ABSTRACT
Ischemia/reperfusion (I/R) injury causes complications in early coronary artery reperfusion for acute myocardial infarction (AMI). Ozone (O3) has been reported to be applied for protecting I/R injury, but its detailed mechanism remains unclear. Our study focused on the protective effect of O3 pretreatment on myocardial I/R injury and JAK2/STAT3 signaling and HSP70 regulation involving in the mediation. The rat hearts which were perfused and isolated as well as the cultured cardiomyocytes of neonatal rat were exposed to hypoxia/reoxygenation (H/R) and different concentrations of O3 followed by heat shock protein 70 (HSP70) siRNA treatment. The results showed O3 attenuated the suppression of cell viability induced by H/R and decreased the release of activity of creatine kinase (CK), lactate dehydrogenase (LDH) and apoptosis of cardiomyocytes in vitro. Moreover, O3 also activated the JAK2/STAT3 signaling, upregulated the expression of HSP70 both in vitro and vivo, and decreased the index of apoptosis of cardiomyocytes caused by I/R as well as myocardial infarct area in vivo. In addition, HSP70 siRNA and JAK2 inhibitor AG490 inhibited the cardioprotective effect of O3. And the expression of HSP70 increased by ozone was reduced by AG-490. In conclusion, our results demonstrated that ozone protects cardiomyocytes in I/R injury through regulation of the expression of HSP70 by activating the JAK2/STAT3 pathway.
Introduction
Globally, acute myocardial infarction (AMI) is the most morbid and mortal disease worldwide, and coronary artery reperfusion in the early stage is the most effective and important treatment [Citation1]. But, this can also result in paradoxical ischemia/reperfusion injury (IRI) [Citation2], inducing some fatal reperfusion injury such as myocardial stunning, the phenomenon of no-reflow and arrhythmia caused by reperfusion [Citation3]. However, IRI is a complex process involving oxidative stress, inflammation, intracellular Ca2+ overload, neurohumoral activation cell, death by apoptosis and necrosis [Citation4,Citation5].
Signaling pathway of transcription 3 (STAT3) is a signal transducer and activator that, along with Janus kinase 2 (JAK2), is widely participated in the body’s biological processes. And, it is a mechanism that can transmit signals from the cell surface to the nucleus through a stress response [Citation6]. The JAK2/STAT3 pathway is an important string associated with regulation of cell proliferation, differentiation, apoptosis and inflammatory response [Citation7]. Studies show that the JAK2/STAT3 signaling pathway is particularly participated in the prevention of myocardial IRI [Citation8,Citation9], especially by generating proteins for protection, including LOS and COX-2 through regulation of expression of Bcl-2/Bax [Citation10].
As a member of the heat shock protein 70 (HSP70) superfamily, HSP70 is found in nearly every cellular compartment in eukaryotes [Citation11]. It has an essential role in cellular protein metabolism due to its ability to promote nascent folding of polypeptides and remove denatured proteins [Citation12]. HSP70 expression produces cells that are highly resistant to death caused by oxidative stress, tumor necrosis factor (TNF), heat, overexpression of caspase-3, UV radiation and several chemotherapeutic drugs [Citation13–15]. Numerous reports found that overexpression of HSP70 enhances myocardial tolerance to IRI [Citation16,Citation17]. In addition, it is also allowed to activate HSP70 through the JAK/STAT pathway [Citation18]. Xu [Citation15] et al. demonstrated that blocking the JAK2/STAT3 signaling pathway downregulated HSP70 expression, thereby inhibiting Raji cell proliferation, inducing cell cycle arrest, and promoting oxidative stress and apoptosis. Guo et al. [Citation19] found that matrine could protect cardiomyocytes from IRI by activating the JAK2/STAT3 pathway to upregulated HSP70 expression. Yu et al. [Citation20] similarly showed that DL-3-n-butylphthalide protects H9c2 cardiomyocytes from ischemia/reperfusion injury by increasing the HSP70 expression through activation of the PI3K/AKT pathway.
Presently, clinicians use ozone (O3) to manage diseases related to infections, ischemia, and inflammation, as well as strokes and peripheral vascular disorders, peritonitis, bedsores in diabetic and non-diabetic patients, and other conditions [Citation21]. Studies confirm that the relative risk of ischemic (infarct extension) and complications of arrhythmia can be reduced by ozone [Citation22]. In rat models of renal and hepatic ischemia, ozone oxidative preconditioning protected against IRI [Citation23,Citation24]. Ozone administration also reduced reperfusion injury in isolated rat heart models [Citation25]. Yet another study reported ozone exerted protective effects against liver IRI through regulation of HSP70 [Citation26]. Based on the above studies, we speculate that ozone may also exert a protective effect against cardiac IRI by activating the JAK2/STAT3 pathway and upregulating the expression of HSP70. Therefore, we explored the mechanisms of HSP70 regulation and JAK2/STAT3 signaling pathway in the role of ozone protection in cardiac IRI through in vivo and in vitro experiments.
