ABSTRACT
Formononetin (FMNT), a flavonoid identified from the Chinese herb Astragalus membranaceus, possesses anti-inflammatory or anti-oxidative properties in different human diseases. This study aims to comprehensively elucidate the function of FMNT in atherosclerosis and its underlying mechanisms. Online public databases were used to identify the drug-disease targets. Protein–protein interaction (PPI), Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment were applied to explore the potential targets and signaling pathways involved in FMNT against atherosclerosis. Human umbilical vein endothelial cells (HUVECs) were exposed to oxidized low-density lipoprotein (ox-LDL) to construct an atherosclerosis cell model in vitro. Endothelial cell function was assessed via examining cell proliferation, inflammatory factors, oxidative markers, reactive oxygen species (ROS), and apoptosis. Western blot was performed to detect the expression of cyclooxygenase-2 (COX-2), endothelial nitric oxide synthase (eNOS), cleaved caspase-3, and peroxisome proliferator-activated receptor-γ (PPAR-γ). A total of 39 overlapping target genes of FMNT and atherosclerosis were identified. Through the PPI network analysis, 14 hub genes were screened and found to be closely relevant to inflammation, oxidative stress, and apoptosis. Results of KEGG pathway assays indicated that lots of targets were enriched in PPAR signaling. Functionally, FMNT could protect against ox-LDL-induced inflammatory reaction, oxidative stress, and apoptosis in HUVECs. Moreover, FMNT attenuated ox-LDL-mediated inactivation of PPAR-γ signaling. GW9662, a PPAR-γ antagonist, reversed the inhibitory effect of FMNT on ox-LDL-induced endothelial injury. In conclusion, FMNT alleviates ox-LDL-induced endothelial injury in HUVECs by stimulating PPAR-γ signaling, providing a theoretical basis for employing FMNT as a potential drug to combat atherosclerosis.
Abbreviations: FMNT: formononetin; PPI: protein–protein interaction; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes; HUVECs: human umbilical vein endothelial cells; ox-LDL: oxidized low-density lipoprotein; COX-2: cyclooxygenase-2; eNOS: endothelial nitric oxide synthase; PPAR-γ: peroxisome proliferator-activated receptor-γ; CVD: cardiovascular disease; TCM: traditional Chinese medicines; OGDR: oxygen-glucose deprivation/reoxygenation; ROS: reactive oxygen species; FBS: fetal bovine serum; CCK-8: cell counting kit-8; EdU: 5-Ethynyl-2ʹ-deoxyuridine; SOD: antioxidant enzymes superoxide dismutase; MDA: malondialdehyde; DCFH-DA: 2ʹ,7ʹ-dichlorofluorescein-diacetate; PVDF: polyvinylidene fluoride; ANOVA: one-way analysis of variance; PPARs: peroxisome proliferation-activated receptors
Introduction
Cardiovascular disease (CVD) is considered as a main reason for both death and premature death in China, accounting for 40% of mortality in the Chinese population [Citation1]. Atherosclerosis is a chronic inflammatory and lipid-depository disease within large and medium-sized arteries that can lead to CVD such as coronary artery disease, myocardial infarction, stroke, and peripheral artery disease [Citation2]. As an initial stage of atherosclerosis, dysfunction of vessel endothelium is able to trigger the initiation of plaques and formation of atherosclerotic lesions [Citation3]. Oxidative stress generation and inflammatory reaction activation are important risk factors contributing to endothelial dysfunction in the progression of atherosclerosis [Citation4]. The apoptosis of macrophages, smooth muscle cells, and endothelial cells is also found to be associated with atherosclerosis development [Citation5]. Despite various chemical drugs available for atherosclerosis treatment, some of them display serious adverse effects. Hence, it is very valuable to explore the molecular mechanisms behind endothelial dysfunction for developing effective therapeutic strategies to combat atherosclerosis.
