ABSTRACT
Differentiated thyroid cancer (DTC), such as papillary thyroid cancer, has a good prognosis after routine treatment. However, in the course of treatment, 5% to 20% of cases may dedifferentiate and can be transformed into dedifferentiated DTC (deDTC) or anaplastic thyroid cancer, leading to treatment failure. To date, several drugs have been used effectively for dedifferentiated thyroid cancer, whereas gene therapy may be a potential method. Literature reported that double suicide genes driven by human telomerase reverse transcriptase promoter (hTERTp) can specifically express in cancer cells and kill them. However, the weak activity of hTERTp limits its further research. To overcome this weakness, we constructed a novel chitosan nanocarrier containing double suicide genes driven by a ‘gene switch’ (a cascade of radiation enhancer E9 and a hTERTp). The vector was labeled with iodine-131 (131I). On one hand, E9 can significantly enhance the activity of hTERTp under the weak radiation of 131I, thereby increasing the expression of double suicide genes in deDTC cells. On the other hand, 131I also plays a certain killing role when it enters host cells. The proposed nanocarrier has good specificity for deDTC cells and thus deserves further study.
Introduction
Thyroid cancer has been the most common endocrine cancer in the world [Citation1]. Most cases are differentiated thyroid cancers (DTCs), of which papillary thyroid cancer and follicular thyroid carcinoma (FTC) are the major histological types [Citation2]. Although most DTCs are curable by surgery and iodine treatment, 5% to 20% of patients may suffer disease recurrence with a high mortality rate [Citation3].
The dedifferentiation of DTC into poorly or dedifferentiated DTC (deDTC) and anaplastic thyroid cancer (ATC) is responsible for the poor prognosis in patients, possibly due to radioactive iodine (RAI) refractoriness resulting from the decreased expression of sodium iodide symporter (NIS) [Citation4]. Clinically, DeDTC is characterized by aggressive growth, recurrence, distant metastasis, and resistance to RAI therapy, along with poor prognosis and high early mortality [Citation5].
When the treatment (surgery and RAI administration) fails in high-risk patients with deDTC, effective therapy is lacking [Citation6]. Thus, a new therapeutic strategy is needed, with gene therapy being a promising method.
Previously, the transfer of double suicide gene system, thymidine kinase E. coli cytosine deaminase (TK/CD), which encodes enzymes that convert nontoxic prodrugs to toxic forms and thus kill the host cells, was suggested to cause cell death after the administration of prodrugs [Citation7]. Two-gene directed enzyme prodrug therapies have been intensively studied in various cancer cells, and they exerted marked effects on host cells [Citation8]. To selectively express TK/CD in cancer cells rather than normal cells, scholars have investigated several promoters, such as human telomerase reverse transcriptase promoter (hTERTp) [Citation9], KDR promoter [Citation10], and survivin promoter [Citation11]. Of these promoters, hTERTp has been mostly suggested to drive gene expressions specifically in a number of studies.
Evidence indicated that the suicide gene system driven by hTERTp shows specificity toward thyroid cancer cells [Citation12]. However, hTERTp exhibits a weak driving efficiency in cancer cells [Citation13].
On the basis of the above evidence, we hypothesized that specifically enhancing the activity of hTERTp will effectively drive the expression of its downstream suicide gene in cancer cells, effectively killing them. Thus, we aimed to construct a novel carrier containing double suicide genes (TK/CD) driven by hTERTp. To overcome the shortage of hTERTp, we added a radiation enhancer (E9) before hTERTp and wrapped the plasmids in chitosan-based nanoparticles. Then, the particles were labeled with iodine-131 (131I). Hence, under low radiation, the efficiency of hTERTp can be significantly enhanced. Moreover, the chitosan-based nanoparticles can be devoured easily by cancer cells. Thus, the killing efficacy of the plasmids on cancer cells may be significantly enhanced.
Materials and methods
Cell culture
The human DTC cell line FTC-133 and Nthy-ori3-1, a thyroid follicular epithelial cell line, were obtained from American Type Culture Collection, cultured in the mixture of Dulbecco’s Modified Eagle’s Medium and Ham’s F-12 medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin, and grown at 37°C and 5% CO2.
The dedifferentiated FTC-133 (dFTC-133) cell line was established by limiting dilution analysis after 131I radiation in accordance with the literature [Citation14]. The cells with the stable and lowest RAI uptake (RAIU) were set as dFTC-133, and their iodide-metabolizing molecules were detected.
