ABSTRACT
The function of tubulin polymerization promoting protein family member 3 (TPPP3) in tumor cells is complicated, and the role of TPPP3 in nasopharyngeal carcinoma (NPC) remains unclear. This study aims to explore the expression of TPPP3 in NPC and its effect on NPC cells. The expression of TPPP3 in NPC tissues and other cancers were analyzed by using the Oncomine and Gene Expression Omnibus (GEO) databases. The mRNA and protein of TPPP3 were detected in NPC tissues by quantitative real-time PCR and immunohistochemistry. Furthermore, TPPP3 was overexpressed in 5–8 F and HONE1 cell lines by lentivirus transfection, and functional analysis of TPPP3 in NPC was evaluated through in vitro experiments. The expression of TPPP3 was significantly down-regulated in NPC tissues and cells. Overexpression of TPPP3 significantly inhibited proliferation of 5–8 F and HONE1 cells in vitro. In addition, overexpression of TPPP3 significantly attenuated the invasion ability of 5–8 F, HONE1 cells in vitro, but have no significant effect on migration ability. Furthermore, TPPP3 overexpression diminished the expression of MMP-2 and MMP-9 mRNA. By analyzing dataset GSE12452, it was interesting that TPPP3 high expression group mainly functioned in B cell receptor signaling pathway, cell cycle and DNA replication. In conclusion, our results suggest that TPPP3 may be considered as an antioncogene, which plays an important role in the occurrence and progression of NPC.
Abbreviations: TPPP3: tubulin polymerization promoting protein family member 3; NPC: nasopharyngeal carcinoma; GEO: Gene Expression Omnibus; qRT-PCR: quantitative real-time PCR; GFP: green fluorescence protein; MOI, transfected multiplicity of infection; CCK-8: cell counting kit-8; OD: optical density; GSEA: gene set enrichment analysis; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes; MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9.
Introduction
Nasopharyngeal carcinoma (NPC) is a kind of malignant tumor originated from the mucous epithelium of the top and side walls of the nasopharynx cavity. It has a very obvious regional distribution characteristics, and its morbidity and mortality in southern China rank first in the world [Citation1]. The common clinical symptoms of NPC are nasal obstruction, blood in the nose, ear congestion, hearing loss, headache, etc. The early clinical symptom of NPC is not typical, and most of NPC patients are found in advanced stage of the disease or distant metastasis [Citation2]. Because of its high occult, malignant degree, rapid development and easy distant metastasis, NPC is difficult to diagnose and treatment. Most patients died of distant metastasis and local recurrence, thus the treatment of NPC is still challenging [Citation3].
Tubulin polymerization promoting protein family member 3 (TPPP3) first cloned from human pituitary by Hu RM’s group in 2000, and it belongs to a new member of TPPP family [Citation4]. TPPP3 may specifically bind to microtubule in vitro and in vivo, which may play an important role in promoting microtubule aggregation, cell proliferation and mitosis [Citation5]. Currently, most studies show that TPPP3 has cancer promoting characteristics, and the expression of TPPP3 is increased in a variety of tumor types, such as non-small cell lung cancer, colorectal cancer, endometrial carcinoma, breast cancer, etc [Citation6–11]. However, study showed that TPPP3 protein was up-regulated in long-term survival pancreatic ductal carcinoma patients, and Kaplan–Meier analysis showed that TPPP3 protein was positively correlated with the number of survival months [Citation12]. The study of TPPP3 in tumor is still rare, and its function in tumor cells is complicated.
Therefore, the role of TPPP3 in NPC needs further elucidation. This study aimed to explore the expression of TPPP3 in NPC, and investigate the role of TPPP3 in NPC cells by cell proliferation, cell migration and invasion assays. Experiments showed that TPPP3 overexpression inhibited NPC cells proliferation and invasion. Our results suggest that TPPP3 may be considered as an antioncogene, which plays an important role in the occurrence and progression of NPC.
