ABSTRACT
D-lactate dehydrogenase (D-LDH) is widely used for the clinical detection of alanine aminotransferase (ALT) activity. It is a key enzyme in ALT detection kits, and its enzymatic properties directly determine sensitivity and accuracy of such kits. In this study, D-lactate dehydrogenase (WP_011543503, ldLDH) coding sequence derived from Lactobacillus delbrueckii was obtained from the NCBI database by gene mining. LdLDH was expressed and purified in Escherichia coli, and its enzyme activity, kinetic parameters, optimum temperature, and pH were characterized. Furthermore, stabilizers, including sugars, polyols, amino acids, certain salts, proteins, and polymers, were screened to improve stability of ldLDH during freeze-drying and storage. Finally, a kit based on ldLDH was tested to determine whether the enzyme had potential clinical applications. The results showed that ldLDH had a specific activity of 1,864 U/mg, Km value of 1.34 mM, optimal reaction temperature of 55°C, and an optimal pH between 7.0 and 7.5. When sucrose or asparagine was used as a stabilizer, freeze-dried ldLDH remained stable at 37°C for > 2 months without significant loss of enzymatic activity. These results indicated that ldLDH possesses high activity and stability. Test results using the ALT assay kit prepared with ldLDH were consistent with those of commercial kits, with a relative deviation <5%. These results indicated that ldLDH met the primary requirements for ALT assays, laying a foundation for the development of new ALT kits with potential clinical applications.
1. Introduction
Serum alanine aminotransferase (ALT) activity is considered a reliable and sensitive marker for the detection of liver disease [Citation1], and also plays an important role in the prevention and monitoring of liver-related disorders such as obesity, diabetes, and cardiovascular disease [Citation2]. ALT is found in the kidney, blood, muscle, and heart, but it is particularly abundant in liver cells, where ALT activity is 3,000 times higher than that in blood [Citation3]. Therefore, when liver cells are injured or diseased, ALT is released, enhancing its activity in the blood. Therefore, ALT activity in the blood can be used to monitor the progression of liver disorders or other related diseases [Citation1,Citation4].
To quantify ALT activity rapidly and simply, ALT activity assay kits (ALT kits) have been widely used in clinical settings. ALT kits have been extensively used to diagnose and assess liver diseases, such as nonalcoholic fatty liver disease, alcoholic liver disease, hepatitis B virus infection, drug-induced hepatotoxicity, autoimmune and cholestatic liver disease, and metabolic liver disease. It can also be used to monitor patients at risk of developing liver disease [Citation2]. The working principle of ALT kits is shown in . ALT in serum converts alanine to pyruvate in the first step; then, the produced pyruvate is catalyzed by lactate dehydrogenase (D-LDH) to produce lactic acid, and NADH is converted to NAD+. The NADH oxidation rate is proportional to ALT activity. The changed value can be monitored using a biochemical analyzer [Citation5,Citation6]. Therefore, only one key enzyme component, which is the activity of D-LDH, directly determines the accuracy and sensitivity of ALT kits.
However, the practical application of using D-LDH faces challenges. ALT kits are used at room temperature for clinical testing; therefore, the thermal stability of D-LDH is an important consideration for ALT kits. Additionally, purified D-LDH may lose its activity during long-term storage because of degradation or denaturation. D-LDH is derived from Pediococcus acidilactici [Citation7] and Bacillus. coagulans [Citation8], Lactobacillus plantarum [Citation9], L. pentosus [Citation10,Citation11], L. confusus [Citation12], L. jensenii [Citation13], etc. However, some of the reported D-LDHs have low activity, while others have diminished thermal stability. Therefore, D-LDHs with both high activity and thermal stability are needed for clinical diagnosis.
Long-term storage stability is important for successful commercial kit application and use. Freeze-drying has been widely used to retain satisfactory bioactivity of proteins during long-term storage [Citation14]. However, during freeze-drying, proteins suffer from stresses such as pH change, ice formation, crystal formation, and phase separation, resulting in significant changes in conformation and loss of bioactivity [Citation14–16]. The addition of protectants is the most commonly used method for increasing bioactivity during long-term storage of proteins [Citation17,Citation18]. Many stabilizers, such as sugars, polyols, amino acids, certain salts, and some proteins or polymers, have been extensively studied and applied in freeze-drying and storage [Citation14,Citation19,Citation20]. In a previous study, residual activity was up to 91% when organophosphorus hydrolase was stored in lyophilized form at 25°C for 60 days after adding maltose and trehalose [Citation21] . The activity of freeze-dried catalase recovered in the presence of sucrose was up to 96.3%, higher than that in the presence of glycerol, sorbitol, and dextran [Citation14]. However, the protective effects of these stabilizers vary from protein to protein depending on other parameters, such as storage pH, temperature, and concentration of additives [Citation14]. Therefore, the optimal protectant for a protein needs to be screened for freeze-drying and storage.