Materials and methods
Animal model of ischemia/reperfusion (I/R) injury
This study was authorized by the Ethics Committee on Animal Experiments, Jinan University (Guangzhou, China) and followed the principles of People’s Republic of China National Guidelines for Laboratory Animal Welfare (https://oacu.oir.nih.gov/animal research advisory committee guidelines).
Thirty male adult Sprague-Dawley (SD) rats, aged 7 weeks of (200–220 g), were acquired from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Raised in a light/dark circle for 12 h in an environment of controlling the temperature and humidity, the rats were supplied with regular food and water ad libitum. Ligation was performed for 30 min to induce I/R, and then the left anterior descending (LAD) coronary was reperfused for 2 h according to the previous studies [Citation27,Citation28]. Briefly, after being fully anesthetized with inhalation of 1.5–2% isoflurane, the hearts were exposed and the LAD coronary artery was ligated by 6–0 suture. Myocardial ischemia was confirmed once the saddleback-type ST segment elevation and a significant T wave increase was recorded by electrocardiogram. After occlusion for 30 min, the ligation was taken away and the LAD was reperfused for another 2 h. The sham operation group was performed the same surgical procedure but the LAD was not ligated.
Animal experiment groups
Thirty rats were separated to five groups randomly (n = 6 per group): sham operation group, I/R group, I/R + 50 μg/kg O3 group, I/R + 100 μg/kg O3 group and I/R + 100 μg/kg O3 + AG490 group. Rats in I/R+ O3 groups had continuous intraperitoneal injection of 50 μg/kg or 100 μg/kg O3 for 5 days before I/R treatment, and the rest of the groups were performed the same volume of air. Rats in the I/R + 100 μg/kg O3 + AG490 group received JAK2 inhibitor AG-490 injection before ozone treatment.
Evaluation of LDH and CK-MB levels in serum
The LDH and CK levels in blood samples and supernatant in each group were determined by ELISA using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) according to the manufacturer’s protocols. The blood samples (5 ml) were collected after reperfusion. Separated and stored the serum at −80°C until assayed.
Myocardial infarct size measurement
The myocardial infarct size was assessed by staining with triphenyl tetrazolium chloride (TTC; Sigma‐Aldrich, USA) [Citation29]. Heart tissues were isolated after the blood sample collection and the ventricles were cut to 5 slices, and then were kept at 37°C of 1% TTC for 10 min and fixed in 4% paraformaldehyde solution. The software of Image J (NIH, Bethesda, MD) could quantify the infarct area (white unstained by TTC) and non-infarcted area (red stained by TTC) and capture images afterward.
Isolation and culture of rat cardiomyocytes
Cardiomyocytes were separated from the ventricles of SD rats (24 h Postnatal) of primary neonatal [Citation30]. In short, the rats were anesthetized with 5% isoflurane. The thoracic cavity was opened under sterile conditions, and the heart was then removed and cut into small pieces. They were then digested by collagenase/dispase (Roche, Germany). Filter the cell suspension through a cell strainer to get rid of debris of larger tissue. After preplanting for 2 h, fibroblasts and endothelial cells were removed and non-adherent cells were collected. Subsequently, the cells were seeded in 6-well plates with DMEM (Thermo, USA) medium which contains 10% fetal bovine serum (FBS; Gibco, USA), and then incubated at 5% CO2, 37°C for 72 h. We digested and seeded the cardiomyocytes into appropriate cell culture plates for the next step.
Hypoxia/reoxygenation (HR) and cell treatment
The cardiomyocytes were subject to the following treatments: H/R treatment hypoxia (4 h, 5% CO2 and 1% O2/94% N2) and reoxygenation (6 h, 95% O2/5% CO2); ozone treatment (30 min) followed by H/R treatment; AG-490 treatment (2 μm, 1 h), ozone treatment and H/R treatment; HSP70 siRNA treatment, ozone treatment and H/R treatment.
CCK8 assay
Cell viability was evaluated through analyzing Cell Counting Kit-8 (CCK-8). Cardiomyocytes were seeded in a 96-well plate. H/R injury and Ozone treatment were conducted like described above. Several concentrations of O3 (0, 5, 10, 20, 30, 40, 80 μg/ml) were used for reoxygenation and after 6 h, 10 μL CCK8 (Nanjing Jiancheng, China) was seeded to each of the well and kept in dark for 2 h at 37°C. A microplate reader (Thermo, USA) was used to measure the absorbance at 450 nm.
