?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Tracking enzyme, substrate, and surfactant interactions to reach maximum reducing sugar production during enzymatic hydrolysis of plant biomass may provide a better understanding of factors that limit the lignocellulosic material degradation in native rice straw. In this study, enzymes (Cellic Ctec2 cellulase and Cellic Htec2 xylanase) and Triton X-100 (surfactant) were used as biocatalysts for cellulose and xylan degradation and as a lignin blocking agent, respectively. The response surface model (R2 = 0.99 and R2-adj = 0.97) indicated that Cellic Ctec2 cellulase (p < 0.0001) had significant impacts on reducing sugar production, whereas Cellic Htec2 xylanase and Triton X-100 had insignificant impacts on sugar yield. Although FTIR analysis suggested binding of Triton X-100 to lignin surfaces, the morphological observation by SEM revealed similar surface features (i.e., smooth surfaces with some pores) of rice straw irrespective of Triton X-100. The reducing sugar yields from substrate hydrolysis with or without the surfactant were comparable, suggesting similar exposure of polysaccharides accessible to the enzymes. The model analysis and chemical and structural evidence suggest that there would be no positive effects on enzymatic hydrolysis by blocking lignins with Triton X-100 if high lignin coverage exists in the substrate due to the limited availability of hydrolyzable polysaccharides.
1 Introduction
Excessive use of fossil fuels for transportation has created several global environmental degradation effects. The high level of carbon dioxide emissions to the atmosphere has resulted in global warming and climate changes [Citation1]. These severe matters lead to the transition from fossil-based fuel products to the utilization of lignocellulose (or plant biomass)-based material to produce biofuels and other bio-based products [Citation2]. This is of more significant potential because lignocellulosic biofuel production appears to be a zero-net carbon emission process, thus representing a green platform for biofuel production and gaining long-term environmental benefits [Citation2,Citation3].
Rice straw represents a potential lignocellulosic feedstock for biofuel production. It is readily available, under-utilized, non-edible, and present in abundance in several countries [Citation4]. The polysaccharides in rice straw (specifically in plant cell walls) can be converted into simple sugar, including glucose, xylose, galactose, and arabinose, for microbial fermentation to yield biofuel [Citation5]. These sugars are formed as structural polymers (i.e., cellulose and hemicellulose) to support the plant’s growth and strength [Citation6]. Moreover, the structural polymers are covered with phenolic polymer lignin that helps prevent microbial attack and degradation. Therefore, converting polysaccharides in lignocellulosic material to sugar for biofuel production remains a challenge [Citation6,Citation7].
To date, several pretreatment methods, including physical, chemical, physicochemical, and biological approaches, are developed to reduce biomass recalcitrance and improve enzymatic hydrolysis [Citation8,Citation9]. However, chemical composition, heterogeneity, and structural complexity vary among plant species; therefore, no pretreatment is suitable for all lignocellulosic biomass. In addition, pretreatments employ high chemical input and temperature, resulting in high total pretreatment costs and extensive waste treatment [Citation10,Citation11]. Moreover, a high amount of sugar and/or lignin degradation products are formed during pretreatment, leading to sugar loss and inhibitory effects on microorganisms and/or enzymes [Citation8].
Efficient enzymatic hydrolysis is an appropriate means to convert polysaccharides in plant cell walls to sugar. It appears to be a rate-limiting step for lignocellulose-based biofuel and biochemical production [Citation6]. It is known that the effectiveness of enzymatic hydrolysis of polysaccharides is severely affected by biomass property and structure [Citation12]. These factors include a degree of crystallinity, porosity and polymerization, pore and particle size, a degree of substitution of hemicellulosic components, and the presence of phenolic polymer lignin on the substrates [Citation7]. Several studies have shown that lignin appears to be a significant physical barrier to enzymes and limits the accessibility of enzymes to target substrates [Citation13,Citation14].
Moreover, lignin can adsorb enzymes, resulting in their nonproductive binding [Citation14–17]. For example, β-glucosidases and endoglucanases, the key enzymes for cellulose hydrolysis, completely lost their activities in the presence of organosolv lignin from corn stover [Citation18]. Cellobiohydrolases are quickly inactivated (45.5%) by lignin adsorption during hydrolysis of ammonia-pretreated corn stover [Citation19]. The enzyme-lignin adsorption is governed by hydrophobic interactions, electrostatic interactions, and hydrogen bonding forces, where hydrophobic interactions appear to play a vital role in adsorption [Citation13,Citation20].
Addition of additives, including non-enzymatic proteins (e.g., bovine serum albumin, casein) [Citation21,Citation22], polymers (e.g., polyethylene glycol (PEG)) [Citation23,Citation24], and nonionic surfactants (e.g., Tween 20 and Triton X-100) [Citation25–27], to enzymatic hydrolysis of lignocellulosic material, has been reported to reduce adverse effects of lignin. Surfactants are of interest here due to their low cost and the fact that they maintain enzyme stability during hydrolysis. The enzymes can be recycled for several rounds [Citation28]. Triton X-100, an amphiphilic nonionic surfactant, contains hydrophilic heads and hydrophobic tails. It has been shown that surfactants can interact with lignin through hydrophobic interaction between its hydrophobic tails and the hydrophobic side groups (e.g., phenyl, CH2, and CH3) of lignin; meanwhile, the hydrophilic heads hydrate the substrates [Citation27]. Thus, lignin blocking with a surfactant can reduce the nonproductive binding of enzymes on lignin and activate enzyme desorption [Citation29,Citation30]. On the contrary, findings of some studies have revealed that the addition of surfactants could reduce enzymatic efficiency and showed no beneficial effects on enzymatic hydrolysis because surfactants can cause enzyme denaturation or compete with enzymes for cellulose-binding sites [Citation23,Citation31].
Moreover, different kinds of substrates (e.g., pure cellulose or lignocellulosic material) and hydrolysis conditions have resulted in different responses to enzymatic activity and surfactant features [Citation27,Citation31]. The mechanism of surfactants in promoting or inhibiting enzymatic hydrolysis has been controversial. Therefore, a clear explanation of the functional roles of surfactants in the enzymatic hydrolysis of certain substrates is thus needed.