To obtain a novel D-LDH with high specific activity and elevated thermal stability, a series of D-LDHs from different species indexed in the NCBI database were mined and screened. Only one D-LDH was selected per species. We heterologously expressed these dehydrogenases in Escherichia coli (E. coli). The yield and activity of these enzymes were evaluated (data not shown). Finally, we selected D-LDH derived from L. delbrueckii (ldLDH) for further systematic studies. LdLDH (WP_011543503) was expressed and purified in E. coli, and its enzymatic properties were characterized. To further improve storage stability, freeze-drying was performed. A total of 22 stabilizers belonging to the six categories mentioned above were screened during freeze-drying and storage. Finally, ALT kits based on ldLDH were prepared according to commercial standards and compared with commercial kits. LdLDH laid the foundation for the development of a new ALT kit with potential clinical applications.
2. Materials and methods
2.1. Strains and plasmids
E. coli BL21 (DE3) cells (Novagen, Madison, WI, USA; genotype: F – ompT hsdSB (rB−mB−) gal dcm(DE3); GenBank accession No. CP001509) were used as hosts for protein expression. The amino acid sequence of D-lactate dehydrogenase (WP_011543503, ldLDH) of L. delbrueckii was obtained from the NCBI database. The full-length gene, containing an NcoI restriction site at the 5ʹ-end and an XhoI restriction site at the 3ʹ-end, was obtained by total gene synthesis (Genewiz, Suzhou, China). The synthetic gene was digested with NcoI and XhoI and inserted into NcoI/XhoI-digested pET-28a(+) plasmid, and the construct was confirmed by DNA sequencing (Genewiz) and referred to as pET-28a-ldLDH. The C-terminus of the ldLDH protein contained a His6-tag, which allows for convenient purification using Ni2+-NTA columns.
2.2. Expression and purification of ldLDH
LdLDH was expressed and purified as described by Zhou et al. [Citation22], with certain modifications. The pET-28a-ldLDH was transformed into E. coli BL21 (DE3) using the heat shock method, and a recombinant ldLDH-expressing strain was constructed. A single colony of the ldLDH recombinant strain was randomly selected and placed into 5 mL of LB medium (10 g/L peptone, 5 g/L yeast powder, and 10 g/L NaCl) with 50 μg/mL kanamycin. After overnight incubation at 37°C, the seed culture was inoculated into 100 mL of LB medium with 1% inoculum. Further incubation at 37°C was performed until the cell density reached 0.6–0.8 (OD600). The expression conditions were optimized at different induction temperatures (16°C, 25°C, and 37°C) and IPTG concentrations (0.1 mM, 0.5 mM, and 1 mM). Then, recombinant ldLDH was expressed by adding IPTG to a final concentration of 0.1 mM and incubation at 25°C for 12 h using 1 L LB medium.
Bacteria were collected by centrifugation at 5,500 × g resuspended in lysis buffer (50 mM Tris/HCl, 100 mM NaCl, 20 mM imidazole, pH 8.0), and homogenized using a high-pressure homogenizer (ATS, Taizhou, China). The solution was centrifuged at 26,000 × g at 4°C for 30 min to remove insoluble precipitates. The supernatant was purified using a nickel affinity chromatography column (Ni2+-NTA, GE, USA). Washing was performed using five column volumes of washing buffer (50 mM Tris/HCl, 100 mM NaCl, 40 mM imidazole, pH 8.0), followed by elution buffer (50 mM Tris/HCl, 100 mM NaCl, 200 mM imidazole, pH 8.0) to elute the ldLDH target protein. After elution, the target protein was placed in a dialysis tube and dialyzed against dialysis buffer (50 mM Tris/HCl, 100 mM NaCl, pH 8.0). The final protein purity was detected using 12% SDS-PAGE (Epizyme, Shanghai, China), and the protein concentration was determined using the BCA protein quantification kit (CWBIO, Beijing, China), which utilizes Cu2+, which can be reduced to Cu+ by proteins under alkaline conditions. Cu+ interacts with bicinchoninic acid (BCA), producing a sensitive color reaction and forming a purple complex, which can be monitored optically at 562 nm [Citation23].
2.3. Enzymatic characterization of ldLDH
2.3.1. Reaction system
The absorbance of the ldLDH cofactor NADH at 340 nm was used to quantify the reaction rate [Citation24] and characterize ldLDH activity. The ldLDH enzyme activity reaction system was modified as previously described [Citation9,Citation25]. Briefly, the reaction system consisted of 10 mM pyruvate, 0.02 mM NADH, and 50 mM Tris/HCl (pH 7.5), and 0.05 U ldLDH was added to initiate the reaction. The absorbance of the reaction system was measured at 340 nm using a spectrometer (SpectraMax M5, CA, USA) over 5 min in a total volume of 200 μL. Enzymatic activity was defined as the amount of enzyme required to oxidize 1 μmol of NADH per minute [Citation26]. The total enzyme activity and specific enzyme activity of the reaction system were calculated using an NADH extinction coefficient of 6.22 cm2/μmol [Citation5,Citation26]. One unit of activity was defined as the amount of enzyme that catalyzed the oxidation of 1 μmol of NADH per minute under standard conditions (37°C, pH 7.5).