Western blot assays
According to the protocol described above, Western blot analysis was performed [Citation31]. Cardiomyocytes were collected and split by RIPA lysis buffer, which contained 1% phenylmethylsulfonyl fluoride. In order to eliminate the insoluble matters, the lysate was centrifuged at 12,000 g, 4°C, lasting for 15 min. By using a commercially available kit (Thermo, USA) and bicinchoninic acid assay, the concentration of protein was measured. Using 12% SDS-PAGE to separate the protein samples (20 μg) of each group, which were then blotted on polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Germany). For blocking the nonspecific binding, the membranes were incubated for 2 h at room temperature in skimmed milk powder of 5% (w/v) in Tris-buffered saline which contained Tween-20 (TBST) of 0.05% (v/v). Thereafter, with the appropriate primary antibodies, the membranes were incubated at 4°C overnight. Information on antibodies is listed as follows: JAK2 (1:1000, ab108596, Abcam, UK); HSP70 (1:1000, ab2787, Abcam, UK); p-JAK2 (1:1000, ab32101, Abcam, UK); Bax (1:1000, ab32503, Abcam, UK); p-STAT3 (1:1000, ab76315, Abcam, UK); Caspase-3 (1:1000, ab184787, Abcam, UK); STAT3 (1:800, ab119352, Abcam, UK); Bcl-2 (1:800, ab196495, Abcam, UK), and β-actin (1:2000, ab179467, Abcam, UK). Washed for 30 min with TBST, the membranes were incubated with the proper secondary antibodies conjugated with HRP at 1:4,000 dilutions. The BioRad imaging system (Bio-Rad, USA) was then used to visualize the membranes with enhanced chemiluminescence.
siRNA transfection
According to the instruction of manufacture, the reagent (Thermo Scientific) was transfected with TurboFect siRNA and the cardiomyocytes were transfected with control siRNA and HSP70 targeted siRNA (GenePharma Co., Ltd, China). The specific siRNA information is manifested below: scrambled siRNA: 5ʹ-CCUCGUGCCGUUCCAUCAGGUAGUU-3ʹ (Sense) and 5ʹ-CUACCUGAUGGAACGGCACGAGGUU-3ʹ (Antisense). Cells were collected and analyzed by western blot to ensure the knockdown efficiency of HSP70 after 24 h.
Real-time RT-PCR analysis
The total RNA from cardiomyocytes was extracted by TRIzol (Invitrogen, USA), and purified by using the RNeasy Mini Kit (Qiagen, Germany). By using Roche Lightcycler 480 Detection System, the specific products were amplified and detected. The difference in HSP70 mRNA expression between groups was expressed using cycle time (Ct) values. The expression of HSP70 in relation to β-actin was determined by 2−ΔΔCt method. The primer sequences for HSP70 gene are: GTGCGGCCTTAGTAGAGGTG (F) and GCTGGTGTCTGTGGCTGTTG (R).
Statistics analysis
All data are showed as the mean ± standard deviation (SD) and analyzed by GraphPad Prism8.0 (GraphPad Prism Software, USA). Using one-way analysis of variance to perform statistical analyses, and followed by Tukey’s post hoc test. When p < 0.05, it was believed to be significant.
Results
Ozone prevented cardiomyocytes injury induced by H/R in vitro
Effects of various ozone concentrations on the viability of cardiomyocytes was first measured with CCK-8 assay ()). Ozone ranging from 5 μg/ml to 20 μg/ml did not affect the viability of cardiomyocytes. Therefore, we used 10 μg/ml and 20 μg/ml to test the effect of ozone on protecting the cardiomyocytes injury caused by H/R. As manifested in ), the cell viability was significantly inhibited, and release of LDH as well as the CK activity were increased by H/R when comparing with the control group. But, the cell viability was notably increased, and the release of LDH as well as the CK activity were decreased by ozone in a manner of dose dependent when comparing with the H/R group. Ozone inhibition on cardiomyocyte apoptosis was further demonstrated by Western blotting ()). H/R-induced Bax/Bcl-2 ratio ()) and the level of active caspase-3 ()) were dramatically decreased by applying ozone in a manner of dose dependent.