RAIU assay
RAIU assay was conducted in accordance with the literature to evaluate the ability of cells to uptake iodine [Citation14]. The radioactivity was normalized to the number of cells present at the time of the assay as cpm every 106 cells.
Synthesis of chitosan nanoparticles
pCDNA3.1-yCDglyTK was provided by Chonqqing Sijun Biotech company. The cDNA product of hTERTp was synthesized in accordance with the literature [Citation15]. The primers for hTERTp: F: GGCGGCATTAATGGCCCCTCCCTCGGGTTACCC; R: TTATTAGCTAGC CGCGGGGGTGGCCGGGGCCAG.
The radiation response element of CArG originating from early growth response 1 was synthesized by Shanghai Qinglan Biotech Company. Each fragment was digested by tool enzymes and linked to the plasmid pCDNA3.1 in the following sequence: pCDNA3.1 E9-hTERTp- yCDglyTK (pE9-hTERTp-yCDglyTK).
The plasmids were wrapped by chitosan to construct nanoparticles in accordance with our previous study [Citation16]. Chloramine-T method was used to label the nanoparticles with 131I [Citation17].
As a result, the following vectors were constructed and prepared for experiments:1) vector 1: 131I-labeled+chitosan+pE9-hTERTp-yCDglyTK; 2) vector 2: chitosan+phTERTp-yCDglyTK; 3) vector 3: 131I-labeled+chitosan; 4) chitosan.
The morphological characteristics of nanoparticles were observed by using transmission electron microscopy.
Cell viability and apoptosis assays
Cells were plated in 96-well plates (1 × 104 cells/well). 3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays were used to assess cell viability, and apoptosis was evaluated by using the Annexin V-FITC apoptosis detection kit (Beytime, China), as described in our previous reports [Citation16].
Quantitative polymerase chain reaction (PCR) assay
Real-time PCR was conducted with a PikoReal Real-Time PCR system (Thermo Fisher Scientific, Vantaa, Finland). The total RNA of cells was extracted and then reversely transcribed to yield cDNA. PCR reactions were conducted. The expression levels of the genes were determined by a threshold cycle number (Ct), which was normalized against the internal reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by using the ΔΔCt method. The primer pairs are listed as follows [Citation14,Citation18]:
GAPDH: F: ATTCCACCCATGGCAAATTC, R: GCATCGCCCCACTTGATT; NIS: F: GACAAACCTCTGAGGACAGGG, R: ATACTGGGGACGGTTGAAGC; TPO: F: ACACAGGCAAATCGGAAATC, R: GCAATGTTTACAAGAAAAGGCC; Tg: F: GGGCATGTTACTGCATGTC, R: TTTGAACACAGGTCTGCCA; TK/CD: F: CGTGGCGGCAAGGTGAT, R: TGCTATGGCCGCGAGAA.
Statistical analysis
Data were expressed as mean value ± standard deviation (SD). Differences between groups were analyzed with one-way analysis of variance (ANOVA) or t-test. The t-test was used for the comparison between two groups. ANOVA was used for the comparison of three or more groups. When the result was positive, Student–Newman–Keuls test was used between groups. The analyses were performed by utilizing SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL). A P value of less than 0.05 was considered statistically significant.
Results
Dedifferentiated thyroid cancer is a serious threat to human health. deDTC cells were established. The double suicide gene driven by hTERTp showed its specificity toward cancer cells. However, the hTERTp exhibited a weak activity. Thus, we constructed a novel chitosan nanocarrier containing double suicide genes driven by a ‘gene switch’ (a cascade of radiation enhancer E9 and hTERTp). The vector was labeled with 131I. The cytotoxic efficacy of the nanocarrier was also evaluated.
Morphological characteristics of the nanoparticles
The nanoparticles of the plasmid were nearly 80–120 nm in size and approximately round shaped with a smooth surface, implying that the nanoparticles were successfully constructed ().