2.3.2. Optimum temperature and pH of ldLDH
Using the above reaction system, enzyme activity was measured at different reaction temperatures to determine the optimal reaction temperature [Citation27]. The temperature was set to 25°C, 35°C, 40°C, 45°C, 50°C, 55°C, 60°C, and 70°C, while the pH was set to 7.5, and all other conditions remained unchanged. The change in absorbance at 340 nm was monitored every 10 s using a microplate analyzer. To determine the optimal pH, the above reaction system was set at 37°C, and changes in enzyme activity were determined over a series of different pH values (5.0–11.0). Changes in absorbance at 340 nm were monitored every 10 s using a microplate analyzer. The different pH buffers used were set as follows: 100 mM citrate buffer (pH 5.0–6.0), 100 mM phosphate buffer (pH 6.0–7.5), 100 mM Tris buffer (pH 7.5–9.0), and 100 mM carbonate buffer (pH 10.0–11.0). Each experiment was repeated three times.
The effects of different metal ions (Na2+, K+, Ca2+, Fe3+, Mn2+, Mg2+, Zn2+, Ba2+, Hg2+, Cu2+, and Co2+) and other additives (ethanol and EDTA) on the enzymatic activity of ldLDH were investigated. LdLDH (100 μg) was mixed with additives (1 mM, 1% for ethanol) at 30 C for 1 h, and activity was measured as described above. Enzyme activity in the absence of any additives was used as the control (100%).
2.4. Determination of ldLDH kinetic parameters
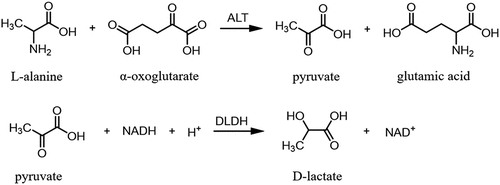
The determination of ldLDH kinetic parameters was performed according to a previous study [Citation7], with some modifications. At 37°C, pH 7.5, different concentrations of pyruvate (0.0625 mM, 0.125 mM, 0.25 mM, 0.5 mM, 1.0 mM, 2.0 mM, 4.0 mM, 8.0 mM, 10.0 mM, 20.0 mM) were added into the enzyme activity reaction system. Changes in absorbance at 340 nm were monitored using a microplate analyzer. The kinetic parameters (Km and Vmax) were calculated using the Lineweaver-Burk (double reciprocal) plot using GraphPad Prism 6.01 (GraphPad Software, CA, USA).
2.5. Determination of ldLDH stability and stabilizer selection
2.5.1. Stability of ldLDH
LdLDH stability was determined based on its pH tolerance and thermal stability [Citation22]. For pH tolerance, the purified enzyme was concentrated and placed in a buffer solution at a pH of 3–11 by ultrafiltration. The enzyme solution was incubated at 4°C for 30 min, the precipitate was removed by centrifugation, and a certain volume of the supernatant was collected to determine enzyme activity, as previously described. To determine thermal stability, the purified enzyme was placed in a water bath at different temperatures, allowed to stand for 20 min, and a certain volume of supernatant was taken to determine enzyme activity, as previously described. Each experiment was repeated three times.
2.5.2. Screening of ldLDH stabilizers
A total of 22 stabilizers were identified from the literature and screened for improved stability of ldLDH after freeze-drying [Citation19,Citation20]. These protective agents can be divided into six groups: (i) sugars, including sucrose, trehalose, glucose, lactose, and sorbose; (ii) amino acids, including glycine, lysine, asparagine, methionine, threonine, and glutamate; (iii) polyols, including sorbitol and xylitol; (iv) salts, including potassium gluconate, EDTA, NaCl, KCl, MgCl2, CaCl2, and citric acid; (v) protein, bovine serum albumin (BSA); and (vi) polymer, polyethylene glycol (PEG-2000). A specific concentration of each stabilizer was mixed with 5 mg/mL ldLDH solution and frozen overnight at −80°C. The sample was vacuum-dried at −40°C using a freeze-dryer (Labconco Freezone 18 L, Kansas, USA) for 48 h. The lyophilized samples were treated and dissolved in 100 mM phosphate buffer (pH 7.5) and used to determine enzyme activity. Some of the samples were placed in an incubator at 37°C for accelerated temperature testing, while the remaining samples were preserved for use in measuring enzyme activity after 60 days.
2.6. Preparation of ALT kit using ldLDH
LdLDH was mixed with optimal stabilizer and freeze-dried at −40°C. The performance of ldLDH was tested using a blank ALT kit (Mindray, Shenzhen, China) without lactate dehydrogenase, including Tris buffer, L-alanine, NADH, α-ketoglutarate, and preservatives [Citation28]. After adding the prepared 20 kU/L ldLDH in a blank ALT kit, a biochemical analyzer (Siemens, ADVIA2400, München, Germany) was used to determine fresh human serum samples. A commercial ALT test kit (Mindray, Shenzhen, China) was used as a positive control to determine serum samples. A total of 80 human serum samples from 64 (81.25%) healthy individuals and 15 (18.75%) hepatopathy patients were included in this study, including 3 (3.75%) patients with severe hepatic disease exhibiting high ALT activity (> 200 U/L). All patients were admitted to Hwa Mei Hospital, University of Chinese Academy of Science (Ningbo, China) between April and May 2021.
2.7. Statistical analysis
All experimental data in this study are the average values of measurements taken in triplicate. Analysis of variance (ANOVA) was carried out using GraphPad Prism 6.01 (GraphPad Software) followed by Dunnett’s multiple comparison analysis. Statistical significance was set at p < 0.05. The kinetic parameters (Km and Kcat) were calculated using Lineweaver-Burk plots.