Materials and Methods
Materials
Tissue specimens
All tissue specimens were extracted from patients who presented to Department of Otorhinolaryngology, the First Affiliated Hospital of Guangxi Medical University during the period of May 2018 to August 2018. The carcinomatous tissues of 16 patients with NPC and the nasopharyngeal mucosal tissues of 16 patients with chronic nasopharyngitis were studied. Among 16 fresh specimens of NPC, 13 were male and 3 were female, aged 32–78 years, with a median age of 51 years. Among 16 specimens of chronic nasopharyngitis patients, there were 10 males and 6 females, aged 25–77 years, with a median age of 44 years.
Paraffin sections of 72 cases of NPC and 51 cases of chronic nasopharyngitis were taken from the Department of Pathology, the First Affiliated Hospital of Guangxi Medical University. In 72 NPC tissues, 50 males and 22 females, aged 16–74 years, with a median age of 49 years. In 51 cases of chronic nasopharyngitis, 34 were male, 17 were female, the age was 16–74 years, the median age was 44 years.
All tissue specimens were confirmed by pathological examination, and patients without radiotherapy or chemotherapy. NPC staging was classified by the TNM stage classification according to the 8th Edition UICC/AJCC. The study was approved by the ethics committee of the First Affiliated Hospital of Guangxi Medical University, and the informed consent of the subjects was obtained.
Cell lines
In this study, NP69 was used as the control group, CNE2, HK1, 5–8 F, HONE1 as the experimental group, all of them were donated by the Otolaryngology Laboratory of Guangxi Medical University. All cells were cultured in 90% DMEM (Gibco, USA) supplemented with 10% FBS (Gibco, USA) and kept in a humid environment of 37℃, 5% CO2.
Methods
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues and cells according to the instructions of RNAiso Plus reagent (Takara, Dalian, China), and mRNA was reverse transcribed into cDNA using the PrimeScript ™ RT reagent Kit (Takara, Dalian, China). Besides, qRT-PCR was performed according to the instructions of the SYBR® Premix Ex TaqTM II (Takara, Dalian, China). Sequences of primers are shown in . The experiment was repeated three times. Fold change (relative expression) = 2−ΔΔCt.
Table 1. Sequences of primers
Western blot
Proteins were extracted by lysis buffer (PMSF: RIPA = 1:100). Subsequently, proteins were electrophoresed by 5% concentrated gel and separated by 12% isolated gel, then transferred by membrane of PVDF. After blocking with 5% skim milk, the PVDF membranes were incubated with anti-TPPP3 antibody (GeneTex, USA, dilution 1: 1000) and anti-β-actin antibody (Sangon Biotech, China, dilution 1:800) overnight at 4°C, respectively. And then, these PVDF membranes should be incubated with fluorescent secondary antibody (CST, USA, dilution 1:10,000) for 60 minutes at room temperature. The Odyssey imaging system (LI-COR Biosciences, Lincoln, USA) was applied to analyze the gray value of corresponding protein [Citation13].
Immunohistochemistry
The primary antibody used for immunostaining was the TPPP3 polyclonal antibody (GeneTex, USA). Immunohistochemical SP method was used to routinely dewax, gradual hydration of gradient alcohol, and repair antigen etc. The diluted TPPP3 primary antibody (dilution 1:100) was incubated at 4°C overnight, and immunohistochemical staining kit SP-9002 (zsbio, Beijing, China) were used. After DAB color reagent was added, hematoxylin was counterstained to the slice.
TPPP3 is mainly distributed in the cytoplasm. Light yellow, brownish yellow or tan fine particles appearing in the cytoplasm are positive staining. Strength of positive cells staining (contrast of staining with background) as follows: 0 (no positive staining), 1 (light yellow), 2 (brown), 3 (tan). Percentage of positive cells as follows: 1 (<10%), 2 (10% −50%), 3 (51% −75%), 4 (>75%). The products of staining intensities and the percentage of positive cell expressions was used as the immunohistochemical score. The immunohistochemical score ≥ 1 is considered positive, otherwise it is considered negative [Citation14].
Cell transfection
Lentivirus vector of TPPP3 overexpression (LV-TPPP3) and the control lentivirus vector (LV-CON307) were completed by Genechem Technology Co., Ltd. (Shanghai, China), and the lentivirus has puromycin resistance and green fluorescence protein (GFP) expression sequence. According to the operation manual of lentivirus, cells were inoculated at the density of 5 × 104/ml, and transfected multiplicity of infection (MOI) was 30. The transfection efficiency of cells was observed under the inverted fluorescence microscope (Olympus, Japan) in 72 hours after transfection. When the fluorescence efficiency over 80%, the transfected cells were used for subsequent experiments. The successful transfection of 5–8 F and HONE1 was determined by qRT-PCR and western blot.