Traditional Chinese medicines (TCM) have been widely applied for the prevention and treatment of atherosclerotic diseases due to their multi-targeted action and low toxicity [Citation6,Citation7]. Formononetin (FMNT), one of the flavonoids identified in the Chinese herb Astragalus membranaceus, displays a broad spectrum of physiological activities conducive to health via dependent and independent mechanisms of estrogen [Citation8]. FMNT is also found to possess anti-inflammatory or anti-oxidative properties in a variety of diseases, such as traumatic brain injury [Citation9], osteoarthritis [Citation10], acute kidney injury [Citation11], inflammatory bowel disease [Citation12], and neurodegenerative diseases [Citation13], via modulating different signaling pathways. Moreover, FMNT is confirmed to be associated with the pathogenesis of myocardial infarction. For instance, Cheng et al. showed that FMNT could alleviate oxygen-glucose deprivation/reoxygenation (OGDR)-induced rat cardiomyocytes injury by suppressing reactive oxygen species (ROS) generation and activating GSK-3β phosphorylation [Citation14]. Wang et al. also depicted that FMNT attenuated myocardial ischemia/reperfusion injury in rats via inactivating the ROS-TXNIP-NLRP3 pathway [Citation15]. To date, there are several documents reporting the improvement of FMNT on endothelial dysfunction [Citation16–18]. However, the action mechanism of FMNT involved in atherosclerosis via affecting endothelial function deserves further clarification.
Network pharmacology is a system-level method to perform medication research by elucidating the potentially complex interaction among diseases, drugs, genes, and protein targets based on system biology, bioinformatics, and high-throughput histology [Citation19]. Network pharmacology is widely used for the action mechanism research of TCM in diseases via screening to identify active ingredients, predicting potential targets, analyzing key pathways, and finally conducting experimental verification [Citation20].
The current study aims to explore the potential targets and pathways of FMNT against atherosclerosis by using a network pharmacology approach. On the other hand, ox-LDL-treated HUVECs were used as an in vitro atherosclerosis model to validate the functions and mechanisms of FMNT in regulating inflammatory response, oxidative stress, and apoptosis. Our findings reveal that FMNT alleviated ox-LDL-mediated inflammation, oxidative stress, and apoptosis at least partly by activating PPAR-γ signaling.
Materials and methods
Candidate targets collection
Target genes related to FMNT were gathered from TargetNet (https://targetnet.scbdd.com) [Citation21], SwissTargetPrediction (https://www.swisstargetprediction.ch/) [Citation22], STITCH (http://stitch.embl.de/) [Citation23], and PharmMpper (http://www.lilab-ecust.cn/pharmmapper/submitfile.html) [Citation24]. Arteriosclerosis-related gene screening was conducted by using OMIM (https://omim.org/) [Citation25], DisGeNET (http://www.disgenet.org/) [Citation26], DrugBank (https://www.drugbank.ca/) [Citation27], and GeneCards (https://www.genecards.org/) [Citation28]. The overlapping genes associated with both FMNT and arteriosclerosis were obtained using an online Venn diagram tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Protein–protein interaction (PPI) network
The 39 candidate targets were introduced into String database (https://string-db.org/) to establish the relationship between proteins. The TSV format was then downloaded and subjected to Cytoscape 3.7.2 software for the visualization and analysis of a PPI network. The genes with a degree greater than the average value (12.16) were screened as important targets for further analysis.
Biological function and pathway enrichment
The GO function annotation and KEGG pathway enrichment were investigated using an online OmicShare platform (http://www.omicshare.com/tools) with the ‘Homo sapiens’ setting. Ranked through P-value, the top 20 relevant biological processes and KEGG pathways (P < 0.05) were plotted as bubble charts with Bioinformatics platform (http://www.bioinformatics.com.cn/).
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin–streptomycin (Gibco) at 37°C. Medium was refreshed every other day unless stated otherwise. HUVECs were incubated 40 μg/mL of ox-LDL (Guangzhou Yiyuan Biotechnologies, Guangzhou, China) for 24 h to establish atherosclerosis cell models in vitro. A PPAR-γ antagonist GW9662 (MedChemExpress, Shanghai, China) was used to disturb PPAR-γ signaling.
Cell counting kit-8 (CCK-8) assay
HUVECs were cultured with various concentrations of FMNT (Bio-Chem Technology Co., Ltd., Shanghai, China; 0, 5, 10, 20, 40, 60, and 80 µM) for 24 h to confirm the optimal dose of FMNT for further assays. To examine the influence of FMNT on cell viability, FMNT (40 µM) was added to HUVECs for 24 h of incubation in the presence of ox-LDL (40 μg/mL). Afterward, cells were incubated with 10 µL CCK-8 solution (Dojindo, Kumamoto, Japan) at 37°C for 2 h. The optical density at 450 nm was determined to calculate the cell viability.
5-Ethynyl-2ʹ-deoxyuridine (EdU) assay
Cell proliferation ability was assessed with a Cell-LightTM EdU DNA Cell Proliferation Kit (RiboBio, Guangzhou, China). Briefly, HUVECs were inoculated into 6-well plates and treated as indicated above. The cells were then incubated with EdU, fixed in paraformaldehyde, and stained with DAPI. The EdU-positive cells were calculated from five randomly selected fields.