3. Results
In this study, a new ALT kit was developed based on a novel ldLDH derived from L. delbrueckii. The enzymatic properties and thermostability of ldLDH, expressed and purified in E. coli, were systematically characterized. To further improve its application prospects, stabilizers were screened for preservation of activity during freeze-drying and long-term storage. Finally, the ALT kit was prepared and used to detect ALT activity in serum.
3.1. Expression and purification of ldLDH
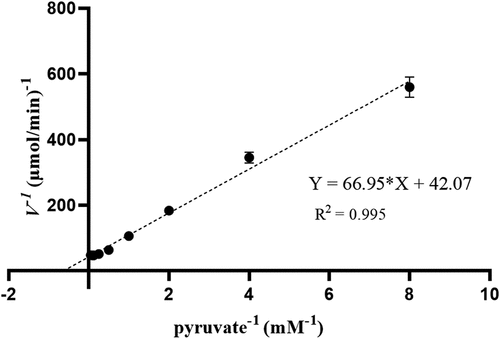
The synthesized ldLDH gene fragment was inserted into pET-28a(+) to form the pET-28a-ldLDH expression plasmid. A His6-tag was included at the C-terminus of ldLDH to minimize any effects on ldLDH activity (). The pET-28a-ldLDH was transformed into E. coli BL21 (DE3) cells, and the incubation temperature and concentration of IPTG were optimized for maximal recombinant protein expression. The results showed that the highest level of ldLDH was expressed and presented in the supernatant in soluble form when induction was performed using 0.1 mM IPTG at 25°C (). LB medium was used to express high amounts of ldLDH based on previously described optimized expression conditions. A pure form of ldLDH was obtained following purification of the homogenized supernatant by Ni2+-NTA affinity column isolation due to the presence of a His6-tag at the C-terminus of ldLDH (). As shown in the chromatogram, the first peak was the flow-through fraction, which did not bind to the Ni2+-NTA column. The second peak represented eluted ldLDH. SDS-PAGE analysis showed that the molecular weight of ldLDH was 30 kDa, which is consistent with the predicted molecular weight (). Pure ldLDH was subsequently used for analysis of enzymatic properties, stabilizer screening, and ALT kit development.
Figure 2. Expression of ldLDH in E. coli and purification analysis
3.2. Optimum temperature and pH of ldLDH
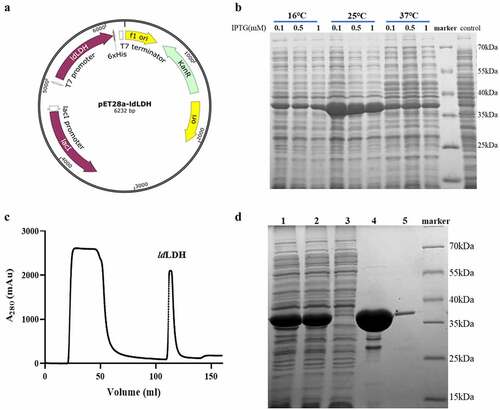
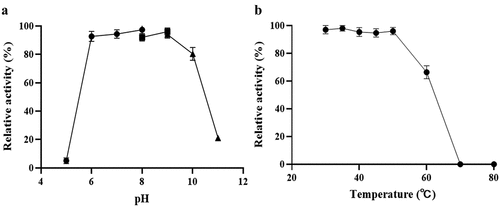
LdLDH catalyzes the conversion of pyruvate to lactic acid, and the cofactor NADH is oxidized to NAD+. NADH in solution has a strong absorbance peak at 340 nm, and can thus be used to monitor changes in NADH concentration. Therefore, the catalytic activity of ldLDH can be determined based on levels of reduced NADH. The reaction system was incubated at different temperatures to determine the influence of ambient temperature on ldLDH activity (). The highest activity was observed at 55°C, and further increases in temperature led to a decline in enzymatic activity. Similarly, the highest ldLDH activity was observed at pH 7.0–7.5, and subsequent increases in pH led to a rapid decrease in enzyme activity (). These results also showed that phosphoric acid buffer or Tris buffer did not have any significant effect on enzyme activity. As a result, the enzyme remained stable at pH 7.5, which met the kit requirements.
Figure 3. pH and temperature dependence of ldLDH activity
3.3. Effects of additives on ldLDH activity
The effects of metal ions and other additives on the activity of ldLDH were characterized. Residual activity was assayed following incubation with metal ions, EDTA, or ethanol (). Mg2+ and Mn2+ slightly stimulated ldLDH activity. To some extent, Ca2+, Fe3+, Zn2+, Ba2+, and ethanol inhibited enzymatic activity. Hg2+ and Cu2+ significantly inhibited ldLDH activity. The other additives had no obvious effects on ldLDH activity. Taken together, ldLDH is a metal ion-independent enzyme, because EDTA chelates metal ions and had no significant effect on ldLDH activity.