Cell counting kit-8 assay (CCK-8) and clone formation assays
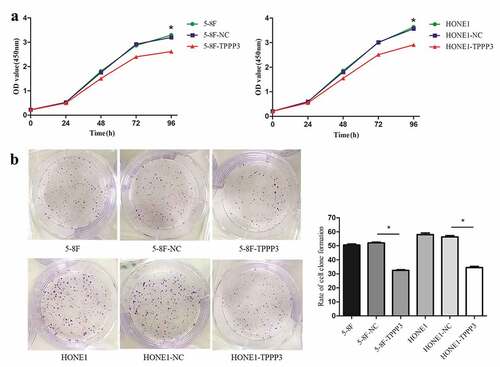
Cell proliferation was detected by CCK-8 (Dojindo Lab, Japan) and clone formation assays. For CCK-8 assay, NPC cells were seeded into 96-well plates at a density of 2500 cells/well and then incubated with CCK-8 solutions at different times (0, 24, 48, 72, and 96 h) for 2 h at 37°C. The optical density (OD) value of cells in each group was detected by Varioskan™ LUX Multimode Microplate Reader (Thermo Scientific, USA) at 450 nm.
For the colony formation assay, 500 cells were plated into each well of 6-well plates. Medium was refreshed every three days and cultured until clones were obviously visible. Colonies were fixed with 4% paraformaldehyde for 20 min, stained with 1% crystal violet for 20 min and counted by visual inspection. Clone formation rate = (number of clone formation/number of seeded cells) × 100% [Citation13].
Cell migration and invasion assays
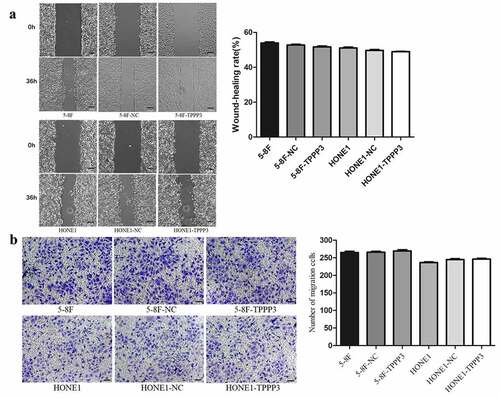
For wound healing assay, cells were seeded into 6-well plates (6 × 105 cells/well) and cultured at 37°C, 5% CO2 until cell grown to a confluent monolayer. Subsequently, a uniform scratch was made down the center of the well using a micropipette tip, and then the cells were cultured in serum-free medium. The migration of each group of cells at the time of scratching 0 h and 36 h later were observed under an inverted microscope. The relative distance of the cells to the scratched area was measured using Image J software, and relative cell migration rate (%) = relative distance of cell migration/original distance of scratched area × 100%.
Cell migration assays were performed using 24-well transwell units with 8 µm pore size polycarbonate inserts (Corning, USA). About 3 × 104 cells were harvested in 200 µl serum-free medium and seeded into the upper chamber, and 500 µl medium containing 10% fetal bovine serum was added to the lower chamber. After 24 h of incubation, the cells on the top of the membrane were wiped off with a cotton swab. Cells on the lower surface were fixed with 4% polymethanol for 30 min, and stained with 1% crystal violet for 20 min. Five random fields of the lower surface were photographed under inverted microscope (Olympus, Japan).
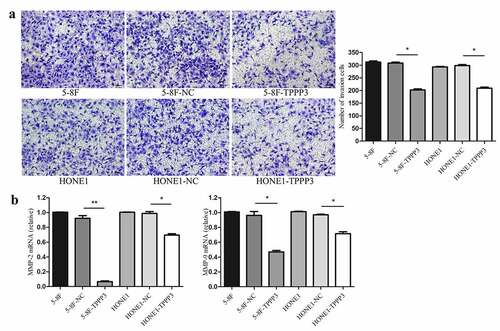
For the invasion assay, the transwell chambers were coated with appropriate concentration of Matrigel (Corning, MA). About 4 × 104 cells were harvested in 200 µl serum-free medium and seeded into the upper chamber. Invasion assay was performed similar to migration assay [Citation13].
