrated that UA could decline COM-induced renal crystal deposition, the effect of 40 mg/kg UA on normal rats is still unclear, which needs to be examined in future work. Besides, a positive drug group was not set in this study, which is the limitation of this study. After treating COM model rats with high- and low-dose UA, the plasma creatinine level in COM rats was manifest reduced. It is known that creatinine is an essential indicator for evaluating glomerular damage, because its content in plasma depends on many factors, like glomerular filtration rate, renal tubular secretion rate, and urine volume [Citation24]. Additionally, the study also found that urea level in COM rats’ plasma elevated, which was reversed via UA intervention. Increased urea and creatinine levels in COM rats may be due to excessively accumulated COM crystals in kidney tissue, whereas reduced plasma creatinine and urea levels via UA may impose an impact via inhibiting COM crystals in kidney tissue. What’s more, NGAL was declined via UA. In the early stages of kidney injury, NGAL in serum is distinctly elevated, prompting that UA plays an important role in the early stages of kidney damage [Citation25]. Importantly, UA treatment signally elevated Na+/K+ATPase activity in HK-2 cells. Sodium, potassium, and mercury are vital for maintaining intracellular environment. Based on previous studies, oligomeric proanthocyanidins up-regulates Na+/K+ATPase activity to repress oxidative stress resulting from COM crystals on HK-2 cells [Citation26], whereas UA features a similar impact.
Renal crystal aggregation has key connection with renal fibrosis. In reference to previous studies, UA is available to suppress TGF-β1-induced renal fibrosis via depressing α-SMA, snail, slug, and other protein expressions [Citation27]. Renal fibrosis is the final universal pathological feature of various chronic kidney diseases, which is closely related to inflammation, oxidative damage, and apoptosis [Citation28,Citation29]. These studies have revealed that COM induces obvious renal fibrosis as well, which is consistent with our findings [Citation30]. A great many studies have revealed that α-SMA and collagen I proteins acts pivotally in renal interstitial fibrosis and epithelial–mesenchymal transition [Citation31,Citation32]. In this work, we found that high- and low-dose UA are available to depress α-SMA and collagen I protein expressions in vivo and in vitro, helping to improve COM-induced renal fibrosist, thus cutting down Com crystal deposit.
Nrf2 is a member of CNC-bZIP (cap’collar-basic leucine zipper) transcription activator family. After forming a heterodimer with the Neh1 domain and Maf protein, it combines with antioxidant reaction element (ARE) to regulate the activity of target genes like SOD, CAT, and phase II detoxification enzymes [Citation33]. HO-1 is among the phase II detoxification enzymes, and the downstream signal pathways it triggers have antioxidant effects on various organs [Citation34]. Based on previous studies, UA is available to regulate Nrf2 pathway in all types of diseases, such as cancer and emphysema [Citation35,Citation36]. The study found that UA increased SOD level and reduced MDA level in COM models in vivo and in vitro via up-regulating Nrf2/HO-1 expression, and repressing COM-induced oxidative stress. It is known that oxidative stress is a key mechanism of COM-induced kidney damage. Excessive ROS is available to activate NF-κB inflammation signaling pathway and induce cell apoptosis [Citation37]. Previous studies have revealed that inhibiting ROS production alleviates COM-induced inflammation in renal tubular epithelial cells. This work found that UA repressed TLR4/NF-κB pathway to impede COM-induced renal inflammation. Whether this effect is achieved through regulating ROS production needs further study.
5. Conclusion
To sum up, our findings indicate that UA is available to reduce COM crystallization in renal tissue and knockdown COM-induced renal cell apoptosis, oxidative damage, inflammation, and renal fibrosis via regulating Nrf2/HO-1 oxidative stress signaling pathway, TLR4/NF-κB inflammatory signaling pathway, as well as α-SMA and collagen I protein expressions. This provides data support for UA as an effective drug for treating kidney stones later.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Khan S. Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron Exp Nephrol. 2004;98(2):e55–60.
- John K, Dean GA, Michael FC, et al. Metabolism of primed, constant infusions of [1,2-13C₂] glycine and [1-13C₁] phenylalanine to urinary oxalate. Metabolism. 2011;60(7):950–956. .
- Robijn S, Hoppe B, Vervaet BA, et al. Hyperoxaluria: a gut-kidney axis? Kidney Int. 2011;80(11):1146–1158. .
- Jiannan L, Kelaier Y, Yinshan J, et al. H3 relaxin protects against calcium oxalate crystal-induced renal inflammatory pyroptosis. Cell Prolif. 2020;53(10):e12902.
- Larissa DA, Juliana MCP, Mariana CDP, et al. Sodium oxalate-induced acute kidney injury associated with glomerular and tubulointerstitial damage in rats. Front Physiol. 2020;11:1076.
- Zizhi L, Linna C, Xiuli R, et al. Modulation of rat kidney stone crystallization and the relative oxidative stress pathway by green tea polyphenol. ACS Omega. 2021;6(2):1725–1731. .
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49(2):57–68.
- Zixi C, Qiaoli L, Zhaowei Z, et al. Ursolic acid protects against proliferation and inflammatory response in LPS-treated gastric tumour model and cells by inhibiting NLRP3 Inflammasome activation. Cancer Manag Res. 2020;12:8413–8424.
- Rai SN, Zahra W, Singh SS, et al. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotox Res. 2019;36(3):452–462. .
- Ramachandran S, Rajendra PN, Umadevi S, et al. Inhibitory effect of ursolic acid on ultraviolet B radiation-induced oxidative stress and proinflammatory response-mediated senescence in human skin dermal fibroblasts. Oxid Med Cell Longev. 2020;2020:1246510.