Table 1. Effects of additives on ldLDH activity
3.4. Kinetic characterization of ldLDH
The catalytic reaction rates of ldLDH at different concentrations of pyruvate were determined using the reaction system described above. When the pyruvate concentration was 10 mM, the reaction rate reached its maximum, and Vmax was 0.024 μmol/min. The specific enzyme activity of ldLDH was 1,864 U/mg at 10 mM pyruvate and 37°C, which was significantly higher than that reported previously (). The Km value of ldLDH for pyruvate was 1.34 mM, the Kcat value was 1,603 s−1, and the Kcat/Km value was 1,198 mM−1 ·s−1, which were calculated using the Lineweaver-Burk method ().
Table 2. Comparison of biochemical properties of D-LDH from various strains
Table 3. LdLDH stabilizer screening
3.5. Stability determination of ldLDH
LdLDH was incubated at different temperatures and pH conditions for specific periods, and residual activity was measured to determine thermal stability and pH tolerance. As shown in , ldLDH was stable between pH 5.5 and 9.0, and 90% of enzyme activity was retained. However, at pH < 5, activity was lost, while at pH 10, > 80% of the catalytic activity was retained, and at pH 11, only 20% of the activity was retained. As shown in , when ldLDH was placed below 50°C, the enzyme activity was stable, and no loss was observed. However, an increase in temperature led to a sharp decrease in enzyme activity, and enzyme activity was lost at 70°C. However, ldLDH retained more than 60% of its activity at 60°C. Its thermal stability was found to be better than that of lactate dehydrogenase from other strains, such as L. janssenii [Citation13] and L. plantarum.
Figure 5. Effects of pH and temperature on ldLDH stability
3.6. Selection of an optimal stabilizer for ldLDH
Freeze-drying enzymes is beneficial for preservation and transportation, but sometimes leads to loss of enzyme activity [Citation15], and the addition of a protective agent is needed for protein stabilization [Citation29,Citation30]. A total of 22 stabilizers, including sugars, polyols, amino acids, salts, proteins, and polymers, were identified from literature sources and used in the experiments reported here () [Citation31,Citation32]. The stabilizers were each mixed with ldLDH in specific proportions and freeze-dried. Freeze-dried samples were used to determine residual enzyme activity. After freeze-drying, 12 of the 22 stabilizers were found to significantly protect ldLDH by retaining > 95% of ldLDH activity. Sorbitol, xylitol, and glutamate effectively reduced the loss of activity caused by lyophilization and significantly increased ldLDH activity by 10%–20% compared to the group without a stabilizer (p < 0.05). For accelerated high-temperature testing, the lyophilized samples were subjected to an ambient temperature of 37°C to determine the stability of ldLDH. Ten of the 22 protective agents were found to be effective in protecting ldLDH for 60 days and retained enzyme activity by > 80%. Notably, when sucrose or asparagine was used as a stabilizer, there was almost no loss of ldLDH activity after 60 days (p > 0.68). In contrast, when lysine, sorbose, and magnesium chloride were used as stabilizers, ldLDH activity was significantly reduced (p < 0.05). Therefore, to retain maximal ldLDH activity, sucrose and asparagine are considered the preferred stabilizers.
3.7. Performance of ldLDH in ALT Kit
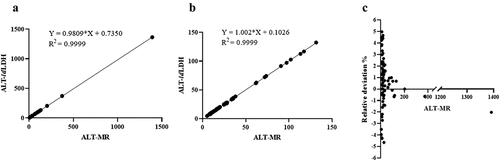
To verify the potential clinical application of ldLDH, with sucrose employed as a stabilizer, the freeze-dried powder was added to an ALT-blank kit (without ldLDH), and serum ALT activity was measured using a biochemical analyzer. At the same time, a commercial ALT kit (ALT-MR) was used to determine serum ALT activity and a comparison of the accuracy of the ldLDH-based ALT kit (ALT-ldLDH) was conducted. As shown in and 6b, the serum ALT activity detected using the developed ALT kit exhibited a highly linear relationship, with values detected using the commercial Mindray ALT kit, with an R2 value of 0.9999. Analysis of the relative deviation degree of the two kits revealed that the relative deviation degree was <5%, which met the requirements of the ALT kit (). Furthermore, outliers with ALT activity >200 can be accurately detected, with a relative deviation <3%, including 1,391 in one group ( and 6c). Therefore, the developed ALT kit can be used to detect the activity of serum ALT with high sensitivity and accuracy.
Figure 6. Performance of ldLDH in ALT kit
4. Discussion
ALT is an important diagnostic indicator, and it provides a simple and reliable method for monitoring liver function during acute liver injury circumstances such as viral hepatitis and toxic hepatitis [Citation33,Citation34]. D-LDH is the core enzyme involved in the preparation of ALT kits, and its activity and stability affect the performance of such kits. However, the D-LDH reported in previous studies has low activity or stability, limiting its practical application. To the best of our knowledge, there are few studies regarding the systematic investigation of D-LDH biochemical properties. Here, a novel ldLDH derived from L. delbrueckiiw was cloned, expressed, purified, and systematically characterized.