Figure 1. (a) Transmission electron microscopic image of the constructed nanoparticles (vector1). (b) The radioiodine uptake was lower in dFTC-133 cells than that in FTC-133 ones (*P < 0.05, FTC-133 vs dFTC-133). (c) Relative mRNA expression of the thyroid specific markers were decreased in dFTC-133 cells relative to FTC-133 cells, respectively (*P < 0.05, FTC-133 vs dFTC-133 in each comparison). (d) The expression level of CD/TK mRNA was significantly higher in dFTC-133+ vector1 than that in dFTC-133+ vector2 (*P < 0.05, ‘dFTC-133+ vector2ʹ vs ‘dFTC-133+ vector2ʹ). (e-f) Marked difference between ‘with prodrug’ and ‘without prodrug’ groups can be shown in vector1 and vector2 group, respectively (*P < 0.05, #P < 0.05, ‘with prodrug’ vs ‘without prodrug’ in each subgroup). Nevertheless, increased cell apoptosis and decreased cell viability can be observed in cells transfected with vector1 compared with those with vector2 (* vs #, P < 0.05, ‘with prodrug’ in ‘vector1ʹ vs ‘vector2ʹ). Increased cell apoptosis and decreased cell viability were observed in vector3 group compared with vector4, regardless of prodrug treatment (†P < 0.05, ‘vector3ʹ vs ‘vector4ʹ in each subgroup)
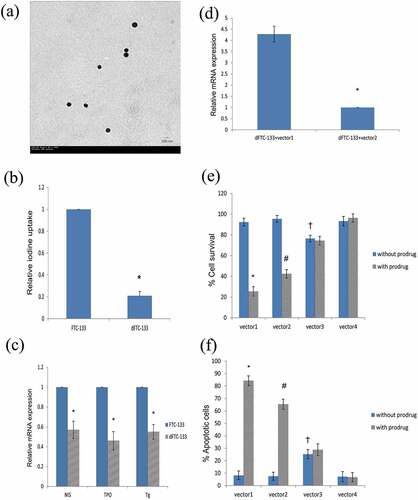
Establishment of dFTC-133 cells
dFTC-133 cells were cultured. The RAIU of the cells was evaluated. The relative RAIU of the dFTC-133 cells was 0.21 relative to the primary FTC-133 cells (). Moreover, the mRNA expression levels of NIS, TPO, and Tg in dFTC-133 cells were significantly lower than those in FTC-133 cells ().
CD-TK expression
CD/TK expression was studied by reverse-transcription PCR (RT-PCR) in cells after transfection to test the driven efficacy of the promoters in the plasmids.
dFTC-133 and Nthy-ori3-1 cells were cultured and divided into six groups: dFTC-133, dFTC-133+ vector1, dFTC-133+ vector2, Nthy-ori3-1, Nthy-ori3-1+ vector1, and Nthy-ori3-1+ vector2. The cells were treated with/without relevant vectors at a dose of 10 µg/ml. After 48 h, the mRNA expression of CD/TK was detected by RT-PCR. The results showed that the mRNA expression of CD/TK was only detected in dFTC-133+ vector1 and dFTC-133+ vector2 groups, suggesting the specificity of hTERTp in cancer cells. Moreover, the mRNA expression level of CD/TK was higher in dFTC-133+ vector1 group than in the dFTC-133+ vector2 group ().
Cytotoxicity analysis
Cytotoxicity experiments were conducted to test the effect of nanoparticle transfection on the cells. The dFTC-133 cells were divided into four groups: 1) dFTC-133+ vector1; 2) dFTC-133+ vector2 3) dFTC-133+ vector3; 4) dFTC-133+ vector4. Each group was subdivided into two groups based on prodrug content.
Cell proliferation was detected by MTT assays. As shown in significantly decreased cell viability and increased cell apoptosis were observed in cells treated with prodrugs in groups 1 and 2 (P < 0.05), in which cell apoptosis markedly increased in group 1 relative to that in group 2 (P < 0.05), indicating that the combination of double suicide gene with the prodrug can result in cell damage, whereas the combination of E9 enhancer and low radiation from 131I may significantly enhance the driving efficiency of the downstream promoter and thus increase cell apoptosis. Notably, in group 3, increased cell apoptosis was observed in cells regardless of the presence of prodrugs, suggesting that the labeled 131I may help exert cytotoxic effects on host cells.
Discussion
In the present study, we first established deDTC cells (dFTC-133), constructed chitosan-encapsulated double suicide gene vectors, and successfully increased CD/TK expression in cultured dFTC-133 cells.
Several effective treatment methods can be used for patients with deDTC. Previously, doxorubicin was the only approved drug for ATC treatment. Nevertheless, the response rate was very low, and numerous side effects may be caused [Citation19]. Tyrosine kinase inhibitors (TKIs), namely, sorafenib and lenvatinib, have also been used for deDTC treatment. However, little evidence indicated that use of TKI can prolong the overall survival time of patients [Citation20]. Hence, novel methods that can effectively treat patients with deDTC are needed.