Figure 1. Ozone prevents cardiomyocytes from injury caused by H/R in vitro. (a) Treat various concentrations (0, 5, 10, 20, 30, 40, 60, 80 μg/ml) of ozone to the cardiomyocytes of primary rat and choose CCK-8 analysis to test cell viability. (b) Treat with 10 μg/ml or 20 μg/ml to cardiomyocytes after 4 hours of hypoxia, and then incubate in a normal incubator with oxygen for 6 hours to reoxygenate, and measure the viability of cell by CCK-8. (c) Release level of LDH. (d) CK activity. (e) analysis of Western blot about the levels of protein of Bax, cleaved caspase-3, Bcl-2 and caspase-3. (f) Quantification of Bax/Bcl-2 ratio. (g) Activity level of caspase-3. n = 3 *p < 0.05, **p < 0.01
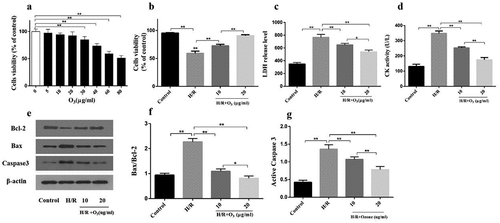
Ozone attenuated myocardial injury induced by I/R in rats
LDH level and CK activity were firstly detected in the serum while examining the effect of ozone in the myocardial injury induced by I/R in the rat model. As it manifested in ), the I/R group had a significant increase in LDH level and CK activity when compared with the Sham group, which was mitigated by ozone treatment in a dose-dependent manner. However, AG-490 treatment almost abolished the effect of ozone pretreatment. These results were consistent with TTC assay ()). Induced by I/R, the infarcted size depended on dose and relieved by applying the ozone while AG-490 significantly attenuated the ozone protective function ()). Next, the Bax/Bcl-2 ratio as well as level of protein of active caspase-3 were tested by Western blot ()). The results manifested that IRI elevated the Bax/Bcl-2 ratio ()) as well as the active caspase-3 level ()), while reduced by applying ozone in a manner of dose dependent, which suggested that ozone can reduce the myocardial injury caused by I/R in animal models.
Figure 2. In animal models, ozone attenuated myocardial injury caused by I/R. (a) The LDH level of Serum. (b) CK-MB activity of Serum. (c) Representative photographs in TTC staining for heart tissues and the myocardial infarct size was calculated. (d) Levels of protein of Bax, caspase-3, cleaved caspase-3 and Bcl-2 were detected by Western blot. (e) Quantification of Bax/Bcl-2 ratio. (f) Activity level of caspase-3. n = 3 **p < 0.01
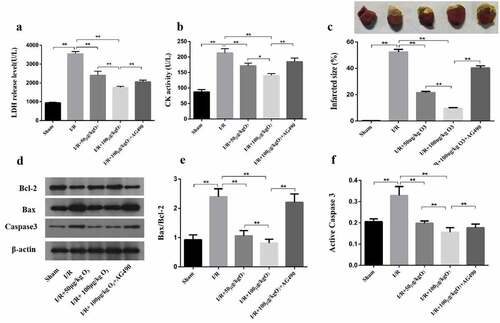
Ozone suppressed cardiomyocyte injury caused by H/R via activating the signaling of JAK2/STAT3
The molecular mechanisms of ozone in myocardial injury caused by H/R were first determined in vitro. To confirm whether ozone protective functions were activated through the signaling pathway of JAK2/STAT3, we detected the protein levels of p-STAT3, STAT3, p-JAK2 and JAK2 as shown in ). H/R stimulation notably reduced the p-JAK2/JAK2 and p-STAT3/STAT3 ratio, which was regained by ozone treatment. 30 min before applying with H/R, the cells were pretreated with 2 mM AG-490, and incubated during the whole process of H/R. This was to confirm whether the ozone’s protection against injury to cardiomyocyte caused by H/R was functioned or not through activating the signaling of JAK2/STAT3. It showed that AG-490 had reversed the LDH level and CK activity of 20 μg/ml of ozone ()), and the active level of caspase-3 and Bax/Bcl-2 ratio ()). Taken together, the data indicated that the cardiomyocyte injury caused by H/R was inhibited by ozone through activating the signaling of JAK2/STAT3.