The ldLDH in this study showed excellent enzymatic properties and high thermostability. The specific activity of ldLDH was 1,864 U/mg, which was significantly higher than that of D-LDHs from other strains reported previously (). LdLDH has moderate kinetic parameters, which are lower than those of several other D-LDHs, indicating that there is a considerable improvement space by site-directed mutagenesis. Therefore, the bioactivity and kinetic parameters of ldLDH can be further improved by directed evolution methods, such as error-prone PCR, rational design, and DNA shuffling, based on sequence alignment, homology modeling, and computational analysis [Citation35]. The Km value of D-LDH from Sporolactobacillus inulinus was reduced from 0.114 mM to 0.08 mM by an N174Y mutation designed based on crystal structure analysis [Citation36].
In addition, ldLDH exhibited high thermostability. When ldLDH was incubated at 50°C for 20 min, ldLDH retained most of its catalytic activity, while at 60°C, > 60% of its activity was retained. D-LDH derived from Pediococcus acidilactici is reported to exhibit a rapid decrease in enzyme activity at temperatures > 30°C [Citation7]. The T50 value (the temperature at which 50% of enzyme activity is lost following heat treatment for 10 min) of D-LDH derived from L. coryniformis is 39.5°C [Citation27]. The activity of D-LDH derived from P. pentosaceus is markedly decreased at 45°C [Citation37]. The D-LDH identified here by genomic mining was stable at 70°C with a half-life of 84 h, however, its specific activity was only 30.2 U/mg [Citation38], much lower than that of ldLDH. Therefore, to meet the requirements for clinical testing, both high thermostability and activity are required, and the developed ldLDH meets the requirements for clinical diagnosis.
To improve the chemical and physical stability of proteins that facilitate commercial distribution and storage, the freeze-drying method has been widely used. Additionally, after the freeze-drying of proteins, the costs involved in product preservation and transportation can be reduced. However, during freeze-drying, protein structural perturbation, aggregation, denaturation, or loss of activity may be observed due to a variety of stresses such as crystallization, dehydration stress, interface stress, pH change, ionic strength change, and ice crystal formation [Citation39,Citation40]. Therefore, it is necessary to add protective agents to stabilize the protein, protect the protein native structure from denaturation, and reduce the loss of enzyme activity [Citation29]. Various compounds, including sugars, polyols, amino acids, certain salts, proteins, and polymers have been proven to be effective in minimizing protein denaturation during the freeze-drying process [Citation20,Citation21,Citation40].
To date, several theories have been proposed to explain the protective mechanisms responsible for the effects of protectants on proteins during lyophilization. Vitrification (glass formation) and water replacement theory are the two main mechanisms [Citation40]. The vitrification mechanism depends on the immobilization of protein molecules, accompanied by glassification, preventing protein-protein interactions. The latter mechanism involves the formation of hydrogen bonds between stabilizers and polar groups of protein molecules, inhibiting the unfolding of proteins. Other stabilization mechanisms include ligand binding, protectant-protein interactions via amino protons, formation of hydration spheres, and accumulation of stabilizers around specific amino acid types [Citation14,Citation40–42].
In this study, the effects of different stabilizers on the freeze-drying and long-term storage stability of ldLDH were tested. During freeze-drying, sorbitol, xylitol, and glutamate exhibited good protective effects against ldLDH denaturation. However, when ldLDH was stored at 37°C, the bioactivity of freeze-dried ldLDH mixed with sorbitol, xylitol, and glutamate were significantly reduced. In contrast, sucrose and asparagine maximally preserved the bioactivity of freeze-dried ldLDH stored at 37°C for 2 months. This difference may be attributed to several factors, such as the glass transition temperature (Tg), stabilizer phase separation, and additional stress factors during storage. The Tg of the stabilizer is directly related to its long-term stabilizing ability; sucrose may have a higher Tg than sorbitol, xylitol, and glutamate. High Tg may contribute to maintaining the freeze-dried ldLDH in a stable, glassified state during storage [Citation43,Citation44]. Additionally, the observed decline in protective action may result from a greater tendency to undergo phase separation compared with sucrose and asparagine [Citation45,Citation46]. Furthermore, additional stress factors during storage, such as unintended temperature excursions, may result in different protective effects between freeze-drying and long-term storage [Citation46].
In terms of practical applications, the solubility of asparagine is relatively low and may cause a small amount of precipitation at different environmental temperatures. Moreover, the addition of asparagine can lead to a significant change in pH, which needs to be readjusted. Therefore, we selected sucrose, which is more stable and convenient for further study. Notably, sucrose preserved 99.5% of ldLDH activity after 60 days of storage. To the best of our knowledge, this is the most stable ldLDH reported to date. Our findings were in accord with previous studies in which a significant protective effect of sucrose was observed on protein stability during freeze-drying and storage [Citation14,Citation40,Citation47]. The mechanism by which sucrose enhances the stability of ldLDH may involve remaining amorphous, water replacement, and vitrification [Citation43,Citation44], as described above. LdLDH with high stability has great advantages in clinical applications. This is because when ALT kits are used for liver function tests, they are generally performed at room temperature, and ldLDH, which is highly stable, can help obtain reliable data, which is highly significant in clinical detection of disease.
Finally, freeze-dried ldLDH was used to prepare an ALT assay kit, and its clinical application was compared with that of a commercial kit. The developed ALT kit based on ldLDH was found to accurately detect ALT activity in serum, with a margin error of <5% compared with the commercial kit. In addition, its sensitivity and detection range met the kit requirements for clinical applications. This study lays the foundation for the development of new ALT kits.