Gene set enrichment analysis (GSEA)
Based on GSE12452, the expression profile data of 31 NPC tissues were grouped by the mean value of TPPP3 expression. The GSEA was performed to analyze the biological processes involved in the expression of TPPP3 gene in NPC and the possible related pathways with the molecular functions and biological processes of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). |NES| >1, NOM p-val < 0.05, FDR q-val < 0.25 were set as the threshold of significant enrichment.
Statistical analysis
We used R 3.5.1 to analyze the differential expression of TPPP3 mRNA in NPC and normal nasopharyngeal tissues from Gene Expression Omnibus (GEO) databases. SPSS 22.0 (Chicago, IL, USA) was used for statistical analysis. Measurement data were expressed as mean ± standard deviation, and p < 0.05 was considered statistically significant (two-sided test). Student’s t-test was used to compare the difference between two groups. One-way ANOVA was used to compare the difference among multiple groups. Chi-square test or Fisher exact probability method was used to compare categorical data between groups.
Results
TPPP3 was down-regulated and may serve as an antioncogene in NPC. Experiments revealed that TPPP3 inhibited NPC cells proliferation and invasion. All of the results suggested TPPP3 may be considered as an antioncogene, which plays an important role in the occurrence and progression of NPC.
Expression of TPPP3 is reduced in NPC tissues and cell lines
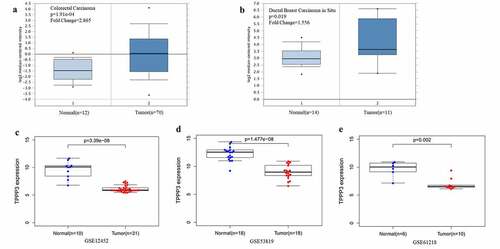
The mRNA expression levels of TPPP3 from the Oncomine database were significantly elevated in colorectal carcinoma and invasive ductal breast carcinoma (), which is consistent with previous published results [Citation9,Citation11]. However, this study analyzed NPC datasets GSE12452, GSE53819 and GSE61218, and found the mRNA expression levels of TPPP3 in NPC tissues were significantly lower than those in normal nasopharyngeal tissues ().
Figure 1. TPPP3 expression in human cancers
Subsequently, we measured the expression level of TPPP3 in NPC tissues by qRT-PCR and immunohistochemistry assays. Our results indicated that the expression of TPPP3 mRNA in NPC tissues was 0.114 ± 0.069 while in chronic nasopharyngitis was 1.340 ± 0.707, TPPP3 mRNA level was significantly lower in NPC tissues than that in chronic nasopharyngitis tissues ()). Consistent with this, the relative expression of TPPP3 mRNA in CNE2, HK1, 5–8 F and HONE1 cells was significantly lower than that in NP69 cells ()). Similarly, immunohistochemistry showed that positive staining intensity of TPPP3 protein was significantly lower in NPC tissues (), ). These data show that TPPP3 is significantly reduced in NPC. However, there was no significant correlation between TPPP3 expression and clinicopathological parameters in NPC ().