Figure 3. Ozone inhibited the injury of cardiomyocyte caused by H/R through activating the signaling of JAK2/STAT3. (a) Western blot to detect the protein levels of STAT3, p-JAK2, p-STAT3 and JAK2. (b) Quantification of p-STAT3/STAT3 ratios. (c) Quantification of p-JAK2/JAK2 ratios. 30 min before applying the H/R, cardiomyocytes, treated with JAK2 inhibitor AG-490 (2 mM), were incubated during the whole process of H/R. (d) The release level of LDH. (e) CK activity. (f) After assaying, the levels of expression of Bax, caspase-3, cleaved caspase-3 and Bcl-2 were determined by Western blot. (g) Quantification of Bax/Bcl-2 ratio. (h) Activity level of caspase-3. each group n = 3, **p < 0.01
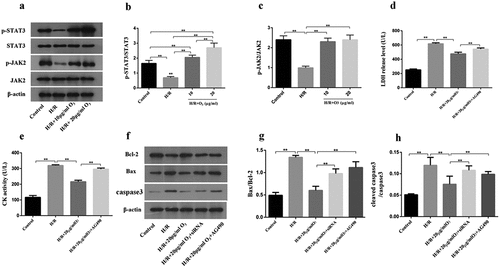
The effect of ozone mediated by HSP70 to cardiomyocytes injury caused by H/R
Next, we studied the HSP70 function in cardiomyocytes protection during myocardial injury caused by H/R. We determined the protein levels and mRNA of HSP70 in cardiomyocytes. Compared with the control group, the protein levels and mRNA of HSP70 were elevated due to H/R; But ozone dose-dependently upregulated the HSP70 expression ()). To further understand the ozone functions via upregulating the HSP70 expression, which had a similar function as AG-490 on the cardiomyocytes, we used HSP70 siRNA 24 h before the treatment with H/R to abolish HSP70 expression in cardiomyocytes. And we found that HSP70 siRNA had a similar function as AG-490 on the cardiomyocytes ()). The results indicated that the effects of ozone to the CK activity and LDH level were attenuated by HSP70 siRNA ()).
Figure 4. The effect of ozone mediated by HSP70 to Cardiomyocytes Injury induced by I/R. (a) The relative expression of HSP70 mRNA was detected by RT-PCR. (b) The protein level of HSP70 was assayed by Western blot. (c) Cardiomyocytes were transfected by HSP70 siRNA, control or pretreated with AG-490 followed by 20 μg/ml ozone treatment and I/R treatment. HSP70 protein level was determined. LDH release level (d) and CK activity (e) in Cardiomyocytes were detected. n = 3 per group, **p < 0.01
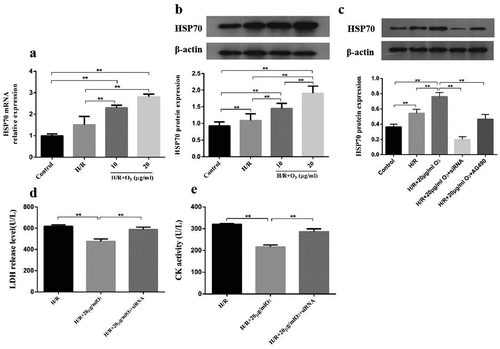
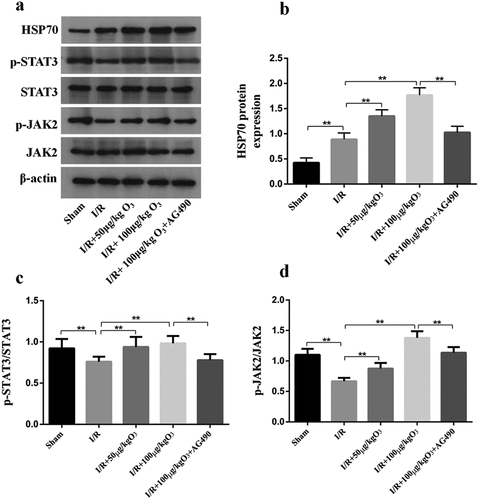
Ozone attenuated myocardial injury induced by I/R via upregulating HSP70 and activating JAK2/STAT3 in vivo
We investigated the roles of JAK2/STAT2 signaling in ozone pretreatment to cardiomyocytes in vivo and cleared the levels of p-JAK2, JAK2, p-STAT3, STAT3 by Western blot. As manifested in ), when compared with the Sham group, I/R injury led to a significant decrease in the ratio of pJAK2/JAK2 and pSTAT3/STAT3, as well as an increase in the expression of HSP70, which could be reversed by pretreating with the AG-490 ()). We also found that rats exposed to AG-490 before I/R treatment and ozone therapy had decreased HSP70 levels. These results are consistent with those in vivo. In general, these results indicate ozone protects cardiomyocytes from injury caused by I/R through activating the pathway of JAK2/STAT3 and upregulating the HSP70.