5. Conclusion
LdLDH is the key enzyme in ALT kits and has important application value in clinical detection of disease. In this study, ldLDH from L. delbrueckii was expressed in an E. coli expression system, purified, and a series of studies were conducted. The results showed that ldLDH had high specific activity and thermal stability, with a specific activity of 1,864 U/mg. When placed at 50°C for 20 min, enzyme activity was not lost, and the kit met the requirements for clinical diagnosis. The use of sucrose as a stabilizer significantly improved ldLDH stability during freeze-drying and storage. The results of the ALT test kit prepared with ldLDH were consistent with those reported using commercial kits. Based on the findings of this study, the developed ALT kit meets the requirements for clinical diagnostic application.
Ethical approval
This study was approved by the ethics committee of Human Research Ethics Committee of Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China.
Acknowledgements
This study was supported by Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province (Grant No.2019E10020); Ningbo Public Service Techology Foundation, China (Grant No.2019C50035, 202002N3156).
Disclosure statement
The authors declare no competing financial interest.
References
- Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med. 2002;137:49–51.
- Kim WR, Flamm SL, Di Bisceglie AM, et al. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363–1370.
- Rej R. Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin Chem. 1978;24(11):1971–1979.
- Tsai JF, Jeng JE, Ho MS, et al. Serum alanine aminotransferase level in relation to hepatitis B and C virus infections among blood donors. Liver. 1997;17(1):24–29.
- Kochhar S, Chuard N, Hottinger H. Serum alanine aminotransferase level in relation to hepatitis B and C virus infections among blood donors. J Biol Chem. 1992;267(28):20298–20301.
- Kochhar S, Hunziker PE, Leong-Morgenthaler P, et al. Primary structure, physicochemical properties, and chemical modification of NAD(+)-dependent D-lactate dehydrogenase. Evidence for the presence of Arg-235, His-303, Tyr-101, and Trp-19 at or near the active site. J Biol Chem. 1992;267(12):8499–8513.
- Mu W, Yu S, Jiang B, et al. Characterization of D-lactate dehydrogenase from Pediococcus acidilactici that converts phenylpyruvic acid into phenyllactic acid. Biotechnol Lett. 2012;34(5):907–911.
- Zheng ZJ, Ma CQ, Gao C, et al. Efficient conversion of phenylpyruvic acid to phenyllactic acid by using whole cells of bacillus coagulans SDM. PLoS One. 2011;6(4):e.19030.
- Jia JH, Mu WM, Zhang T, et al. Bioconversion of phenylpyruvate to phenyllactate: gene cloning, expression, and enzymatic characterization of D- and L1-lactate dehydrogenases from Lactobacillus plantarum SK002. Appl Biochem Biotechnol. 2010;162(1):242–251.
- Tokuda C, Ishikura Y, Shigematsu M, et al. Conversion of Lactobacillus pentosus d-Lactate Dehydrogenase to a d-Hydroxyisocaproate dehydrogenase through a single amino acid replacement. J Bacteriol. 2003;185(16):5023–5026.
- Zhu YB, Hu FG, Zhu YY, et al. Enhancement of phenyllactic acid biosynthesis by recognition site replacement of D-lactate dehydrogenase from lactobacillus pentosus. Biotechnol Lett. 2015;37(6):1233–1241.
- Hummel W, Schutte H, Kula MR. Large-scale production of D-lactate dehydrogenase for the stereospecific reduction of pyruvate and phenylpyruvate. Eur J Appl Microbiol Biotechnol. 1983;18(2):75–85.
- Jun C, Sa YS, Gu S-A, et al. Discovery and characterization of a thermostable D-lactate dehydrogenase from Lactobacillus jensenii through genome mining. Process Biochem. 2013;48(1):109–117.
- Liao YH, Brown MB, Quader A, et al. Protective mechanism of stabilizing excipients against dehydration in the freeze-drying of proteins. Pharm Res. 2002;19(12):1854–1861.
- Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12(5):505–523.
- Manning MC, Chou DK, Murphy BM, et al. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27:544–575.
- Ohtake S, Kita Y, Arakawa T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliv Rev. 2011;63:1053–1073.
- Jeong SH. Analytical methods and formulation factors to enhance protein stability in solution. Arch Pharm Res. 2012;35(11):1871–1886.
- Zhao G, Zhang G. Effect of protective agents, freezing temperature, rehydration media on viability of malolactic bacteria subjected to freeze-drying. J Appl Microbiol. 2005;99(2):333–338.
- Zhan Y, Xu Q, Yang MM, et al. Screening of freeze-dried protective agents for the formulation of biocontrol strains, Bacillus cereus AR156, Burkholderia vietnamiensis B418 and Pantoea agglomerans 2Re40. Lett Appl Microbiol. 2012;54(1):10–17.
- Iyengar ARS, Tripathy RK, Bajaj P, et al. Improving storage stability of recombinant organophosphorus hydrolase. Protein Expr Purif. 2015;111:28–35.
- Zhou XH, Zhou J, Xin FX, et al. Heterologous expression of a novel D-lactate dehydrogenase from Lactobacillus sp ZX1 and its application for D phenyllactic acid production. Int J Biol Macromol. 2018;119:1171–1178.