The suicide gene system can kill cancer cells because it can use an enzyme to convert nontoxic prodrugs into cytotoxic compounds. Ganciclovir (GCV) is often used as an antiviral compound, and the HSV-TK gene encodes thymidine kinase that can phosphorylate GCV to GCV-toxic triphosphate. Hence, the activity of DNA polymerase may be inhibited [Citation21]. The CD gene encodes cytosine deaminase which can be metabolized to 5-Fc and then transformed into the cytotoxic agent 5-Fu [Citation22]. The prodrug (5-FC or GCV) is nontoxic to normal human cells when administered alone. However, the presence of enzymes (CD or TK) may catalyze the prodrug into its cytotoxic form. Then, the generated metabolites will exert antitumor activity. Evidence showed that the combination of the two suicide gene systems (CD/TK) can enhance antitumor activity without damaging normal cells [Citation23]. Therefore, the double suicide gene system was used in the present study, and the results confirmed its antitumor activity.
A previous study used suicide gene driven by hTERTp to selectively kill thyroid cancer cells [Citation12]. Nevertheless, the intense research and application have been limited due to the weak efficiency of the promoter, although the use of the plasmids has achieved encouraging results. Enhancers can significantly intensify the driving efficiency of promoters [Citation24], and radio-sensitive promoters can greatly improve the driving efficiency under radiations [Citation25]. Thus, to overcome the shortage of plasmids, radiation enhancer ‘E9’ was added prior to hTERTp to construct a ‘gene switch’ that can selectively control the CD/TK expression in cancer cells. The driving efficiency of the promoter may be enhanced by low radiation. Then, the plasmid was wrapped by chitosan to construct nanoparticles that can be easily taken up by thyroid carcinoma cells. Afterward, the nanoparticles were labeled by RAI 131. Chitosan is a nano-carrier with numerous advantages, such as good biocompatibility, nontoxicity, and ease of preparation; its absorption-enhancing properties make it an ideal carrier for the delivery of genes [Citation26]. Therefore, the vector we constructed can achieve the anti-tumor effect from two aspects. On the one hand, the labeled 131I can act as low-dose radiation source to drive the expression of CD/TK, and on the other hand, the 131I that entered may directly kill the host cells to a certain extent. Hence, the constructed nanoparticles may increase the efficiency and specificity of suicide gene expression in cancer cells.
Conclusion
In conclusion, we constructed a nanoparticle that may selectively and effectively express double suicide gene in cultured dFTC-133 cells, which may be a potential effective strategy for DeDTC treatment.
Abbreviations
DTCs: differentiated thyroid cancers; FTC: follicular thyroid carcinoma; deDTC: dedifferentiated DTC; ATC: Anaplastic thyroid cancer; TK/CD: Thymidinekinase/E.colicytocinedeaminase; 131I: Iodine-131; DMEM: Dulbecco’s modified Eagle’s medium; dFTC-133: dedifferentiated FTC-133; TEM: transmission electron microscopy; Ct: cycle number; ANOVA: Analysis of Variances; RAIU: radioiodine uptake; TKIs: Tyrosine kinase inhibitors; GCV: Ganciclovir; hTERTp: human telomerase reverse transcriptase promoter; RAI: radioactive iodine; NIS: sodium iodide symporter; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; SD: standard deviation
Data availability statement
All data generated or analyzed during this study are included in this published article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ma B, Xu W, Wei W, et al. Clinicopathological and survival outcomes of well-differentiated thyroid carcinoma undergoing dedifferentiation: a retrospective study from FUSCC. Int J Endocrinol. 2018;2018:2383715.
- Li Q, Liang J, Zhang S, et al. Overexpression of centromere protein K (CENPK) gene in differentiated thyroid carcinoma promote cell proliferation and migration. Bioengineered. 2021;12(1):1299–1310.
- Ma B, Jiang H, Wen D, et al. Transcriptome analyses identify a metabolic gene signature indicative of dedifferentiation of papillary thyroid cancer. J Clin Endocrinol Metab. 2019;104(9):3713–3725.
- Oh JM, Ahn BC. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. 2021;11(13):6251–6277.
- Chen H, Luo D, Zhang L, et al. Restoration of p53 using the novel MDM2-p53 antagonist APG115 suppresses dedifferentiated papillary thyroid cancer cells. Oncotarget. 2017;8(26):43008–43022.
- Klein Hesselink EN, Zafon C, Villalmanzo N, et al. Increased global DNA hypomethylation in distant metastatic and dedifferentiated thyroid cancer. J Clin Endocrinol Metab. 2018;103(2):397–406.