In this study, three variables affecting the hydrolysis of untreated rice straw were examined using the response surface methodology technique. These factors were Cellic Ctec2 cellulase, Cellic Htec2 hemicellulase cocktails, and nonionic surfactant Triton X-100. The commercially available Cellic Ctec2 cellulase and Cellic Htec2 xylanase preparations were used because these enzymatic cocktails are formulated to produce second-generation lignocellulosic ethanol. These enzymatic cocktails contain cellulases and hemicellulases with a wide range of abilities to hydrolyze lignocellulosic biomass [Citation32]. Triton X-100 was used because of its good lignin-binding ability [Citation27,Citation28,Citation30,Citation33]. Box-Behnken design and ANOVA analysis were used to create an experimental matrix, analyze and optimize models, respectively. The Cellic Ctec2 cellulase was combined with the Cellic Htec2 xylanase to reduce the effects of soluble hemicellulose on cellulose hydrolysis. At the same time, Triton X-100 was added to the enzymatic reaction as it is supposed to bind lignin present in untreated rice straw. Our model predicted the critical roles of variables, including enzyme, substrate, and surfactant, in the hydrolysis of untreated rice straw and explained the interaction among them. This study extends knowledge of biomass degradation to the next development level of lignocellulosic biofuel production.
2 Material and methods
2.1 Materials, enzymes, and chemicals
Rice straw (Oryza sativa) was collected from rice fields in Ayutthaya Province of Thailand. It was air-dried and cut into smaller pieces (1–2 cm) using scissors and ground with a kitchen blender. The chemical composition of rice straw used in the present study was previously determined [Citation29], and it contains 30.0% glucan, 10.4% xylan, and 23.3% lignin [Citation34]. Triton X-100 was purchased from Sigma-Aldrich. All chemicals used were analytical grade.
The commercial cellulase (Cellic Ctec2) and xylanase (Cellic Htec2) preparations, having enzymatic activities of 148 filter paper units (FPU)/mL and 1040 xylanase units (XU)/mL, respectively, were purchased from Novozymes (Bagsværd, Denmark). The enzymatic cocktails were dialyzed against buffer before use to remove sugar and additives.
2.2 Design of experiment and optimization
Box-Behnken was used to design a matrix to study the combined effects of variables affecting the hydrolysis of untreated rice straw. These variables included two enzyme compartments (Cellic Ctec2 and Cellic Htec2) and one surfactant Triton X-100. The optimum levels of those variables were identified for maximizing reducing sugar production. The three variables were examined at three levels: low (−1), central (0), and high (+1) to gauge the variability in the measurements (). The designed matrix comprised 17 experimental runs, including five replicates at the center points (). Each experimental run was repeated four times, and the average reducing sugar released was opted for the response values for the combination of independent variables.
Table 1. Factors and their levels for Box-Behnken design
Table 2. Box-Behnken design matrix with actual and coded values (in parenthesis), and the response and predict values of reducing sugar from the hydrolysis of untreated rice straw
The obtained experimental response values were used to construct a second-order polynomial model based on Equation (1), where ‘Y’ is the predicted response, β0 is constant, βi represents the linear coefficient, βii implies the coefficient of the squared terms, βij expresses the coefficient of the cross-term products. In contrast, xi and xj are the independent variables.
The experimental responses were analyzed and used to generate a model using JMP version 13 (SAS Institute Inc., USA). The statistical significance level of the model was assessed through an analysis of variance (ANOVA, p < 0.05). The regression coefficients were evaluated from the standardized effects based on Student’s t test (p < 0.05). The quality of fit of the polynomial model equations was evaluated by the coefficient of determination (R2), the adjusted coefficient of determination (R2-adj), and the lack-of-fit F-test.
2.3 Enzymatic hydrolysis
The enzymatic hydrolysis reactions were prepared in a 1.5 mL microcentrifuge tube. Each tube contained 5% (w/v) of untreated rice straw in 0.05 M sodium acetate buffer saline (ABS) at pH 5.5. The commercial enzymes Cellic Ctec2 and Cellic Htec2 and Triton X-100 were added at different dosages as shown in or stated otherwise. The reaction mixture was then added with distilled water to a final total volume of 200 µL. The reactions were then incubated at 55°C in an incubator with a shaking speed of 150 rpm for 24 h to reach the maximal sugar release. After being centrifuged at 10,000 rpm and 4°C for 15 min, the supernatant (100 µL) was moved to a new microcentrifuge tube. The amount of reducing sugar released from the substrate was determined by the 3, 5-dinitrosalicylic acid (DNS) reagent method (Miller, 1959), using glucose as a standard. The absorbance of samples was measured at 540 nm using a Thermo Scientific™ Multiskan™ GO Microplate spectrophotometer (ThermoFisher Scientific, USA).
2.4 Thin-layer chromatography (TLC) analysis
TLC analyzed the hydrolysis product in the supernatant. The samples (8 µL) were spotted on a silica gel 60 F245 plate and dried. The plate was placed in the TLC chamber with a mobile phase containing n-butanol, acetic acid, and water (2:1:1). After that, the plate was dried and sprayed with visualizing agents (4 g of α-diphenylamine, 4 mL of aniline, 200 mL of acetone, and 30 mL of 80% phosphoric acid) to detect sugars. The sugar spots were developed by heating the plate at 95°C for 10–15 min.
2.5 Structural analysis
A scanning electron microscope (A JEOL JSM-6610LV, Tokyo, Japan) was used to observe the morphology of samples in the presence or absence of Triton X-100. The surface imaging of the sample was conducted under low vacuum or variable pressure modes at room temperature, allowing us to observe the non-conductive samples without gold coating.
Fourier transform infrared (FTIR) spectrometry was used to analyze functional groups of samples. The FTIR spectra of the samples were run on a Perkin-Elmer UATR Two (Waltham, MA, USA) spectrometer.
3 Results and discussion
3.1 Effects of variables on reducing sugar release from untreated rice straw
In this study, the Box-Behnken matrix design was used to create enzymatic hydrolysis conditions to identify the influence of individual variables on the release of reducing sugar (response values) and understand the relationship between the independent variables (i.e., Cellic Ctec2, Cellic Htec2, and Triton X-100). The ranges of enzyme and surfactant loads (per gram rice straw) were chosen according to our preliminary study and literature (). These ranges were previously studied with both untreated and pretreated biomasses [Citation17,Citation35–39].