Figure 5. Ozone alleviated myocardial injury caused by I/R through up-regulating HSP70 and activating JAK2/STAT3 in vivo. (a) Analyzed the levels of protein of JAK2, p-STAT3, p-JAK2, HSP70 and STAT3 in I/R animal models. (b–d) Quantification of HSP70 level, as well as p-STAT3/STAT3 and p-JAK2/JAK2 ratios. n = 6, **p < 0.01
Discussion
Myocardial IRI is caused by a direct result of blood flow restoration to the ischemic tissue and can lead to cell death and added cardiac dysfunction [Citation32]. Ozone administration is used in treatment for conditions associated with the tolerance to ischemia-induced damage [Citation33], also called ozone oxidative preconditioning (OOP) [Citation34]. However, ozone is a potential toxic substance, and continued exposure to high levels of ozone might increase the produce of reactive oxygen and inflammatory mediators [Citation35]. We tested the therapeutically nontoxic ozone concentrations of 10–80 μg/ml in vitro and found that the ozone concentration of less than 30 μg/ml did not affect cardiomyocytes viability. Consequently, we chose 10 μg/ml and 20 μg/ml ozone for the following experiments in vitro. In vivo, we selected an ozone dose of 100 μg/kg as described in a previous study [Citation36]. OOP provides protective effects against apoptosis, inflammation and oxidative stress in IRI [Citation37,Citation38]. In the present study, we found that ozone treatment reduced the area of I/R-induced myocardial infarction in rats. In addition, ozone significantly reduced CK activity and LDH release in serum of I/R rats and H/R-induced cardiomyocytes, and protected against myocardial injury by inhibiting active caspase-3 and Bax protein expression. These results are consistent with the findings of Chen et al. [Citation39]. They found that interference with lncRNA MIAT reduced H/R-induced LDH release from cardiomyocytes, I/R-induced myocardial infarct size, and inhibited the expression of apoptotic proteins.
JAKs and STATs families of protein transduce extracellular signals into nucleus and activate the transcription of target genes [Citation40]. STAT proteins are substrates of JAKs and can be phosphorylated to transform into an activated form whereby p-STATs translocate to the nucleus and transactivate responsive genes [Citation40]. The JAK2/STAT3 signaling pathway regulates the expression of genes involved in cell-cycle progression, cell survival, cell proliferation, and angiogenesis [Citation41]. JAK2/STAT3 signaling is important for myocardial protection. In the LAD ligation of a rat model, as early as 5 minutes after myocardial infarction, we observed JAK2, STAT1, STAT3, STAT5a, and STAT6 phosphorylation [Citation42]. We also found that scutellarin modulates IRI-induced oxidative stress and apoptosis by enhancing JAK2/STAT3 pro-survival signaling [Citation43]. Several genes related to apoptosis including Bcl-xl and Bcl-2, were classified as STAT3’s target genes [Citation44,Citation45]. Shinji Negoro et al. found that in rats AMI with coronary ligation and AG-490 treatment, apoptotic cells were notably increased, while pSTAT3 was significantly inhibited. Meanwhile, the expression of activity of caspase-3 and Bax in the myocardium were increased notably, which indicated that in AMI myocardium, the JAK2/STAT3 pathway, acting as a pivotal role in signaling of cytoprotective, was activated [Citation46]. Considering the importance of JAK-STAT3 in preconditioning and cardioprotection, it was sufficient for activated JAK2/STAT3 to protect the myocardium from apoptosis. In this study, ozone showed a myocardial protective function in IRI, which led to a reduced number of apoptotic cardiomyocytes, a smaller myocardial infarct size and a decrease in LDH release and CK activity. In this process, ozone treatment could restore the reduced ratios of p-STAT3/STAT3 and p-JAK2/JAK2 caused by IRI, with up-regulating the expression of the anti-apoptotic protein Bcl2, down-regulating the expression of the pro-apoptotic protein Bax, as well as the Caspase-3. JAK2 inhibitor AG-490 reverses the above results. All data indicate cardioprotection was induced by ozone through activating the signaling pathway of JAK2/STAT3.
HSP70, a conserved protein, is widely expressed and related to the cytoprotection fighting against stresses [Citation47]. It contributes to cardioprotection in IRI by suppressing reactive oxygen species generation [Citation48], inhibiting cell apoptosis [Citation49], preventing the process of cell death by influencing the activation of caspase-3 and caspase-7, or even further downstream events of caspase-3 and caspase-7 activation [Citation50]. Our study showed that ozone can significantly increase the expression of HSP70 in a manner of dose dependent, which can be inhibited by AG-490. Also, HSP70 siRNA could inhibit HSP70 expression and attenuate the effects of ozone to the release of LDH as well as CK activity, demonstrating that ozone protected cardiomyocytes from IRI by upregulating the HSP70 expression.
Conclusion
In summary, we studied the relationship between the expression of HSP70 and JAK2/STAT3 in the ozone treatment of cardiomyocytes IRI. The results indicate that ozone alleviates myocardial IRI by activating the signaling pathway of JAK2/STAT3 and upregulating the expression of HSP70 as well.
Highlights
Ozone attenuates the suppression of cell viability induced by hypoxia/reoxygenation (H/R).
Ozone decreases the release of activity of CK, LDH and apoptosis of cardiomyocytes.
Ozone protects cardiomyocytes in I/R injury through regulation of the expression of HSP70 by activating the JAK2/STAT3 pathway.