Detection of inflammatory cytokines
Commercial ELISA kits (R&D Systems Inc., Minneapolis, MN, USA) were applied to evaluate the levels of TNF-α and IL-1β in the culture supernatant.
Oxidative markers examination
The antioxidant enzymes superoxide dismutase (SOD) activity and malondialdehyde (MDA) content in the culture supernatant were measured with commercial assay kits (Abcam, Cambridge, MA, USA) pursuant to the manufacturer’s guideline.
Intracellular ROS measurement
The intracellular ROS level was examined by using 2ʹ,7ʹ-dichlorofluorescein-diacetate (DCFH-DA, Beyotime, Shanghai, China). In brief, HUVECs were placed into 6-well plates and maintained overnight for cell attachment.
After the treatment, 10 μM of DCFH-DA was added to the cells for 30-min incubation in the dark. The fluorescence intensity was detected using FLUOstar Omega Reader (BMG Labtech GmbH, Ortenberg, Germany).
Flow cytometry assay
Apoptotic cells were double-stained with Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Western blot assay
Total protein extraction was performed using RIPA buffer (Sigma–Aldrich). After separation on 10% SDS-PAGE gels, proteins were blotted into polyvinylidene fluoride (PVDF) membranes (GE Healthcare Corp, Piscataway, NJ, USA). The membranes were then probed with the following primary antibodies including COX-2 (1:500; ab62331), eNOS (1:500; ab76198), cleaved caspase-3 (1:500; ab32042), PPAR-γ (1:500; ab59256), and GAPDH (1:2000; ab181602) overnight at 4°C. The immunoactive blots were visualized by ELC detection reagents (Millipore, Billerica, MA, USA). All primary antibodies were purchased from Abcam (Cambridge, MA, USA).
Statistics
All experimental values are presented as mean ± SD. One-way analysis of variance (ANOVA) coupled with Dunnett’s multiple comparison or Tukey–Kramer post hoc test was used for comparison among groups. P < 0.05 indicates a statistically significant difference. GraphPad Prism 7.0 (GraphPad software, La Jolla, CA, USA) was applied for all statistical analysis.
Results
The protective effects of FMNT against atherosclerosis and possible action mechanisms have not been comprehensively elucidated. Here, we used a network pharmacology approach to predict the candidate targets and potential pathways of FMNT involved in atherosclerosis. Ox-LDL-induced HUVECs were applied as a cell model to validate the role and mechanism of FMNT in regulating atherosclerosis.
Screening of candidate targets
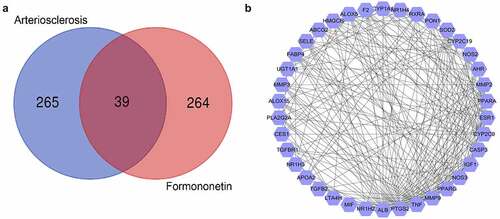
According to OMIM, DisGeNET, DrugBank, and GeneCards databases, 304 genes were found to be associated with arteriosclerosis. A total of 303 FMNT-related targets were collected based on TargetNet, SwissTargetPrediction, STITCH, and PharmMpper databases. As shown in the intersection by Venn diagram, 39 drug-disease common targets were identified ()).
Interactional network analysis of targets
Subsequently, the PPI network was established by inputting the 39 overlapping genes into String database and analyzed with Cytoscape. The degree value was used as an index to reveal their contribution to FMNT against arteriosclerosis. This network comprises 38 nodes and 338 edges. ALB, PTGS2, TNF, MMP9, PPARG, NOS3, CASP3, IGF1, CYP2C9, ESR1, AHR, PPARA, MMP2, and NOS2 with degree value greater the average value (12.16) were identified as hub genes ()). The higher the degree is, the more biological importance the target has. Hence, these genes might be the main targets by which FMNT affects the progression of atherosclerosis.