Seventeen experimental runs were conducted according to the Box-Behnken design (). The reducing sugar release was a function of the interactions among the three independent variables. Under the conditions studied, the release of reducing sugar at 24 h ranged from 0.83 to 2.92 mg/mL, corresponding to 0.02 to 0.10 g/g substrate. The maximum reducing sugar yields were obtained with run no. 15, where the highest amount of Cellic Ctec2 (50 FPU/g) was loaded with a moderate amount of Cellic Htec2 (55 XU/g) and a small amount of Triton X-100. The lowest sugar release was obtained with run no. 2, where 5 FPU/g of Cellic Ctec2, 55 XU/g of Cellic Htec2, and 0.15 g/g of Triton X-100 were loaded together ().
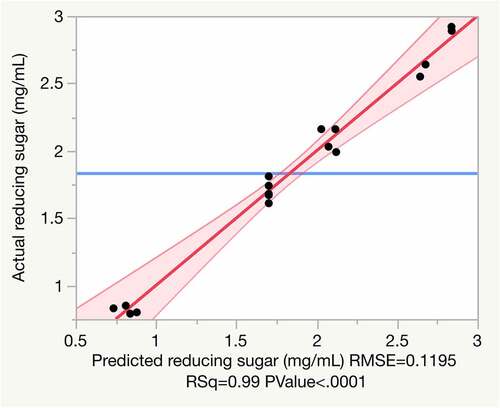
The regression model was established to understand the relationship of variables with reducing sugar release. The experimental data in were analyzed using response surface methodology, and ANOVA analysis was used to evaluate the significance of the model. ANOVA analysis indicated that the established model was significant, as the F value was high (61.30), and the calculated probability values (p-value, <0.0001) were lower than 0.05 (the model terms are significant when p < 0.05), as shown in . Error analysis showed that the lack of fit (F = 4.44, p = 0.09) of the model was insignificant, suggesting that the model equation might be suitable for predicting the release of reducing sugar in this study. The R2 and R2-adj were 0.99 and 0.97, respectively, which means that the model could explain 99% of the total variation of the responses.
Table 3. ANOVA analysis of the model for the response
Multiple regression analysis was used to analyze experimental data in order to fit a second-order polynomial equation. The regression coefficients of intercept, linear, quadratic, and interaction terms are expressed in (EquationEquation 2(2)
(2) ). The positive and negative terms in the equation indicate linear effects to increase Y and antagonistic effects on Y, respectively. Analysis of these coefficients with p-value showed that a linear term C2 (p < 0.0001) and quadratic terms, H2× H2 (p = 0.0109) and TX×TX (p = 0.0164), were significant (). Thus, from Equation (2) and , it can be inferred that the release of reducing sugar from untreated rice straw is heavily dependent on Cellic Ctec2 loading, as it has a significant linear effect on sugar production. The quadratic effect of Cellic Htec2 on the enzymatic reaction is more significant than other quadratic parameters. When the experimental values were plotted against predicted values (), the results were closely clustered along the line of best fit (R2 = 0.99), indicating a high degree of consistency between actual and predicted values. This confirms that the regression model equation is reliable and provides an accurate description of correlations among the three variables in reducing sugar production from untreated rice straw under the experimental ranges tested.
Table 4. Model parameter estimates (regression analysis) of the quadratic model for reducing sugar release from the hydrolysis of untreated rice straw
The interaction profiler was performed to illustrate the dependence of one factor on the level of another factor [Citation40,Citation41]. That is, it can be used to explain the interaction effects of three factors (C2, H2, and TX), interacting with one another in terms of reducing sugar release. The factor lines intersecting each other or lines with different slopes indicate a degree of interaction [Citation40]. Here, it is observed that there are no interactions between all studied factors and reducing sugar release (). For example, the curves of Triton X-100 and Cellic Htec2 are almost parallel, indicating no interaction effects; moreover, the changes in one have no impact on the other. On the contrary, it seems that the Cellic Ctec2 line crosses the Triton X-100 line, suggesting interactive effects among them. However, the slopes of those two lines are almost similar, likely indicating nonsignificant interaction. Thus, this interaction may have negligible effects on reducing sugar release.
Figure 2. Interaction profiler showing the interactive effect of factors for reducing sugar release. Interactions between terms are shown as crossed lines or lines of different slopes. No interactive effects are shown as parallel lines
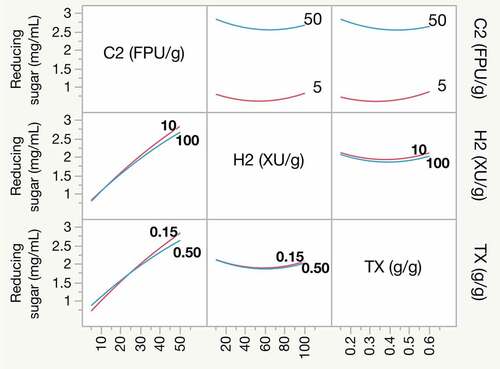
A prediction profiler is a useful tool to predict the response surface and determine the optimum value of each independent variable on response. The prediction plot showed the effects of independent variables on reducing sugar release (). The increase of Cellic Ctec2 loading from 0 to 50 FPU/g showed an increasing trend for reducing sugar release. However, the increased loadings of Cellic Htec2 and Triton X-100 did not show any significant effects on sugar production. Additions of Cellic Htec2 and Triton X-100 showed the curved shape, which agreed well with the critical quadratic terms of the model. These two factors were less pronounced on the dependent variable than Cellic Ctec2 as shown in .