Figure 2. Expression of TPPP3 is reduced in NPC tissues and cell lines
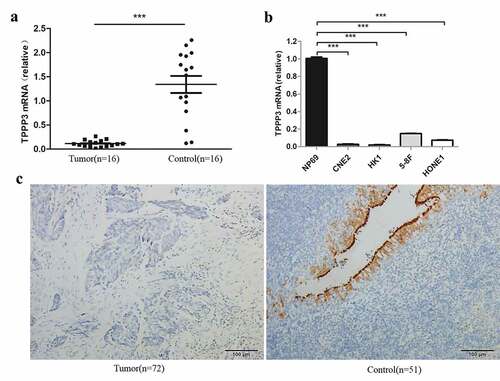
Table 2. The expression of TPPP3 in NPC and chronic nasopharyngitis tissues
Table 3. The correlation between TPPP3 expression and clinicopathological parameters in NPC
Construction of TPPP3 overexpression stable cell lines
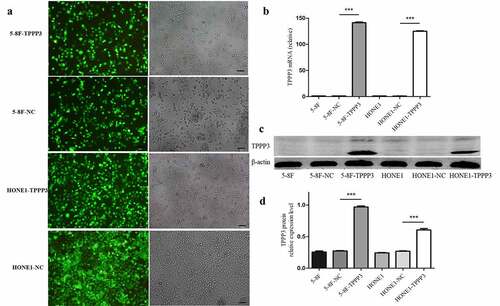
In order to study the effect of TPPP3 on biological behaviors of NPC cell, stable overexpression TPPP3 in 5–8 F and HONE1 cell lines, which had lower endogenous TPPP3 expression, was made. After lentivirus transfected cells were screened with puromycin, the efficiency of GFP was observed under the inverted fluorescence microscope. The expression of TPPP3 mRNA and protein in different cell groups were detected by qRT-PCR and western blot. These results all indicated that TPPP3 overexpression stable cell line was successfully constructed ().
Figure 3. Construction of TPPP3 overexpression stable cell lines
TPPP3 overexpression inhibited NPC cells proliferation
To study the effect of TPPP3 overexpression on cell proliferation, we carried out cell proliferation and clone formation assays. The proliferation of both cells was measured using CCK-8 assay, which revealed that TPPP3 overexpression caused a significant decrease in proliferation of 5–8 F and HONE1 ()). Similarly, colony formation assay suggested TPPP3 overexpression significantly inhibited the proliferation rate of both cells ()). Our results demonstrated that TPPP3 overexpression significantly inhibited proliferation of 5–8 F and HONE1 cells in vitro.
TPPP3 overexpression attenuated NPC cells invasion
To further determined whether TPPP3 affected cell migration and invasion in vitro, wound healing, transwell migration and transwell invasion assays were carried out. Both wound healing and transwell migration assays suggest that TPPP3 overexpression had no significant effect on the migration ability of 5–8 F and HONE1 cells (). Interestingly, transwell invasion assay suggested that TPPP3 overexpression attenuated the invasion ability of 5–8 F, HONE1 cells ()). Furthermore, TPPP3 overexpression diminished the expression of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) mRNA ()).
Enrichment analysis of TPPP3 in NPC tissues
According to GSEA, it revealed that TPPP3 high expression group enriched to 19 up-regulated KEGG pathways and 11 down-regulated KEGG pathways (). Interestingly, TPPP3 high expression group mainly functioned in B cell receptor signaling pathway, cell cycle and DNA replication. However, no enrichment in GO molecular function and GO biological process.
Table 4. Significantly enriched KEGG pathways
Discussion
Recently, the activation of oncogenes and the deletion of tumor suppressor genes have attracted extensive attention in tumor research, and many tumor suppressor genes are considered as important factors in the occurrence and development of NPC [Citation15,Citation16]. With the deepening of research between TPPP3 gene and tumors, TPPP3 has been reported highly expressed in lung cancer, HeLa cells of cervical cancer and colorectal cancer [Citation6–9,Citation17]. However, Hu D et al used proteomics to analyze prognostic biomarkers for pancreatic ductal adenocarcinoma, they found that TPPP3 protein was up-regulated in patients with long-term survival of pancreatic ductal carcinoma, and TPPP3 protein is associated with a good prognosis of pancreatic ductal adenocarcinoma [Citation12]. At present, research of TPPP3 in tumors is still unclear, and its function in tumor cells is complex. Therefore, the function of TPPP3 gene may be different in different tumors.
The relationship between TPPP3 and NPC has not been reported, and its biological function in NPC is not clear. Firstly, we found that the expression of TPPP3 mRNA in NPC tissues is lower than those in normal healthy nasopharyngeal tissues (GSE12452, GSE53819 and GSE61218). Further investigation found that TPPP3 mRNA and protein levels in NPC tissues were significantly lower. Consistent with tissue assays, the relative expression levels of TPPP3 mRNA in NPC cell lines (CNE2, HK1, 5–8 F and HONE1) were significantly lower than that in normal nasopharyngeal epithelial cell line NP69. Based on the mutual verification of the above experiments, we found that the expression level of TPPP3 in NPC was significantly downregulated, suggesting TPPP3 may play an important role in the occurrence and development of NPC.