Biological function and pathway enrichment
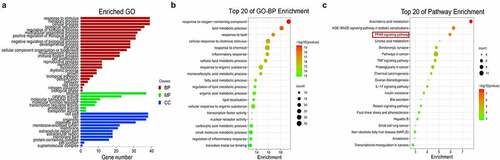
GO function annotation and KEGG pathway enrichment of the 39 overlapping genes were conducted with the OmicShare platform. As shown in ), there were 26 biological process (BP)-, 7 molecular function (MF)-, and 12 cellular component (CC)-related GO terms. As described in the bubble chart, the main BP involved in FMNT against atherosclerosis was response to oxygen-containing compounds, lipid metabolic process, response to lipids, cellular response to chemical stimulus, response to chemical, inflammatory response, and so on ()). The KEGG pathway enrichment manifested that many targets were enriched in the AGE-RAGE, PPAR, TNF, and IL-17 signaling pathways ()). The detailed information for the top 20 relevant BP and KEGG pathway is presented in Supplemental Table 1 and Table 2. Peroxisome proliferation-activated receptors (PPARs) process therapeutic potential for cardiovascular disorders, and PPAR agonists are currently undergoing pre-clinical and clinical studies [Citation29]. The PPI network also showed that PPARG was among the top seven key genes involved in FMNT against atherosclerosis. Thus, FMNT might affect atherosclerosis through regulating PPAR-γ signaling.
Figure 2. Biological function and pathway assays. (a) GO second class enrichment assay of 39 potential targets for FMNT against atherosclerosis. (b) Bubble chart showing the top 20 biological processes (BP) of GO terms. (c) Bubble chart of top 20 signaling pathways linked to FMNT against atherosclerosis
FMNT attenuates ox-LDL-triggered proliferation suppression in HUVECs
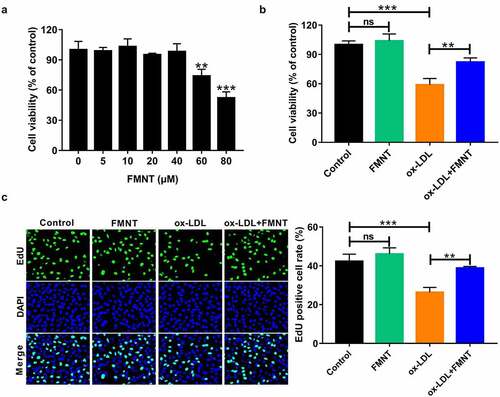
First, the impact of different concentrations of FMNT on cell viability was determined. No significant change in cell viability was observed at 5–40 μM of FMNT, while 60 or 80 μM FMNT significantly repressed cell viability ()). Thus, 40 μM FMNT was selected for further function experiments. In order to elucidate the pharmacological function of FMNT against atherosclerosis, ox-LDL-treated HCMECs were used as the cell model of atherosclerosis. Cells were stimulated with ox-LDL with or without 40 μM FMNT. CCK-8 assays found that the ox-LDL-induced decrease in cell viability was effectively attenuated after treatment with FMNT ()). Consistently, EdU assays showed that the proliferation suppression mediated by ox-LDL was reversed due to FMNT intervention ()). Together, FMNT could protect HUVECs from ox-LDL-induced viability inhibition.
Figure 3. FMNT attenuates ox-LDL-induced viability inhibition in HUVECs. (a) HUVECs were treated with designated concentrations of FMNT for 24 h, followed cell viability determination. (b and c) HUVECs were treated with 40 μg/mL of ox-LDL for 24 h with or without 40 µM FMNT, followed by CCK-8 assay of cell viability (b) and EdU assay of cell proliferation (c). **P < 0.01, ***P < 0.001
FMNT abates ox-LDL-mediated inflammation in HUVECs
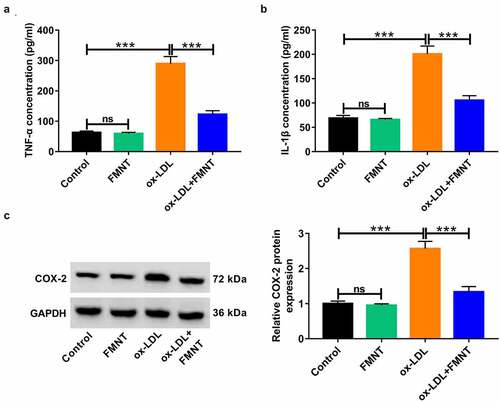
Subsequently, the influence of FMNT on inflammatory response in ox-LDL-treated HUVECs was analyzed. Ox-LDL stimulation led to the increase of TNF-α and IL-1β level in culture supernatant, while this effect was greatly abated due to FMNT treatment (). A Western blot was applied to detect whether FMNT could affect key target PTGS2/COX-2. Ox-LDL administration resulted in an increase of COX-2 protein expression, but this effect was weakened in the presence of FMNT ()). Collectively, FMNT could attenuate ox-LDL-mediated inflammation in HUVECs.