- Krieg RC, Dong Y, Schwamborn K, et al. Protein quantification and its tolerance for different interfering reagents using the BCA-method with regard to 2D SDS PAGE. J Biochem Biophys Methods. 2005;65(1):13–19.
- Schumann C, Michlmayr H, Del Hierro AM, et al. Malolactic enzyme from Oenococcus oeni: heterologous expression in Escherichia coli and biochemical characterization. Bioengineered. 2013;4(3):147–152.
- Li XF, Jiang B, Pan BL, et al. Purification and partial characterization of lactobacillus species SK007 Lactate Dehydrogenase (LDH) Catalyzing Phenylpyruvic Acid (PPA) Conversion into Phenyllactic Acid (PLA). J Agric Food Chem. 2008;56(7):2392–2399.
- Kochhar S, Lamzin VS, Razeto A, et al. Roles of His205, His296, His303 and Asp259 in catalysis by NAD+-specific D-lactate dehydrogenase. Eur J Biochem. 2000;267(6):1633–1639.
- Gu SA, Jun C, Joo JC, et al. Higher thermostability of L-lactate dehydrogenases is a key factor in decreasing the optical purity of D-lactic acid produced from Lactobacillus coryniformis. Enzyme Microb Technol. 2014;58-59:29–35.
- Yang XL, Liu BZ, Sang Y, et al. Kinetic analysis of the lactate-dehydrogenase-coupled reaction process and measurement of alanine transaminase by an integration strategy. Anal Sci. 2010;26(11):1193–1198.
- Gouda MD, Singh SA, Rao AGA, et al. Thermal inactivation of glucose oxidase - Mechanism and stabilization using additives. J Biol Chem. 2003;278(27):24324–24333.
- Sawangwan T, Goedl C, Nidetzky B. Glucosylglycerol and glucosylglycerate as enzyme stabilizers. Biotechnol J. 2010;5(2):187–191.
- Dabulis K, Klibanov AM. Dramatic enhancement of enzymatic activity in organic solvent by lyoprotectants. Biotechnol Bioeng. 1993;41(5):566–571.
- Roy I, Gupta MN. Freeze-drying of proteins: some emerging concerns. Biotechnol Appl Biochem. 2004;39(2):165–177.
- Xu H-M, Chen Y, Xu J, et al. Drug-induced liver injury in hospitalized patients with notably elevated alanine aminotransferase. World J Gastroenterol. 2012;18(41):5972–5978.
- Galvin Z, McDonough A, Ryan J, et al. Blood alanine aminotransferase levels >1,000 IU/l – causes and outcomes. Clin Med (Lond). 2015;15(3):244–247.
- Tamaki FK, Reece R. Directed evolution of enzymes. Emerg Top Life Sci. 2020;4(2):119–127.
- Zhu LF, Xu XL, Wang LM, et al. NADP+-Preferring d-Lactate dehydrogenase from sporolactobacillus inulinus. Appl Environ Microbiol. 2015;81(18):6294–6301.
- Yu S, Jiang H, Jiang B, et al. Characterization of D-Lactate Dehydrogenase producing D-3-Phenyllactic acid from pediococcus pentosaceus. Biosci Biotechnol Biochem. 2012;76(4):853–855.
- Li C, Tao F, Xu P. Carbon Flux Trapping: highly Efficient Production of Polymer-Grade d-Lactic Acid with a Thermophilic d-Lactate Dehydrogenase. Chembiochem. 2016;17(16):1491–1494.
- Fang R, Bogner RH, Nail SL, et al. Stability of freeze-dried protein formulations: contributions of ice nucleation temperature and residence time in the freeze-concentrate. J Pharm Sci. 2020;109(6):1896–1904.
- Butreddy A, Janga KY, Ajjarapu S, et al. Instability of therapeutic proteins - An overview of stresses, stabilization mechanisms and analytical techniques involved in lyophilized proteins. Int J Biol Macromol. 2021;167:309–325.
- Allison SD, Chang B, Randolph TW, et al. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch Biochem Biophys. 1999;365(2):289–298.
- Wang W, Ohtake S. Science and art of protein formulation development. Int J Pharm. 2019;568:118505.
- Hansen LJJ, Daoussi R, Vervaet C, et al. Freeze-drying of live virus vaccines: a review. Vaccine. 2015;33(42):5507–5519.
- Tonnis WF, Mensink MA, de Jager A, et al. Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol Pharm. 2015;12(3):684–694.
- Forney-Stevens KM, Pelletier MJ, Shalaev EY, et al. Optimization of a Raman microscopy technique to efficiently detect amorphous–amorphous phase separation in freeze-dried protein formulations. J Pharm Sci. 2014;103(9):2749–2758.
- Thakral S, Sonje J, Munjal B, et al. Stabilizers and their interaction with formulation components in frozen and freeze-dried protein formulations. Adv Drug Del Rev. 2021;173:1–19.
- Bravo-Ferrada BM, Brizuela N, Gerbino E, et al. Effect of protective agents and previous acclimation on ethanol resistance of frozen and freeze-dried Lactobacillus plantarum strains. Cryobiology. 2015;71(3):522–528.