At the cellular level of tumorigenesis and development, unlimited cell proliferation, cell cycle imbalance and cell apoptosis disorder are important biological characteristics of tumor cells [Citation18,Citation19]. In the present study, both CCK-8 assay and clone formation assay revealed that TPPP3 overexpression inhibited the proliferation of NPC cells in vitro. Notably, TPPP3 high expression group enriched in 11 down-regulated KEGG pathways, especially in cell cycle and DNA replication, which is consistent with the result that TPPP3 overexpression inhibited the proliferation of NPC cells. These results demonstrate TPPP3 may inhibit NPC proliferation by regulating cell cycle and DNA replication pathways.
Invasion and metastasis of tumor cells are not only basic biological characteristics of tumor, but also key factors of tumor malignant progression [Citation20–22]. The clinical treatment of NPC is mainly radiotherapy, and the treatment effect for stage I patients is good, and the 10-year survival rate can reach 98%; but the treatment effect of advanced patients is poor, and the median survival time of patients with advanced metastasis is only 3 years [Citation23–25]. Thus, it is very important to study the molecular mechanism of NPC metastasis. In this study, transwell invasion assay showed that TPPP3 overexpression attenuated the invasion of NPC cells in vitro, whereas, neither wound healing assay nor transwell migration assay have effect on the migration ability of NPC cell. Further study revealed TPPP3 overexpression diminished the expression of MMP-2 and MMP-9 mRNA, which are involved in the breakdown of extracellular matrix in cancer metastasis. All above suggested that TPPP3 may serve as an important regulator in NPC progression.
In this study, the down-regulation of TPPP3 expression in NPC seems to be in contradiction with other previous reports that TPPP3 expression is up-regulated in colorectal cancer, non-small cell lung cancer and hepatocellular carcinoma; in addition, previous reports demonstrated that knockdown of TPPP3 inhibits cell proliferation, migration and invasion, which is inconsistent with our findings [Citation8,Citation9,Citation26]. In fact, it exactly reflects that the role of epigenetic modification related genes in tumors are not single, reveals the expression of TPPP3 may have specific tissue heterogeneity. Due to the complex heterogeneity in tumors, related genes can play different biological roles in different tumor cells. Therefore, exploring the specific role of epigenetic modification related genes in specific tumors is of great significance for early diagnosis, treatment and prognosis of tumors. TPPP3 has dual effect on tumorigenesis and development. The difference may be explained by the fact that different tumors have different genetic backgrounds, and are subjected to different upstream regulation, but the specific mechanism needs further study.
Conclusion
In summary, our study demonstrated that TPPP3 is significantly down-regulated in NPC tissues and cells. Furthermore, TPPP3 overexpression significantly attenuated the proliferation and invasion abilities of NPC cells in vitro. These findings suggested that TPPP3 may be considered as an antioncogene, which plays an important role in the occurrence and progression of NPC, whereas the underlying mechanism require further research.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012 [J]. CA Cancer J Clin. 2015;65(2):87–108.
- Junlin Y, Li G, Xiaodong H, et al. Symptoms and Prognosis of Nasopharyngeal Carcinoma [J]. Acta Acad Med Sin. 2006;28(3):315–317.
- Li G, Liu Y, Liu C, et al. Genome-wide analyses of long noncoding RNA expression profiles correlated with radioresistance in nasopharyngeal carcinoma via next-generation deep sequencing [J]. BMC Cancer. 2016;16(1):719.
- Hu R-M, Han Z-G, Song H-D, et al. Gene expression profiling in the human hypothalamus-pituitary-adrenal axis and full-length cDNA cloning [J]. Proc Natl Acad Sci U S A. 2000;97(17):9543–9548.
- Vincze O, Tökési N, Oláh J, et al. Tubulin polymerization promoting proteins (TPPPs): members of a new family with distinct structures and functions [J]. Biochemistry. 2006;45(46):13818–13826.