Figure 4. FMNT alleviates ox-LDL-induced inflammatory response in HUVECs. HUVECs were exposed to 40 μg/mL of ox-LDL for 24 h in the presence or absence of 40 µM FMNT. (a and b) The TNF-α and IL-1β levels in culture supernatant were determined by corresponding ELISA kits. (c) Western blot assays of COX-2 protein expression in HUVECs. ***P < 0.001
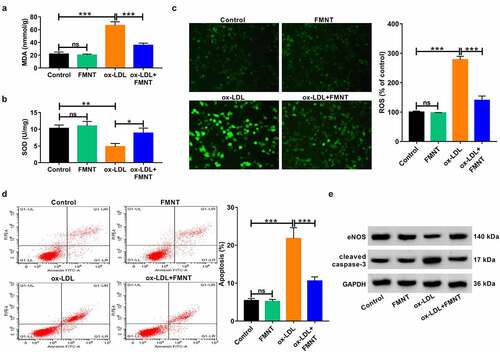
FMNT weakens ox-LDL-mediated oxidative stress and apoptosis in HUVECs
We further observed the oxidative stress and apoptosis in ox-LDL-treated HUVECs under FMNT treatment. Ox-LDL treatment increased MDA content and lowered SOD activity in culture supernatant, while these changes were significantly eliminated in the presence of FMNT (). Moreover, the ox-LDL-induced ROS increase was significantly abrogated following FMNT treatment ()). Flow cytometry assays revealed that FMNT could reverse ox-LDL-induced apoptosis of HUVECs ()). NOS3 and CASP3 were also identified as key genes involved in FMNT against atherosclerosis. Hence, the protein levels of eNOS and cleaved caspase-3 expression were correspondingly determined. The results revealed that ox-LDL reduced eNOS protein expression and enhanced cleaved caspase-3 protein level, but these changes were counteracted by FMNT ()). These data indicated that FMNT suppressed ox-LDL-induced oxidative stress and apoptosis in HUVECs.
Figure 5. FMNT relieves oxidative stress and apoptosis in ox-LDL-treated HUVECs. HUVECs were stimulated with 40 μg/mL of ox-LDL for 24 h with or without 40 µM FMNT. (a and b) The MDA content and SOD activity in culture supernatant were measured with corresponding assay kits. (c) Intracellular ROS generation was examined by DCFH-DA staining. (d) Flow cytometry assays of apoptotic rate. (e) Western blot assays of eNOS and cleaved caspase-3 expression. *P < 0.05, **P < 0.01, ***P < 0.001
FMNT alleviates ox-LDL-induced cytotoxicity in HUVECs by activating PPAR-γ signaling
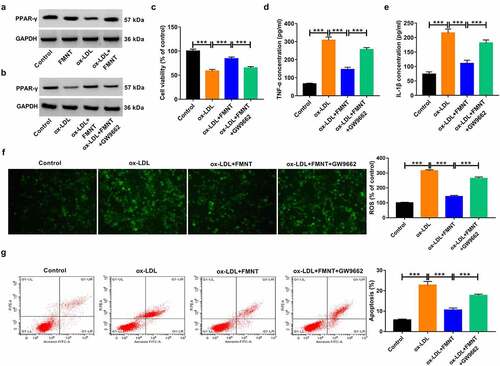
Subsequently, we clarified whether PPAR-γ signaling was implicated in the inhibitory effects of FMNT on ox-LDL-induced endothelial injury. Ox-LDL suppressed the expression of PPAR-γ, whereas this effect was reversed by FMNT treatment ()), suggesting that FMNT could activate PPAR-γ signaling in ox-LDL-treated HUVECs. The HUVECs were then pretreated with a PPAR-γ antagonist (GW9662, 5 μM) before treatment with ox-LDL and FMNT. The results showed that pretreatment with GW9662 attenuated the promotive effect of FMNT on PPAR-γ expression in ox-LDL-treated HUVECs ()). CCK-8 assays found that the viability improvement mediated by FMNT in ox-LDL-stimulated HUVECs was abrogated due to GW9662 treatment ()). Moreover, the suppressive effects of FMNT on TNF-α and IL-1β level were impaired by GW9662 (). In addition, treatment with GW9662 counteracted the inhibitory action of FMNT on ROS production in ox-LDL-treated HUVECs ()). Furthermore, the anti-apoptotic role induced by FMNT in ox-LDL-treated HUVECs was weakened due to the use of GW9662 ()). Hence, it was concluded that FMNT mitigated the ox-LDL-induced cytotoxicity in HUVECs by stimulating PPAR-γ signaling.