Figure 3. Prediction profiler and desirability of reducing sugar release. The prediction profiler depicts the effects of Cellic Ctec2 (C2) cellulase, Cellic Htec2 (H2) xylanase, and Triton X-100 (TX) on reducing sugar release. The black lines indicate the prediction trace; the dotted vertical red line refers to the current dosage of factor; the value in red on the vertical axis predicted response based on loads of individual independent variables
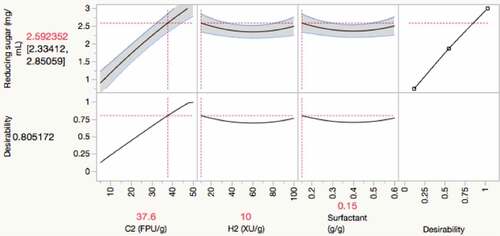
The desirability optimization was performed to maximize reducing sugar release. The prediction can be evaluated by the desirability of the predicted values [Citation42]. The values of the scale lie between 0 and 1, where 0 represents a completely undesirable response, and 1 represents the most desirable one. The maximum reducing sugar concentration (desirability = 1) was projected to be 3.11 mg/mL by loading 50 FPU/g Cellic Ctec2, 10 XU/g Cellic Htec2, and 0.15 g/g Triton X-100, using a maximum desirability optimization approach. However, a high dose of Cellic Ctec2 is required at this hydrolysis condition, making the process highly expensive. For this reason, we reduced the desirability value from 1.00 to 0.805 to reduce enzyme loads while achieving the desired sugar concentration.
When the desirability of the predicted values was judged at 0.805, the optimal values of Cellic Ctec2, Cellic Htec2, and Triton X-100 were 37.6 FPU/g, 10.0 XU/g, and 0.15 g/g, with the predicted reducing sugar release value of 2.58 mg/mL (). Three trials using the optimal conditions were performed to confirm this predicted sugar yield and test the model’s validity. The resulting sugars from Trial-1 to Trial-3 were 2.45, 2.28, and 2.66 mg/mL. The resulting sugar yield was consistent with the predicted values. The actual values differed from the predicted ones with a margin of around 10%, thus verifying the precision of the model for prediction. Therefore, our model analysis is reliable and offers the ease of obtaining optimal conditions with a rapid response and reducing enzyme loads for enhanced reducing sugar release.
3.2 Comparison of reducing sugar released from hydrolysis of untreated rice straw in the presence or absence of Triton X-100
The addition of nonionic surfactants has been shown to promote enzymatic hydrolysis of lignocellulosic materials and increase sugar release [Citation43,Citation44]. However, this study and regression analysis suggested that the addition of Triton X-100 to enzymatic hydrolysis of untreated rice straw showed no improvement in sugar release. To confirm this prediction, we tested the hydrolysis of untreated rice straw in the absence of the surfactant. The enzymatic reaction was carried out following the design matrix shown in , with no Triton X-100 in the individual run.
Hydrolysis of untreated rice straw in the absence of Triton X-100 yielded reducing sugars from 0.75 to 3.09 mg/mL, whereas in the presence of Triton X-100, it yielded reducing sugars from 0.79 to 2.92 mg/mL (). Hence, in the same run, the yields of reducing sugar with and without the surfactant were not statistically different. For example, at Run no. 11, the reducing sugar concentrations were 1.96 ± 0.16 and 1.61 ± 0.12 mg/mL for hydrolyzed rice straws with and without Triton X-100, respectively. The comparable yields indicate that the addition of Triton X-100 does not facilitate or promote enzymatic hydrolysis of untreated rice straw and, in turn, reducing sugar release.
Figure 4. Comparison of reducing sugar release from enzymatic hydrolysis of untreated rice straw in the absence and presence of Triton X-100
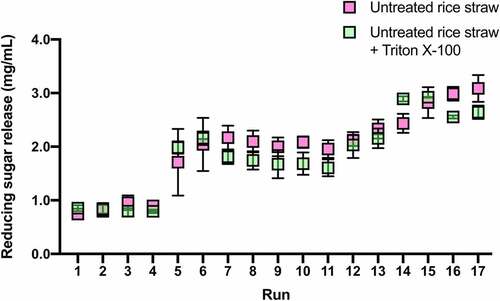
The result of reducing sugar yield is consistent with our regression model, which indicates that Triton X-100 is not significant for enzymatic hydrolysis efficiency or sugar yield. The plausible explanation for this phenomenon is that 1) Triton X-100 might not bind to the native form of lignin, resulting in no blocking of lignin-enzyme binding; 2) Triton X-100 might bind to lignin and prevent enzyme adsorption. However, it does not improve enzymatic hydrolysis because of the limited availability of free active surfaces of polysaccharides (i.e., cellulose). Our result differs from the previous observation by Guerfali, et al. [Citation45], who found that Triton X-100 increased enzymatic digestibility of phosphoric acid-pretreated newspaper by 45%. We suggest that the effects of surfactants on enzymatic hydrolysis might be more pronounced with pretreated substrates, having modified lignin structure or content.
3.3 TLC analysis of hydrolysis product
To understand the actions of the enzymes against the substrate in the absence or presence of Triton X-100, we further determined the kinds of sugars derived from the hydrolysis of untreated rice straw. The hydrolysis products from 17 hydrolysis conditions with and without the surfactant were taken and analyzed by the TLC technique (). It was found that, in the absence of Triton X-100, hydrolysis of untreated rice straw exclusively yielded glucose (). A similar profile was observed with the hydrolysis of untreated rice straw in the presence of Triton X-100 ().
Figure 5. TLC analysis of hydrolysis products from untreated rice straw hydrolyzed in (a) the absence and (b) the presence of Triton X-100. The samples were incubated at 55°C with an agitation rate of 180 rpm for 24 h. The molecular size marker lane (G1-G6) contains a mix of celloligosaccharides, including glucose (G1), cellobiose (G2), cellotriose (G3), cellotetraose (G4), cellopentaose (G5), and cellohexaose (G6). The molecular size marker lane (X1-X6) contains a mix of xylooligosaccharides, including xylose (X1), xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6). Lanes 1–17 show the hydrolysis products from Runs 1–17 in the absence or presence of Triton X-100
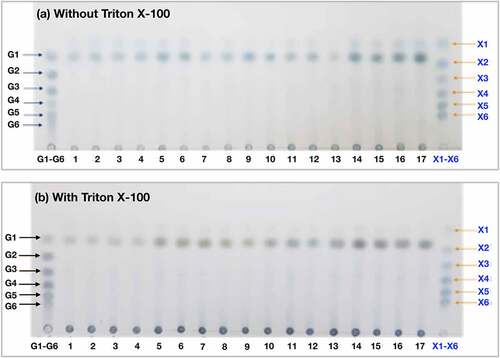
Hydrolysis of untreated rice straw, with and without Triton X-100, exclusively yielding glucose suggests that cellulose in untreated rice straw is the main component to be hydrolyzed by the Cellic Ctect2 cellulase, possibly due to its high content (threefold higher than xylan) and exposure to the enzymes. This result is consistent with the regression model, indicating Cellic Ctec2 was the most significant factor for the hydrolysis of untreated rice straw. In addition, López-Gutiérrez, et al. [Citation32] reported that Cellic Ctec2 contained aggressive cellulases with high β-glucosidase activity, which might explain the accumulation of glucose as a final product. However, when we determined the yield of glucan conversion to glucose, the results were not impressive, as the maximum yield from the hydrolysis conditions with or without the surfactant was about 20% glucan conversion (data not shown). This low value indicates a poor digestibility of the untreated biomass. Accordingly, the low glucan conversion yield may reflect the limited availability of the free surfaces or free ends of cellulose in untreated rice straw for accessibility to cellulases. Moreover, the addition of Triton X-100 might have no significant effects on biomass structure modification to improve enzymatic hydrolysis.