- Zhou W, Li J, Wang X, et al. Stable knockdown of TPPP3 by RNA interference in Lewis lung carcinoma cell inhibits tumor growth and metastasis [J]. Mol Cell Biochem. 2010;343(1-2):231–238.
- Pastor MD, Nogal A, Molina-Pinelo S, et al. Identification of proteomic signatures associated with lung cancer and COPD [J]. J Proteomics. 2013;89:227–237.
- Li Y, Xu Y, Ye K, et al. Knockdown of Tubulin Polymerization Promoting Protein Family Member 3 Suppresses Proliferation and Induces Apoptosis in Non-Small-Cell Lung Cancer [J]. J Cancer. 2016;7(10):1189–1196.
- Ye K, Li Y, Zhao W, et al. Knockdown of Tubulin Polymerization Promoting Protein Family Member 3 inhibits cell proliferation and invasion in human colorectal cancer [J]. J Cancer. 2017;8(10):1750–1758.
- Aiqun S, Xiaowen T, Huaifang L, et al. TPPP3 inhibits the proliferation, invasion and migration of endometrial carcinoma targeted with miR‐1827 [J]. Clin Exp Pharmacol Physiol. 2021;48(6)890-901.
- Ren Q, Hou Y, Li X, et al. Silence of TPPP3 suppresses cell proliferation, invasion and migration via inactivating NF-κB / COX2 signal pathway in breast cancer cell. Cell Biochem Funct. 2020;38(6):773–781.
- Hu D, Ansari D, Pawłowski K, et al. Proteomic analyses identify prognostic biomarkers for pancreatic ductal adenocarcinoma [J]. Oncotarget. 2018;9(11):9789–9807.
- Zhang Z, Zhang Y, Qiu Y, et al. Human/eukaryotic ribosomal protein L14 (RPL14/eL14) overexpression represses proliferation, migration, invasion and EMT process in nasopharyngeal carcinoma [J]. Bioengineered. 2021;12(1):2175–2186.
- Yang Z, Li X, Li J, et al. TPPP3 Associated with Prognosis and Immune Infiltrates in Head and Neck Squamous Carcinoma [J]. Biomed Res Int. 2020;2020:3962146.
- Gao Q, Tang L, Wu L, et al. LASP1 promotes nasopharyngeal carcinoma progression through negatively regulation of the tumor suppressor PTEN [J]. Cell Death Dis. 2018;9(3):393.
- Song L-B, Li J, Liao W-T, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells [J]. J Clin Invest. 2009;119(12):3626–3636.
- Zhou W, Wang X, Li L, et al. Depletion of tubulin polymerization promoting protein family member 3 suppresses HeLa cell proliferation [J]. Mol Cell Biochem. 2010;333(1-2):91–98.
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm [J]. Nat Rev Cancer. 2009;9(3):153–166.
- Preston-Martin S, Pike MC, Ross RK, et al. Increased cell division as a cause of human cancer [J]. Cancer Res. 1990;50(23):7415–7421.
- Hanahan D, Weinberg R. Hallmarks of cancer: the next generation [J]. Cell. 2011;144(5):646–674.
- McSherry EA, Donatello S, Hopkins AM, et al. Molecular basis of invasion in breast cancer [J]. Cell Mol Life Sci. 2007;64(24):3201–3218.
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms [J]. Nat Rev Cancer. 2003;3(5):362–374.
- Yang C-F, Peng L-X, Huang T-J, et al. Cancer stem-like cell characteristics induced by EB virus-encoded LMP1 contribute to radioresistance in nasopharyngeal carcinoma by suppressing the p53-mediated apoptosis pathway [J]. Cancer Lett. 2014;344(2):260–271.
- Chua DTT, Sham JST, Kwong DLW, et al. Treatment outcome after radiotherapy alone for patients with Stage I-II nasopharyngeal carcinoma [J]. Cancer. 2003;98(1):74–80.
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099 [J]. J Clin Oncol. 1998;16(4):1310–1317.
- Jianhua L, Wenzhi G, Jiakai Z, et al. Expression of tubulin polymerization promoting protein 3 in hepatocellular carcinoma and its biological function in hepatocellular carcinoma cells [J]. Chin J Exp Surg. 2015;32(8):1892–1895.