- Lee M, Kim YS, Lee K, et al. Novel semi-replicative retroviral vector mediated double suicide gene transfer enhances antitumor effects in patient-derived glioblastoma models. Cancers (Basel). 2019;11(8). DOI:10.3390/cancers11081090
- Navarro SA, Carrillo E, Grinan-Lison C, et al. Cancer suicide gene therapy: a patent review. Expert Opin Ther Pat. 2016;26(9):1095–1104.
- Liu T, Wu HJ, Liang Y, et al. Tumor-specific expression of shVEGF and suicide gene as a novel strategy for esophageal cancer therapy. World J Gastroenterol. 2016;22(23):5342–5352.
- Ma J, Li M, Mei L, et al. Double suicide genes driven by kinase domain insert containing receptor promoter selectively kill human lung cancer cells. Genet Vaccines Ther. 2011;9:6.
- Liu C, Wen C, Wang X, et al. Golgi membrane protein GP73 modified-liposome mediates the antitumor effect of survivin promoter-driven HSVtk in hepatocellular carcinoma. Exp Cell Res. 2019;111496. DOI:10.1016/j.yexcr.2019.111496.
- Takeda T, Inaba H, Yamazaki M, et al. Tumor-specific gene therapy for undifferentiated thyroid carcinoma utilizing the telomerase reverse transcriptase promoter. J Clin Endocrinol Metab. 2003;88(8):3531–3538.
- Shepelev MV, Kalinichenko SV, Saakian EK, et al. Xenobiotic response elements (XREs) from human CYP1A1 gene enhance the hTERT promoter activity. Dokl Biochem Biophys. 2019;485(1):150–152.
- Feng F, Wang H, Hou S, et al. Re-induction of cell differentiation and (131)I uptake in dedifferentiated FTC-133 cell line by TSHR gene transfection. Nucl Med Biol. 2012;39(8):1261–1265.
- Higashi K, Hazama S, Araki A, et al. A novel cancer vaccine strategy with combined IL-18 and HSV-TK gene therapy driven by the hTERT promoter in a murine colorectal cancer model. Int J Oncol. 2014;45(4):1412–1420.
- Zhuo X, Chang A, Huang C, et al. Nanoparticle-mediated down-regulation of TWIST increases radiosensitivity of nasopharyngeal carcinoma cells via ERK pathway. Am J Cancer Res. 2015;5(4):1571–1579.
- Li C, Tan J, Chang J, et al. Radioiodine-labeled anti-epidermal growth factor receptor binding bovine serum albumin-polycaprolactone for targeting imaging of glioblastoma. Oncol Rep. 2017;38(5):2919–2926.
- Niu Y, Li JS, Luo XR. Enhancement of expression of survivin promoter-driven CD/TK double suicide genes by the nuclear matrix attachment region in transgenic gastric cancer cells. Gene. 2014;534(2):177–182.
- Marano F, Frairia R, Rinella L, et al. Combining doxorubicin-nanobubbles and shockwaves for anaplastic thyroid cancer treatment: preclinical study in a xenograft mouse model. Endocr Relat Cancer. 2017;24(6):275–286.
- Viola D, Valerio L, Molinaro E, et al. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016;23(4):R185–205.
- Yi QY, Bai ZS, Cai B, et al. HSVTK/GCV can induce cytotoxicity of retinoblastoma cells through autophagy inhibition by activating MAPK/ERK. Oncol Rep. 2018;40(2):682–692.
- Liu Y, Zhu P, Huang Z, et al. Simultaneous detection of 5-fluorocytosine and 5-fluorouracil in human cells carrying CD/5-FC suicide gene system by using capillary zone electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1076:1–7.
- Duzgunes N. Origins of suicide gene therapy. Methods Mol Biol. 2019;1895:1–9.
- Xu ZJ, Jia YL, Wang M, et al. Effect of promoter, promoter mutation and enhancer on transgene expression mediated by episomal vectors in transfected HEK293, Chang liver and primary cells. Bioengineered. 2019;10(1):548–560.
- Ogawa R, Morii A, Watanabe A, et al. Development of a therapeutically important radiation induced promoter. Bioengineered. 2013;4(1):44–49.
- Lv Y, Zhang J, Wang C. Self-assembled chitosan nanoparticles for intranasal delivery of recombinant protein interleukin-17 receptor C (IL-17RC): preparation and evaluation in asthma mice. Bioengineered. 2021;12(1):3029–3039.