It is known that the Cellic Htec2 xylanase can convert xylan to simple sugars and increase cellulose hydrolysis when combined with Cellic Ctec2 [Citation32,Citation46]. In this study, the hydrolysis products from xylan with and without Triton X-100 were observed on TLC plates with minimal amounts of xylose ( a and b). The low concentration of xylose indicates that xylan is not easy to hydrolyze, implying that xylan, in its natural state, is recalcitrant to degrade. This resistance is possibly due to the heterogeneity, a high degree of substitution, and the complexation with lignin [Citation47]. The recalcitrant structure of xylan poses a challenge for enzymatic hydrolysis. It may also support the above reason for the poor glucose yield as xylan covers most parts of cellulose surfaces, thus, impeding cellulose hydrolysis.
Overall, it is likely that the hydrolysis of untreated rice straw with or without Triton X-100 is equivalent in terms of yield and the type of sugar. However, to better understand the function and interaction with the substrate, further investigation regarding the effect of Triton X-100 on biomass structure and its ability to bind lignin is needed.
3.4 Chemical, morphological, and structural analyses
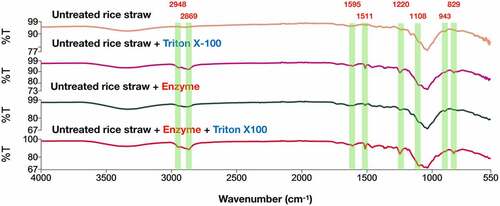
To prove our assumption and reveal whether Triton X-100 can bind lignin on untreated rice straw, we qualitatively determined the chemical structure of samples using the FTIR technique to observe chemical change (or functional groups). Triton X-100 (0.375 g/g) was added to 5% (w/v) untreated rice straw alone or the hydrolysis reaction with enzyme loads of 50 FPU/g and 100 XU/g substrates. All samples were incubated in ABS at 55°C for 24 h and then centrifuged to obtain the solid residues. Later, four kinds of samples, including untreated rice straw, untreated rice straw with Triton X-100, hydrolyzed untreated rice straw samples with and without Triton X-100, were obtained and assessed.
We first investigated the FTIR spectra of pure Triton X-100 from 500 to 4000 cm−1, revealing significant peak intensities at 829, 943, 1108, 1220, 1511, 1610, 2869, and 2948 cm−1 (data not shown), agreeing with previous observation [Citation48,Citation49]. The FTIR spectrum of untreated rice straw was used as a control, and some differences were observed in the intensity with the addition of Triton X-100 (). The peaks at 1108, 2869, and 2948 cm−1 were reported to be the maximum intensities for Triton X-100 [Citation49]. These peaks appeared on both hydrolyzed and untreated rice straw samples with the added surfactant, suggesting the physical adsorption of Triton X-100 on the surfaces of the samples. The wavenumbers of 1220–1330 and 1400–1590 cm−1 were attributed to lignin components [Citation50], whereas 1260 and 1350 cm−1 were assigned to C–O vibration of guaiacyl and syringyl rings [Citation49]. The sharp increase in peak intensities was observed at 1220 and 1511 cm−1 for the hydrolyzed and untreated rice straw samples in the presence of Triton X-100. The increased intensity at 1220 cm−1 is consistent with the previous study by Eckard, et al. [Citation49], who showed that incubation of extrusion-pretreated corn stover resulted in the maximum value of IR absorbance at 1220 cm−1. The wavenumber around 1500–1513 cm−1 was attributed to the aromatic ring vibration of guaiacyl and syringyl rings [Citation51]. However, the peak intensities at 1260 and 1350 cm−1 did not differ among the control and the samples with and without TritonX-100. It has been reported that Triton X-100 was less effective in lignin removal as evidenced by a small decrease (around 15%) in the peak intensities at 1260 and 1350 cm−1 [Citation49]. Therefore, the predominated peak at 1511 cm−1 was attributed to the strong IR absorption by Triton X-100. It was proposed that surfactants could disrupt hydrogen bonds [Citation37,Citation52]. Here, a broad band at around 3100–3500 cm−1, corresponding to O-H stretching of hydrogen bonds of cellulose [Citation51], was decreased when the untreated and hydrolyzed rice straw samples were added with Triton X-100, showing a sign of partial cellulose disruption.