Abbreviations
Acute myocardial infarction: AMI
Cell Counting Kit-8: CCK8
Creatine kinase: CK
Heat shock protein 70: HSP70
Hypoxia/reoxygenation: H/R
Ischemia/reperfusion: I/R
Janus kinase 2: JAK2
Lactate dehydrogenase: LDH
Left anterior descending: LAD
Signal transduction and activator of transcription 3: STAT3
Triphenyltetrazolium chloride: TTC
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Acknowledgements
The authors grateful acknowledge the helpful comments from copyeditors of TopEdit which greatly improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Hausenloy DJ. Conditioning the heart to prevent myocardial reperfusion injury during PPCI. Eur Heart J Acute Cardiovasc Care. 2012;1(1):13–32.
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100.
- Yang C. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue Za Zhi. 2018;30:209–215.
- Baines CP. How and when do myocytes die during ischemia and reperfusion: the late phase. J Cardiovasc Pharmacol Ther. 2011;16:239–243.
- Zhang Z, Ma BJ, Li LT, et al. Pterostilbene attenuates inflammation in rat heart subjected to ischemia-reperfusion via eNOS activation. Anal Quant Cytology Histol. 2018;40:132–140.
- Wilson T, Omelchenko I, Foster S, et al. JAK2/STAT3 inhibition attenuates noise-induced hearing loss. PLoS One. 2014;9(10):e108276.
- Shi X, Franko B, Frantz C, et al. JSI-124 (cucurbitacin I) inhibits Janus kinase-3/signal transducer and activator of transcription-3 signalling, downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and induces apoptosis in ALK-positive anaplastic large cell lymphoma cells. Br J Haematol. 2006;135(1):26–32.
- Wu J, Yu J, Xie P, et al. Sevoflurane postconditioning protects the myocardium against ischemia/reperfusion injury via activation of the JAK2-STAT3 pathway. PeerJ. 2017;5:e3196.
- Luan HF, Zhao ZB, Zhao QH, et al. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz J Med Biol Res. 2012;45(10):898–905.
- Eid RA, Alkhateeb MA, Eleawa S, et al. Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res Cardiol. 2018;113(2):13.
- Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006;6(11):1215–1225.
- Pantos C, Mourouzis I, Dimopoulos A, et al. Enhanced tolerance of the rat myocardium to ischemia and reperfusion injury early after acute myocardial infarction. Basic Res Cardiol. 2007;102(4):327–333.
- Lepore DA, Knight KR, Anderson RL, et al. Role of priming stresses and Hsp70 in protection from ischemia-reperfusion injury in cardiac and skeletal muscle. Cell Stress Chaperones. 2001;6(2):93–96.
- Mao RF, Rubio V, Chen H, et al. OLA1 protects cells in heat shock by stabilizing HSP70. Cell Death Dis. 2013;4(2):e491.
- Xu NW, Chen Y, Liu W, et al. Inhibition of JAK2/STAT3 signaling pathway suppresses proliferation of Burkitt’s lymphoma raji cells via cell cycle progression, apoptosis, and oxidative stress by modulating HSP70. Med Sci Monit. 2018;24:6255–6263.
- Huang BP, Lin CS, Wang CJ, et al. Upregulation of heat shock protein 70 and the differential protein expression induced by tumor necrosis factor-alpha enhances migration and inhibits apoptosis of hepatocellular carcinoma cell HepG2. Int J Med Sci. 2017;14(3):284–293.
- Suzuki K, Sawa Y, Kaneda Y, et al. In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia-reperfusion injury in rat. J Clin Invest. 1997;99(7):1645–1650.
- Madamanchi NR, Li S, Patterson C, et al. Thrombin regulates vascular smooth muscle cell growth and heat shock proteins via the JAK-STAT pathway. J Biol Chem. 2001;276(22):18915–18924.
- Guo S, Gao C, Xiao W, et al. Matrine protects cardiomyocytes from ischemia/reperfusion injury by regulating HSP70 expression via activation of the JAK2/STAT3 pathway. Shock. 2018;50(6):664–670.
- Yu Y, Zhu Y, Sun X, et al. DL-3-n-butylphthalide protects H9c2 cardiomyoblasts from ischemia/reperfusion injury by regulating HSP70 expression via PI3K/AKT pathway activation. Exp Ther Med. 2021;22(3):1008.
- Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13(1):3–11.
- Ciampa S, Dequerquis G, Lettieri B. The role of ozone in the treatment of the acute phase of ischemic heart disease. Ozone Ther. 2017;1(3):6549
- Wang L, Chen Z, Weng X, et al. Combined ischemic postconditioning and ozone postconditioning provides synergistic protection against renal ischemia and reperfusion injury through inhibiting pyroptosis. Urology. 2019;123:296.e1–296.e8.