- Wang Z, Dakai G, Jie J, et al. Protective effect of ursolic acid on the intestinal mucosal barrier in a rat model of liver fibrosis. Front Physiol. 2019;10:956.
- Jun Z, Haoyi Z, Zhongguo S, et al. Ursolic acid exhibits anti-inflammatory effects through blocking TLR4-MyD88 pathway mediated by autophagy. Cytokine. 2019;123:154726.
- Tian-Kui M, Li X, Ling X, et al. Ursolic acid treatment alleviates diabetic kidney injury by regulating the ARAP1/AT1R signaling pathway. Diabetes Metab Syndr Obes. 2019;12:2597–2608.
- Hsu CL, Hong BH, Yu YS, et al. Antioxidant and anti-inflammatory effects of Orthosiphon aristatus and its bioactive compounds. J Agric Food Chem. 2010 Feb 24;58(4):2150–2156.
- Du X, Tao Q, Du H, et al. Tengdan capsule prevents hypertensive kidney damage in shr by inhibiting periostin-mediated renal fibrosis. Front Pharmacol. 2021;12:638298.
- Kim JH, Jeong SJ, Kim B, et al. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J Pineal Res. 2012 Mar;52(2):244–252.
- Emre C, Do KV, Jun B, et al. Age-related changes in brain phospholipids and bioactive lipids in the APP knock-in mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2021 Jun 29;9(1):116.
- Daniyal M, Liu Y, Yang Y, et al. Anti-gastric cancer activity and mechanism of natural compound “Heilaohulignan C” isolated from Kadsura coccinea. Phytother Res. 2021 Jun 21. DOI:10.1002/ptr.7114.
- Yang C, Shi J, Wang J, et al. Circ_0006988 promotes the proliferation, metastasis and angiogenesis of non-small cell lung cancer cells by modulating miR-491-5p/MAP3K3 axis[J]. Cell cycle (Georgetown, Tex.), 2021, 20(13):1334–1346
- Yasir F, Wahab AT, Choudhary MI. Protective effect of dietary polyphenol caffeic acid on ethylene glycol-induced kidney stones in rats. Urolithiasis. 2018 Apr;46(2):157–166.
- Fan QX, Gong SQ, Hong XZ, et al. Clinical-grade Garcinia cambogia extract dissolves calcium oxalate crystals in Drosophila kidney stone models. Eur Rev Med Pharmacol Sci. 2020 Jun;24(11):6434–6445.
- Huang F, Sun XY, Ouyang JM. Preparation and characterization of selenized Astragalus polysaccharide and its inhibitory effect on kidney stones. Mater Sci Eng C Mater Biol Appl. 2020 May;110:110732.
- Mlala S, Oyedeji AO, Gondwe M, et al. Its derivatives as bioactive agents. Molecules. 2019 Jul 29;24(15):15.
- Moran S, Myers B. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27(6):928–37.
- Shang W, Wang Z. The Update of NGAL in acute kidney Injury. Curr Protein Pept Sci. 2017;18(12):1211–1217.
- Shuo W, Peng D, Ning Z, et al. Oligomeric proanthocyanidins protect against HK-2 cell injury induced by oxalate and calcium oxalate monohydrate crystals. Urolithiasis. 2016;44(3):203–210. .
- Xu CG, Zhu XL, Wang W, et al. Ursolic acid inhibits epithelial-mesenchymal transition in vitro and in vivo. Pharm Biol. 2019;57(1):169–175. .
- Chen H, Fan Y, Jing H, et al. Emerging role of lncRNAs in renal fibrosis. Arch Biochem Biophys. 2020;692:108530.
- Fan Y, Chen H, Huang Z, et al. Emerging role of miRNAs in renal fibrosis. RNA Biol. 2020;17(1):1–12. .
- Yueyan L, Rui Z, Guofeng X, et al. Generation and characterization of a novel rat model of primary hyperoxaluria type 1 with a nonsense mutation in alanine-glyoxylate aminotransferase gene. Am J Physiol Renal Physiol. 2021. 320(3): F475-F484.
- Mengkui S, Wei Z, Fei Y, et al. MicroRNA-302b mitigates renal fibrosis via inhibiting TGF-β/Smad pathway activation. Braz J Med Biol Res = Rev Bras Pesqui Med Biol. 2021;54(3):e9206.
- Zhou Y, Chai P, Wang J, et al. Wingless/int-1 induced secreted protein-1: a new biomarker for renal fibrosis. J Biol Regul Homeost Agents. 2021;35(1):97-103.
- Agnieszka L, Milena D, Elzbieta P, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247. .
- Can AA, Mehmet T, Armagan H, et al. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol. 2014;71:116–121.
- Lin L, Yin Y, Hou G, et al. Ursolic acid attenuates cigarette smoke-induced emphysema in rats by regulating PERK and Nrf2 pathways. Pulm Pharmacol Ther. 2017;44:111–121.
- Chao W, Limin S, Chengyue Z, et al. Histone methyltransferase setd7 regulates Nrf2 signaling pathway by phenethyl isothiocyanate and ursolic acid in human prostate cancer cells. Mol Nutr Food Res. 2018;62(18):e1700840. .
- Shuai Z, Fan C, Qiliang Y, et al. Reactive oxygen species interact with NLRP3 inflammasomes and are involved in the inflammation of sepsis: from mechanism to treatment of progression. Front Physiol. 2020;11:571810.