Figure 6. FTIR spectra for untreated rice straw samples with and without Triton X-10 and hydrolyzed untreated rice straw samples with and without Triton X-100 at 24-h incubation. Triton X-100 (0.375 g/g) was added to 5% (w/v) of untreated rice straw alone and the hydrolysis reaction with enzyme loads of 50 FPU/g and 100 XU/g substrates. All samples were incubated in ABS at 55°C for 24 h and then centrifuged to obtain the solids for FTIR analysis
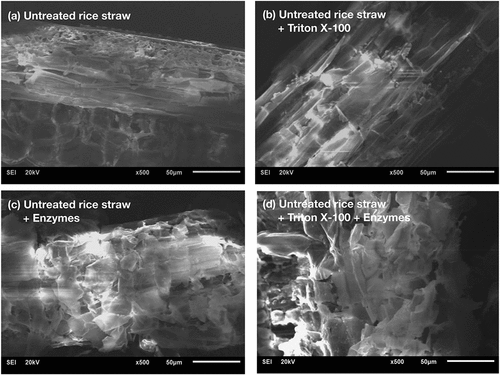
Macroscopic structures of the untreated and hydrolyzed samples were observed by SEM to investigate whether Triton X-100 could modify surface morphology (). It was found that untreated rice straw samples both with and without Triton X-100 showed even and smooth surfaces ( and b). However, in the presence of Triton X-100, the rice straw fibers appeared swollen, possibly due to the wetting ability of the surfactant (). It was reported that Tween 20 could disrupt cellulose by adding water molecules between or inside cellulose microfibrils, resulting in fiber swelling [Citation21]. The hydrolyzed rice straw with and without Triton X-100 showed similar disruptive features of the fiber surfaces, revealing some porosity and disintegration of the fibers ( and d). These distortion areas might be less ordered or compact; thus, they are prone to enzymatic hydrolysis. However, most parts of the fiber surfaces were not degraded and remained thick and rigid, indicating that the external surfaces are evenly protected or layered with networks of lignin-(hemi)cellulose ( and d). A similar result was observed by Seo, et al. [Citation37], who found that Tween 20 is unable to break down the cell walls of the pinewood chip, containing high lignin content (25.94%), and Avicel, which has high crystallinity (around 88%). Therefore, our result suggests that, despite adsorption on lignin, Triton X-100 could not disrupt a rigid and sophisticated form of carbohydrate-lignin layers of untreated rice straw.
3.5 Proposed mechanisms of Triton X-100 on enzyme-substrate (untreated rice straw) interactions
The effects of surfactants on enzyme-substrate interactions have been proposed [Citation17,Citation31,Citation35,Citation37,Citation39]. For example, adsorbed enzymes are prevented from inactivation by adding surfactants, facilitating the desorption of enzymes from the substrate [Citation17,Citation39,Citation53]. In this study, the reducing sugar concentrations derived from hydrolysis of untreated rice straw in the absence or presence of Triton X-100 were not statistically different. This result differs from other previous studies in that the addition of surfactant generally prevents enzyme adsorption on lignin and, in turn, promotes hydrolysis of polysaccharides.
According to the model of plant cell wall structure, cellulose is formed as microfibrils. The cellulose microfibrils are integrated into bundles, where hemicellulose binds to cellulose surfaces. Lignin is linked with hemicellulose to form a lignin-hemicellulose matrix (), which covers cellulose microfibrils [Citation6]. Regarding the regression model (Equation (2) and ), the Cellic Ctec2 cellulase is the most significant factor to produce reducing sugar. It is known that the adsorption of cellulolytic enzymes on cellulose surfaces is significant and correlated with hydrolysis or yield [Citation39]. The distribution of hemicellulose (xylan) and lignin content restricted cellulase accessibility to cellulose. Also, the inherent structure of hemicellulose, complexed with lignin, is recalcitrant to degradation by xylanase enzymes, as evident by a tiny amount of xylose (). The manufacturer recommends that Cellic Htec2 xylanase is highly specific to soluble hemicellulose [Citation32]. Therefore, only available cellulose surfaces and readily soluble or exposed xylan can be degraded by the enzymes ().
Figure 8. Possible effects of surfactant on enzyme-substrate interaction during hydrolysis of untreated rice straw
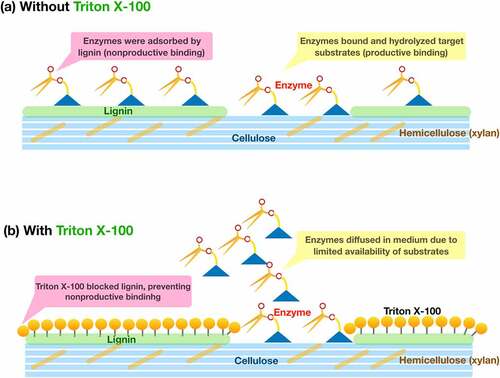
When added to the hydrolysis reaction, Triton X-100 bound to the untreated rice straw, as evident by the FTIR analysis (). Seo, et al. [Citation37] showed that nonionic surfactant, e.g., Tween 20, bound lignin surfaces of raw pine wood chips, and its high adsorption capacity was due to the existence of lignin. Our result agrees well with the previous study and confirms that the surfactant blocks lignin, preventing enzyme adsorption onto lignin. In contrast, the hydrophilic head of the surfactant may activate the enzymes to access target substrates [Citation17].
In terms of reducing sugar production, we found that the surfactant-lignin binding is not advantageous for the enzymatic hydrolysis of untreated rice straw. It is plausible that Triton X-100 cannot increase reaction sites in the substrate [Citation17]. Seo, et al. [Citation37] found that surfactants positively affected enzymatic hydrolysis when the lignin structure and content were modified, and lignin content is not directly proportional to the potential surfactant effect [Citation54]. In our case, the lignin blocking with Triton X-100 might have had no pronounced effect on enzymatic hydrolysis because of the high lignin content. The high lignin coverage on untreated rice straw allows a few enzyme molecules to access the limited availability of polysaccharides, while the rest of the enzymes may diffuse in the reaction medium. Thus, they do not participate in the hydrolysis of polysaccharides [Citation28,Citation55] (). This thought is supported by the fact that the fiber surfaces of untreated rice straw samples both with and without Triton X-100 remained smooth. Also, the fibers after hydrolysis exhibited similar surfaces, showing burst and disintegrated fibers as a result of enzyme attacks at easily hydrolyzable parts ( and d).
It seems that Triton X-100 could likely disrupt intra- and inter-hydrogen bonds of cellulose chains as evidenced by the decrease in FTIR spectra at 3500 cm−1. This is possible because Triton X-100 might interact with surface hydroxyl groups of cellulose through its oxygen atoms of hydrophilic side chains [Citation48]. However, this interaction may have had no beneficial effects on enzymatic hydrolysis, as the surfactant may pose steric hindrance or interference (e.g., hydrophobic side chains) to enzymes. Thus, there are no substantial effects on sugar yield.