Figure 6. Inactivation of PPAR-γ signaling reversed the effects of FMNT on ox-LDL-induced endothelial injury. (a) Western blot assay was applied to evaluate the effect of FMNT on PPAR-γ expression in ox-LDL-treated HUVECs. (b-g) PPAR-γ antagonist (GW9662, 5 μM) was used to block PPAR-γ signaling in HUVECs prior to ox-LDL and FMNT treatment, followed by assays of PPAR-γ protein expression (b), cell viability (c), TNF-α and IL-1β level in culture supernatant (d and e), intracellular ROS level (f) and apoptotic rate (g). ***P < 0.001
Discussion
Atherosclerosis remains the most important pathological basis of vascular disease [Citation30]. Cigarette smoking, hypertension, blood cholesterol, and diabetes mellitus are demonstrated as major risk factors associated with atherosclerosis [Citation31]. Endothelial dysfunction is reported to be involved in the initiation and progression of atherosclerosis [Citation32]. Thus, improving endothelial function might be an effective strategy for atherosclerosis treatment.
TCM has been used for the prevention and treatment of atherosclerosis for a long time due to its smaller side effects and higher efficacy [Citation33]. Network pharmacology is employed for TCM discovery and research by establishing a ‘drug–gene–disease’ interaction network with a holistic view [Citation34]. A recent document disclosed that FMNT improved endothelial function and enhances angiogenesis in vitro and in vivo by stimulating Erk1/2-and Akt-mediated eNOS/NO signaling pathway [Citation16]. In addition, Ma et al. illustrated that FMNT mitigated the progression of atherosclerosis in apoE−/- mice by regulating the interplay between KLF4 and SRA [Citation17]. Liang et al. discovered that FMNT exhibited a protective role in HUVECs by increasing cell proliferation and migration via promoting IGF-1 and ICAM-1 expression [Citation18]. Herein, we found that FMNT alleviated ox-LDL-induced inflammatory response, oxidative stress, and apoptosis in HUVECs via activating PPAR-γ signaling based on a network pharmacology method (). A recent document revealed that FMNT could protect against liver injury possibly by regulating inflammatory molecular pathways via network pharmacology combined with biochemical determination [Citation35].
Figure 7. A flow chart using a network pharmacology method to illustrate that FMNT alleviates ox-LDL-induced endothelial injury in HUVECs via activating PPAR-γ signaling
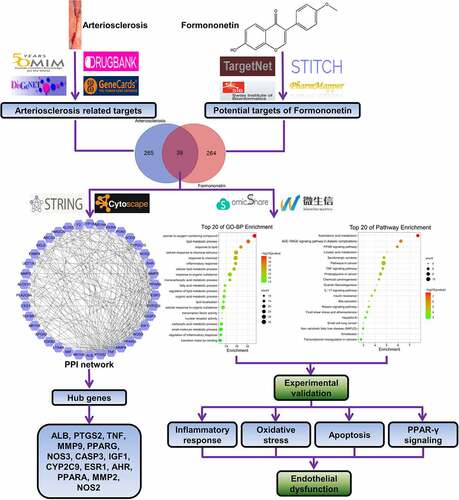
According to the public databases, a total of 39 overlapping genes were predicted to be related to arteriosclerosis and FMNT. By constructing and analyzing the PPI network of these genes with String and Cytoscape, ALB, PTGS2, TNF, MMP9, PPARG, NOS3, CASP3, IGF1, CYP2C9, ESR1, AHR, PPARA, MMP2, and NOS2 were identified as key genes involved in FMNT against arteriosclerosis. HUVECs have been extensively used as an experimental model to investigate cardiovascular disease [Citation36] and ox-LDL is involved in atherosclerosis via acting on various cells including endothelial cells [Citation37]. Hence, ox-LDL-treated HUVECs were employed as a cell model of arteriosclerosis for further experimental validation.
The onset of inflammation and the generation of ROS destruct the function of endothelial cells and are a dominant factor in CVD progression [Citation38]. As we have known, a critical event in the pathogenesis of atherosclerosis is inflammatory reaction, and drug discovery based on anti-inflammatory strategy has gained extensive attentions in atherosclerotic disease [Citation39]. Oxidative stress triggered by superabundant ROS is regarded as a critical, final general mechanism in atherosclerosis [Citation40]. Inflammatory response and oxidative stress are able to induce endothelial cell apoptosis, finally causing the dysregulation of lipid homeostasis and immunity [Citation41]. Here, we found that FMNT attenuated ox-LDL-induced inflammation, oxidative stress, and apoptosis in HUVECs via regulation of COX-2, eNOS, and cleaved caspase-3 expression.