- Ajamieh HH, Menéndez S, Martínez-Sánchez G, et al. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia-reperfusion. Liver Int. 2004;24(1):55–62.
- Merin O, Attias E, Elstein D, et al. Ozone administration reduces reperfusion injury in an isolated rat heart model. J Card Surg. 2007;22(4):339–342.
- León Fernández OS, Ajamieh HH, Berlanga J, et al. Ozone oxidative preconditioning is mediated by A1 adenosine receptors in a rat model of liver ischemia/ reperfusion. Transpl Int. 2008;21:39–48.
- Wu X, Qin Y, Zhu X, et al. Increased expression of DRAM1 confers myocardial protection against ischemia via restoring autophagy flux. J Mol Cell Cardiol. 2018;124:70–82.
- Di Filippo C, Marfella R, Capodanno P, et al. Acute oxygen-ozone administration to rats protects the heart from ischemia reperfusion infarct. Inflamm Res. 2008;57(10):445–449.
- Dong S, Cheng Y, Yang J, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284(43):29514–29525.
- Hou CJ, Qi YM, Zhang DZ, et al. The proliferative and migratory effects of physical injury and stromal cell-derived factor-1α on rat cardiomyocytes and fibroblasts. Eur Rev Med Pharmacol Sci. 2015;19:1252–1257.
- Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol. 2010;105(1):1–5.
- Prasad A, Stone GW, Holmes DR, et al. Reperfusion injury, microvascular dysfunction, and cardioprotection: the “dark side” of reperfusion. Circulation. 2009;120(21):2105–2112.
- Sagai M, Bocci V. Mechanisms of action involved in ozone therapy: is healing induced via a mild oxidative stress? Med Gas Res. 2011;1(1):29.
- Rodríguez ZZ, Guanche D, Alvarez RG, et al. Effects of ozone oxidative preconditioning on different hepatic biomarkers of oxidative stress in endotoxic shock in mice. Toxicol Mech Methods. 2011;21(3):236–240.
- Fakhrzadeh L, Laskin JD, Gardner CR, et al. Superoxide dismutase-overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-alpha. Am J Respir Cell Mol Biol. 2004;30:280–287.
- Meng W, Xu Y, Li D, et al. Ozone protects rat heart against ischemia-reperfusion injury: a role for oxidative preconditioning in attenuating mitochondrial injury. Biomed Pharmacother. 2017;88:1090–1097.
- Ajamieh HH, Berlanga J, Merino N, et al. Role of protein synthesis in the protection conferred by ozone-oxidative-preconditioning in hepatic ischaemia/reperfusion. Transpl Int. 2005;18(5):604–612.
- Chen H, Xing B, Liu X, et al. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur J Pharmacol. 2008;581:306–314.
- Chen L, Zhang D, Yu L, et al. Targeting MIAT reduces apoptosis of cardiomyocytes after ischemia/reperfusion injury. Bioengineered. 2019;10(1):121–132.
- Igarashi K, Garotta G, Ozmen L, et al. Interferon-gamma induces tyrosine phosphorylation of interferon-gamma receptor and regulated association of protein tyrosine kinases, Jak1 and Jak2, with its receptor. J Biol Chem. 1994;269(20):14333–14336.
- Raible DJ, Frey LC, Brooks-Kayal AR. Effects of JAK2-STAT3 signaling after cerebral insults. Jakstat. 2014;3:e29510.
- El-Adawi H, Deng L, Tramontano A, et al. The functional role of the JAK-STAT pathway in post-infarction remodeling. Cardiovasc Res. 2003;57(1):129–138.
- Wang Z, Yu J, Wu J, et al. Scutellarin protects cardiomyocyte ischemia-reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 2016;157:200–207.
- Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104(18):7391–7396.
- You L, Li L, Xu Q, et al. Postconditioning reduces infarct size and cardiac myocyte apoptosis via the opioid receptor and JAK-STAT signaling pathway. Mol Biol Rep. 2011;38(1):437–443.
- Negoro S, Kunisada K, Tone E, et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47(4):797–805.
- Shrestha L, Young JC. Function and chemotypes of human Hsp70 chaperones. Curr Top Med Chem. 2016;16:2812–2828.
- Chen Z, Shen X, Shen F, et al. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70. Mol Cell Biochem. 2013;377(1–2):35–44.
- Thompson J, Hu Y, Lesnefsky EJ, et al. Activation of mitochondrial calpain and increased cardiac injury: beyond AIF release. Am J Physiol Heart Circ Physiol. 2016;310(3):H376–84.
- Komarova EY, Afanasyeva EA, Bulatova MM, et al. Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones. 2004;9(3):265–275.