Overall, our ideas could explain why the addition of Triton X-100 does not promote enzyme hydrolysis of untreated rice straw, and its reducing sugar yield was comparable to that of the hydrolysis of untreated rice straw in the absence of Triton X-100. This study primarily suggests that exposure of carbohydrates to enzymes is more important than lignin blocking by Triton X-100.
4 Conclusion
Herein, we used a response surface methodology with Box-Behnken design to design experimental conditions to determine the relationships of enzyme, substrate, and surfactant on hydrolysis of untreated rice straw and explained the effects of the surfactant on enzyme-substrate interactions. Regarding model analysis, Cellic Ctec2 cellulase was the most influential factor in producing reducing sugar. Although FTIR analysis showed that Triton X-100 bound to lignin on the untreated substrate, it did not enhance enzymatic hydrolysis. Furthermore, the reducing sugar yields from hydrolysis of untreated rice straw with and without Triton X-100 were comparable, which indicated that the addition of the surfactant did not create more reaction sites, and the availability of polysaccharides accessible to enzymes was almost identical. Therefore, we suggest that the effects of surfactants on enzymatic hydrolysis depend on the substrate features. This study provides a clearer mechanism on activity of cellulase, xylanase, and surfactant in hydrolysis of natural plant biomass. This study gives information on further development of biological pretreatment using enzyme/microbe and composting process of solid waste rich in lignocellulosic material.
Authorship contributions
Sengthong Lee: Methodology, Investigation, Data curation, Writing-original draft;
Saengchai Akeprathumchai: Formal analysis, Validation, Writing-original draft;
Damkerng Bundidamorn: Investigation;
Lakha Salaipeth: Formal analysis, Validation;
Kanokwan Poomputsa: Conceptualization, Formal analysis;
Khanok Ratanakhanokchai: Conceptualization, Writing-review & editing;
Ken-Lin Chang: Formal analysis, Conceptualization, Writing-review & editing;
Paripok Phitsuwan: Conceptualization, Methodology, Investigation, Supervision, Data curation, Funding acquisition, Writing-original draft, review & editing.
Highlights
Effect of interaction between enzyme and surfactant on hydrolysis of native rice straw was studied.
The mathematical model indicates that only Cellic Ctec2 cellulase is significant for sugar release
Triton X-100 binds onto rice straw but does not promote enzymatic hydrolysis
Exposure of carbohydrates to enzymes is more important than lignin blocking
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen R. A paradigm shift in biomass technology from complete to partial cellulose hydrolysis: lessons learned from nature. Bioengineered. 2015;6:69–72.
- Islam MK, Wang H, Rehman S, et al. Sustainability metrics of pretreatment processes in a waste derived lignocellulosic biomass biorefinery. Bioresour Technol. 2020;298:122558.
- Zhu P, Abdelaziz OY, Hulteberg CP, et al. New synthetic approaches to biofuels from lignocellulosic biomass. Curr Opin Green Sust Chem. 2020;21:16–21.
- Satlewal A, Agrawal R, Bhagia S, et al. Rice straw as a feedstock for biofuels: availability, recalcitrance, and chemical properties. Biofuel Bioprod Biorefin. 2017;12:83–107.
- Abraham A, Mathew AK, Sindhu R, et al. Potential of rice straw for bio-refining: an overview. Bioresour Technol. 2016;215:29–36.
- Zoghlami A, Paës G. Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem. 2019;7:874.
- Yoo CG, Meng X, Pu Y, et al. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: a comprehensive review. Bioresour Technol. 2020;301:122784.
- Jin X, Song J, Liu G-Q. Bioethanol production from rice straw through an enzymatic route mediated by enzymes developed in-house from Aspergillus fumigatus. Energy. 2020;190:116395.
- Lorenci Woiciechowski A, Dalmas Neto CJ, Porto de Souza Vandenberghe L, et al. Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance - Conventional processing and recent advances. Bioresour Technol. 2020;304:122848.
- Li P, He C, Li G, et al. Biological pretreatment of corn straw for enhancing degradation efficiency and biogas production. Bioengineered. 2020;11:251–260.
- Song HT, Gao Y, Yang YM, et al. Synergistic effect of cellulase and xylanase during hydrolysis of natural lignocellulosic substrates. Bioresour Technol. 2016;219:710–715.
- Haldar D, Purkait MK. Lignocellulosic conversion into value-added products: a review. Process Biochem. 2020;89:110–133.
- Li M, Pu Y, Ragauskas AJ. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front Chem. 2016;4:45.
- Thoresen M, Malgas S, Pletschke BI. Enzyme adsorption-desorption and evaluation of various cellulase recycling strategies for steam pre-treated Eucalyptus enzymatic degradation. Biomass Conv Bioref. 2020. DOI:10.1007/s13399-020-00670-9
- Dos Santos AC, Ximenes E, Kim Y, et al. Lignin-enzyme interactions in the hydrolysis of lignocellulosic biomass. Trends Biotechnol. 2019;37:518–531.
- Palonen H, Tjerneld F, Zacchi G, et al. Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol. 2004;107:65–72.
- Eriksson T, Börjesson J, Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol. 2002;31:353–364.
- Yarbrough JM, Mittal A, Mansfield E, et al. New perspective on glycoside hydrolase binding to lignin from pretreated corn stover. Biotechnol Biofuels. 2015;8:214.
- Xin D, Yang M, Chen X, et al. Improving the hydrolytic action of cellulases by Tween 80: offsetting the lost activity of cellobiohydrolase Cel7A. ACS Sust Chem Eng. 2017;5:11339–11345.
- Liu H, Sun J, Leu S-Y CS. Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuel Bioprod Biorefin. 2016;10:648–663.
- Bhagia S, Kumar R, Wyman CE. Effects of dilute acid and flowthrough pretreatments and BSA supplementation on enzymatic deconstruction of poplar by cellulase and xylanase. Carbohydr Polym. 2017;157:1940–1948.
- Eckard AD, Muthukumarappan K, Gibbons W. Enhanced bioethanol production from pretreated corn stover via multi-positive effect of casein micelles. Bioresour Technol. 2013;135:93–102.
- Li J, Li S, Fan C, et al. The mechanism of poly(ethylene glycol) 4000 effect on enzymatic hydrolysis of lignocellulose. Colloids Surf B Biointerfaces. 2012;89:203–210.