PPARs are ligand-inducible transcription factors and consist of three members: PPAR-α, PPAR-β/δ, and PPAR-γ. As reported, PPARs participate in the pathogenesis of metabolic diseases, including metabolic syndrome, type 2 diabetes mellitus, nonalcoholic fatty liver disease, and cardiovascular disease, by modulating a variety of genes [Citation42]. PPAR-γ agonists have the potential to attenuate inflammation processes, lower oxidative stress, improve endothelial function, and affect lipid metabolism [Citation29]. There have been many documents elucidating the involvement of PPAR-γ signaling in endothelial dysfunction. For instance, Mukohda et al. reported that PPAR-γ could combat against IL-1β-mediated endothelial dysfunction by suppressing oxidative stress responses [Citation43]. Zhang et al. found that inhibition of vascular PPAR-γ aggravated oxidative stress and inflammation in high-fat-diet-induced obese mice exposed to chronic intermittent hypoxia [Citation44]. Jin et al. disclosed that interference with endothelial PPAR-γ accelerated thrombosis by enhancing leukocyte recruitment to the vessel wall via increasing P-selectin expression [Citation45]. In the current study, we found that FMNT could activate PPAR-γ signaling in ox-LDL-treated HUVECs. Moreover, FMNT-mediated anti-inflammatory, anti-oxidative, and anti-apoptotic effects in ox-LDL-treated HUVECs were reversed by inhibition of PPAR-γ signaling, indicating that FMNT protected against ox-LDL-mediated cytotoxicity in HUVECs by activating PPAR-γ signaling. Our study used an in vitro cell model to elucidate the potential of FMNT against endothelial inflammation, oxidative stress, and apoptosis. However, animal models in vivo are warranted to further verify the function and mechanism of FMNT in regulating atherosclerosis.
Conclusion
In summary, our study identified the key targets and possible pathways of FMNT against atherosclerosis using network pharmacology method. In vitro experiments were performed by using ox-LDL-induced HUVECs to confirm the mechanism by which FMNT regulates atherosclerosis. Our findings show that FMNT alleviates ox-LDL-mediated inflammation reaction, oxidative stress, and apoptosis in HUVECs by activating PPAR-γ signaling, contributing to a more profound understanding of the action mechanism by which FMNT protects against atherosclerosis and providing a promising alternative treatment strategy for atherosclerosis.
Highlights
FMNT alleviates ox-LDL-induced inflammation in HUVECs.
FMNT attenuates ox-LDL-induced oxidative stress and apoptosis in HUVECs.
FMNT activates PPAR-γ signaling in ox-LDL-treated HUVECs.
FMNT inhibits ox-LDL-induced cytotoxicity in HUVECs through activating PPAR-γ signaling.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
References
- Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–212.
- Kobiyama K, Ley K. Atherosclerosis. Circ Res. 2018;123:1118–1120.
- Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73:924–967.
- Gimbrone MA Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636.
- Paone S, Baxter AA, Hulett MD, et al. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell Mol Life Sci. 2019;76:1093–1106.
- Wang C, Niimi M, Watanabe T, et al. Treatment of atherosclerosis by traditional Chinese medicine: questions and quandaries. Atherosclerosis. 2018;277:136–144.
- Guo J, Chen W, Bao B, et al. Protective effect of berberine against LPS-induced endothelial cell injury via the JNK signaling pathway and autophagic mechanisms. Bioengineered. 2021;12:1324–1337.
- Machado Dutra J, Espitia PJP, Andrade Batista R. Formononetin: biological effects and uses - A review. Food Chem. 2021;359:129975.
- Li Z, Zeng G, Zheng X, et al. Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomed Pharmacother. 2018;106:349–354.
- Cho IA, Kim TH, Lim H, et al. Formononetin antagonizes the interleukin-1β-induced catabolic effects through suppressing inflammation in primary rat chondrocytes. Inflammation. 2019;42:1426–1440.
- Aladaileh SH, Hussein OE, Abukhalil MH. Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel). 2019;8:430.
- Wu D, Wu K, Zhu Q, et al. Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting NLRP3 inflammasome signaling pathway. Mediators Inflamm. 2018;2018:3048532.
- Sugimoto M, Ko R, Goshima H, et al. Formononetin attenuates H2O2-induced cell death through decreasing ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells. Neurotoxicology. 2021;85:186–200.
- Cheng Y, Xia Z, Han Y, et al. Plant natural product formononetin protects rat cardiomyocyte H9c2 cells against oxygen glucose deprivation and reoxygenation via inhibiting ros formation and promoting GSK-3β phosphorylation. Oxid Med Cell Longev. 2016;2016:2060874.
- Wang DS, Yan LY, Yang DZ, et al. Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem Biophys Res Commun. 2020;525:759–766.
- Wu J, Kong M, Lou Y, et al. Simultaneous activation of Erk1/2 and Akt signaling is critical for formononetin-induced promotion of endothelial function. Front Pharmacol. 2020;11:608518.
- Ma C, Xia R, Yang S, et al. Formononetin attenuates atherosclerosis via regulating interaction between KLF4 and SRA in apoE(-/-) mice. Theranostics. 2020;10:1090–1106.
- Liang C, Zhou A, Sui C, et al. The effect of formononetin on the proliferation and migration of human umbilical vein endothelial cells and its mechanism. Biomed Pharmacother. 2019;111:86–90.
- Boezio B, Audouze K, Ducrot P, et al. Network-based approaches in pharmacology. Mol Inform. 2017;36:1700048.
- Wang X, Wang ZY, Zheng JH, et al. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19:1–11.
- Yao ZJ, Dong J, Che YJ, et al. TargetNet: a web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Comput Aided Mol Des. 2016;30:413–424.
- Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–W364.
- Szklarczyk D, Santos A, von Mering C, et al. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016;44:D380–D384.
- Wang X, Shen Y, Wang S, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45(W1):W356–W360.
- Amberger JS, Bocchini CA, Schiettecatte F, et al. OMIM.org: online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–D98.
- Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–D855.
- Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082.
- Safran M, Dalah I, Alexander J, et al. GeneCards Version 3: the human gene integrator. Database (Oxford). 2010;2010:baq020.
- Ivanova EA, Myasoedova VA, Melnichenko AA, et al. Peroxisome Proliferator-Activated Receptor (PPAR) gamma agonists as therapeutic agents for cardiovascular disorders: focus on atherosclerosis. Curr Pharm Des. 2017;23:1119–1124.
- Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546.
- Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56.
- Little PJ, Askew CD, Xu S, et al. Endothelial dysfunction and cardiovascular disease: history and analysis of the clinical utility of the relationship. Biomedicines. 2021;9:699.
- Li TT, Wang ZB, Li Y, et al. The mechanisms of traditional Chinese medicine underlying the prevention and treatment of atherosclerosis. Chin J Nat Med. 2019;17:401–412.
- Luo TT, Lu Y, Yan SK, et al. Network pharmacology in Research of Chinese Medicine formula: methodology, application and prospective. Chin J Integr Med. 2020;26:72–80.
- Liao L, Huang L, Wei X, et al. Bioinformatic and biochemical studies of formononetin against liver injure. Life Sci. 2021;272:119229.
- Medina-Leyte DJ, Domínguez-Pérez M, Mercado I, et al. Use of human umbilical vein endothelial cells (HUVEC) as a model to study cardiovascular disease: a review. Appl Sci. 2020;10:938.
- Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786.
- Haybar H, Shahrabi S, Rezaeeyan H, et al. Endothelial cells: from dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc Toxicol. 2019;19:13–22.
- Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond). 2018;132:1243–1252.
- Kattoor AJ, Pothineni NVK, Palagiri D, et al. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42.
- Choy JC, Granville DJ, Hunt DW, et al. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33:1673–1690.
- Han L, Shen WJ, Bittner S, et al. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017;13:279–296.
- Mukohda M, Stump M, Ketsawatsomkron P, et al. Endothelial PPAR-γ provides vascular protection from IL-1β-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2016;310:H39–48.
- Zhang Y, Zhang C, Li H, et al. Down-regulation of vascular PPAR-γ contributes to endothelial dysfunction in high-fat diet-induced obese mice exposed to chronic intermittent hypoxia. Biochem Biophys Res Commun. 2017;492:243–248.
- Jin H, Gebska MA, Blokhin IO, et al. Endothelial PPAR-γ protects against vascular thrombosis by downregulating P-selectin expression. Arterioscler Thromb Vasc Biol. 2015;35:838–844.