- Rocha-Martin J, Martinez-Bernal C, Perez-Cobas Y, et al. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour Technol. 2017;244:48–56.
- Chen Y-A, Zhou Y, Qin Y, et al. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: pretreatment, hydrolysis conditions and lignin structure. Bioresour Technol. 2018;269:329–338.
- Seo DJ, Fujita H, Sakoda A. Effects of a non-ionic surfactant, Tween 20, on adsorption/desorption of saccharification enzymes onto/from lignocelluloses and saccharification rate. Adsorption. 2011;17:813–822.
- Zhu S, Sui J, Liu Y, et al. Effects of washing, autoclaving, and surfactants on the enzymatic hydrolysis of negatively valued paper mill sludge for sugar production. Energy Fuels. 2019;33:1219–1226.
- Jain L, Kurmi AK, Agrawal D. Feasibility studies with lignin blocking additives in enhancing saccharification and cellulase recovery: mutant UV-8 of T. verruculosus IIPC 324 a case study. Enzyme Microb Technol. 2018;118:44–49.
- Agrawal R, Satlewal A, Kapoor M, et al. Investigating the enzyme-lignin binding with surfactants for improved saccharification of pilot scale pretreated wheat straw. Bioresour Technol. 2017;224:411–418.
- Cheng J, Yu Y, Zhu M. Enhanced biodegradation of sugarcane bagasse by Clostridium thermocellum with surfactant addition. Green Chem. 2014;16:2689–2695.
- Zhou Y, Chen H, Qi F, et al. Non-ionic surfactants do not consistently improve the enzymatic hydrolysis of pure cellulose. Bioresour Technol. 2015;182:136–143.
- López-Gutiérrez I, Razo-Flores E, Méndez-Acosta HO, et al. Optimization by response surface methodology of the enzymatic hydrolysis of non-pretreated agave bagasse with binary mixtures of commercial enzymatic preparations. Biomass Conv Bioref. 2020. DOI:10.1007/s13399-020-00698-x
- CKC DA, de Oliveira Campos A, de Araujo Padilha CE, et al. Enhancing enzymatic hydrolysis of coconut husk through Pseudomonas aeruginosa AP 029/GLVIIA rhamnolipid preparation. Bioresour Technol. 2017;237:20–26.
- Phitsuwan P, Permsriburasuk C, Waeonukul R, et al. Evaluation of fuel ethanol production from aqueous ammonia-treated rice straw via simultaneous saccharification and fermentation. Biomass Bioenergy. 2016;93:150–157.
- Lin W, Chen D, Yong Q, et al. Improving enzymatic hydrolysis of acid-pretreated bamboo residues using amphiphilic surfactant derived from dehydroabietic acid. Bioresour Technol. 2019;293:122055.
- Li Y, Sun Z, Ge X, et al. Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol Biofuels. 2016;9:20.
- Seo D-J, Fujita H, Sakoda A. Structural changes of lignocelluloses by a nonionic surfactant, Tween 20, and their effects on cellulase adsorption and saccharification. Bioresour Technol. 2011;102:9605–9612.
- Olsen SN, Bohlin C, Murphy L, et al. Effects of non-ionic surfactants on the interactions between cellulases and tannic acid: a model system for cellulase-poly-phenol interactions. Enzyme Microb Technol. 2011;49:353–359.
- Qing Q, Yang B, Wyman CE. Impact of surfactants on pretreatment of corn stover. Bioresour Technol. 2010;101:5941–5951.
- Siddiquee K, Zhao C, Stemler MA, et al. Cell-culture growth conditions resulting in the oxidation of a recombinant antigen-binding fragment. Bioresour Bioprocss. 2019;6
- Huang J, Kaul G, Cai C, et al. Quality by design case study: an integrated multivariate approach to drug product and process development. Int J Pharm. 2009;382:23–32.
- Mourabet M, El Rhilassi A, El Boujaady H, et al. Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by Brushite. Arabian J Chem. 2017;10:S3292–S302.
- Parnthong J, Kungsanant S, Chavadej S. The influence of nonionic surfactant adsorption on enzymatic hydrolysis of oil palm fruit bunch. Appl Biochem Biotechnol. 2018;186:895–908.
- Lou H, Zeng M, Hu Q, et al. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour Technol. 2018;249:1–8.
- Guerfali M, Saidi A, Gargouri A, et al. Enhanced enzymatic hydrolysis of waste paper for ethanol production using separate saccharification and fermentation. Appl Biochem Biotechnol. 2015;175:25–42.
- Phitsuwan P, Permsriburasuk C, Baramee S, et al. Structural analysis of alkaline pretreated rice straw for ethanol production. Int J Polym Sci. 2017;2017:9.
- Malgas S, Mafa MS, Mkabayi L, et al. A mini review of xylanolytic enzymes with regards to their synergistic interactions during hetero-xylan degradation. World J Microbiol Biotechnol. 2019;35:187.
- Iyyappan E, Wilson P, Sheela K, et al. Role of triton X-100 and hydrothermal treatment on the morphological features of nanoporous hydroxyapatite nanorods. Mater Sci Eng C. 2016;63:554–562.
- Eckard AD, Muthukumarappan K, Gibbons W. The role of polymeric micelles on chemical changes of pretreated corn stover, cellulase structure, and adsorption. BioEnergy Res. 2014;7:389–407.
- Lu X, Zheng X, Li X, et al. Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stover pretreated with liquid hot water. Biotechnol Biofuels. 2016;9:118.
- Gao AH, Bule MV, Laskar DD, et al. Structural and thermal characterization of wheat straw pretreated with aqueous ammonia soaking. J Agric Food Chem. 2012;60:8632–8639.
- Li H, Wang C, Xiao W, et al. Dissecting the effect of polyethylene glycol on the enzymatic hydrolysis of diverse lignocellulose. Int J Biol Macromol. 2019;131:676–681.
- Wang W, Zhuang X, Tan X, et al. Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuels. 2018;32:5951–5959.
- Kristensen JB, Börjesson J, Bruun MH, et al. Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb Technol. 2007;40:888–895.
- Wang Z, Xu JH, Feng H, et al. Fractal kinetic analysis of polymers/nonionic surfactants to eliminate lignin inhibition in enzymatic saccharification of cellulose. Bioresour Technol. 2011;102:2890–2896.