ABSTRACT
Current study was conducted to design and screen a long-lasting Exendin-4 analog for treating type 2 diabetes via the novel strategy of albumin binding combined with thrombin enzymolysis. First, a series of fusion peptides, containing different albumin-binding tags, a determinate thrombin-cleavable linker and a native Exendin-4, were prepared via chemosynthesis for in vitro and in vivo characterization. Surface plasmon resonance assay, thrombin cleavage assay and plasma stability test were performed for screening the optimal HEX peptide with enhanced albumin-binding affinity, controlled-release as well as plasma stability. The in vivo anti-diabetic efficacies of the selected candidate were further assessed via both acute and chronic pharmacodynamic evaluation in diabetic model animals. HEX15 exhibited either the highest affinity for human serum albumin or the superior in vitro stability and controlled release of Exendin-4 among 21 HEX peptides. Glucose tolerance test and hypoglycemic duration assay both revealed the notably improved the glucose tolerance and prolonged normoglycemic duration, respectively, of diabetic mice after single treatment of HEX15. Furthermore, chronic dosing of HEX15 significantly ameliorated the manifestations of diabetes in the db/db mice, including body weight, food intake, glycometabolism as well as hyperlipemia. Interestingly, combination therapy of HEX15 and long non-coding RNA-ENST00000411554 notably accelerated the wound healing and improved foot ulcer symptoms in model rats with diabetic foot ulcers. In summary, based on the strategy of linking the heptapeptide tag and thrombin-based sustained release, a long-acting Exendin-4 analog, HEX15, holds potential to be developed as a drug for ameliorating T2D as well as diabetic complications.
1. Introduction
In recent years, polypeptide therapeutics hold good application prospect due to the high safety, efficacy, specificity, tolerability and low immunogenicity [Citation1,Citation2]. Unfortunately, the inherent disadvantages of peptides, such as low oral utilization, high enzyme degradation and short half-life, limit further clinical applications [Citation3,Citation4]. The in vivo rapid filtration in therapeutic peptides results from the combination of poor metabolic stability and hydrodynamic radius below the renal glomerular filtration limit, which lead to the frequent administration of peptide-based drugs to achieve the desirable therapeutic levels [Citation4,Citation5]. Therefore, many strategies have been developed to overcome these challenges.
At present, structural modification is an effective means to improve the polypeptide stability [Citation6,Citation7]. For example, in vivo protease degradation is avoided by amino acid substitution, cyclization, or the molecular size of peptides is expanded by glycosylation or PEGylation, thereby effectively reducing the renal clearance [Citation8,Citation9]. However, there are some difficulties in structural modification of peptides. Unnatural amino acid substitution or cyclization is difficult to implement synthetically [Citation4,Citation6]. Another practical problem is that if only factors in terms of peptide stability are considered, it is easy to ignore the effects in terms of peptide bioactivity [Citation10]. For example, glycosylation and PEGylation inevitably affect the biological activity of peptides [Citation11]. Therefore, there is a need to preserve or improve the physiological activity of peptides while improving their stability. Human serum albumin (HSA) is the most abundant protein in serum, with an in vivo half-life of up to 19 days [Citation12]. Recently, the strategy of “hitchhiking” albumin has been widely used to improve the drugability and preserve the biological activity of the short-acting peptide [Citation13].
Exendin-4, a natural analog of glucagon-like peptide-1 (GLP-1) composed of 39 amino acid residues, was discovered from the venom of American great venom lizards and was approved by the FDA in 2005 for the treatment of type 2 diabetes [Citation14]. Exendin-4 holds numerous physiological functions including promoting insulin secretion and islet cell proliferation and regeneration, inhibiting glucagon release and delaying gastric emptying [Citation15]. However, Exendin-4 has a short in vivo circulating half-life of 2.4 hours and requires twice daily dosing [Citation15]. Therefore, it is very necessary to develop long-acting Exendin-4 drugs through further structural modification and dosage form modification.
Here, the heptapeptide tag was fused to Exendin-4 via a thrombin-recognized element to develop a series of novel long-acting Exendin-4 molecules, termed HEX peptides. The aim of current study was to screen the optimal candidate via in vitro evaluation and subjected to the preliminarily pharmacodynamic and kinetic characteristics evaluation in diabetic rodents. Furthermore, previous studies have identified the LncRNA-ENST00000411554 as critically involved in the pathogenesis of diabetic foot ulcers (DFU) [Citation16]. Hence, the improved effects of HEX15 and LncRNA combination therapy on DFU will also be investigated. The hypothesis is that the newly designed Exendin-4 analog may hold the improved in vivo antidiabetic efficacies and exhibited enhanced combined effects on DFU with LncRNA.
2. Materials and methods
2.1 Materials and animals
HEX peptides were synthesized by Hefei KS-V Peptide Biotechnology Co., Ltd. (Hefei, China) using combinatorial Fmoc solid phase synthesis strategy with purity >95%. Semaglutide was acquired from Hefei KS-V Peptide Biotechnology Co., Ltd. (Hefei, China). HSA with a purity of 99% was obtained from Sigma-Aldrich (St. Louis, USA). Six- to eight-week-old male db/db mice (male, ~40 g) and eight- to nine-week-old Sprague-Dawley (SD) rats (male, ~275 g) were purchased from Beijing Maidisiwei Biotechnology Co., Ltd. (Beijing, China). Rodent animals were divided into six per cage. All animals were kept under standard husbandry conditions (25 ± 1°C; 55%–65% relative humidity; 12-hour light/dark cycle). Mouse glycated hemoglobin (HbA1c) ELISA kit was obtained from Sigma-Aldrich (St. Louis, USA). All animal studies were carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals and approved by ShengLunBio IACUC committee with approval number DPR-000145003.
2.2 Albumin binding affinity test
The optimal HEX peptide with the highest binding affinity for HSA was selected by surface plasmon resonance (SPR) analysis based on the previously described procedure [Citation17]. All kinetic constants including equilibrium constant (KD), association constant (ka) and dissociation constants (kd) were calculated by using the BIA evaluation software 4.1 (Biacore AB, Sweden).
2.3 Protease cleavage test
HEX13-HEX15 (60 ng/mL) were subjected to protease cleavage with the thrombin at final concentration of 0.4 U/mL in PBS (pH = 7.4). Reaction mixture was incubated at 37°C for seven days and protected from light, then taken out at predetermined time points of 0, 1, 2, 3, 4, 5, 6 and 7 days. At last, the different hydrolyzed fragments and the released Exendin-4 were detected by ELISA and LC-MS/MS methods, respectively.
2.4 Plasma stability assay
Monkey plasma was brought from Sigma-Aldrich (St. Louis, USA) and stored at −20°C until used. HEX13-HEX15 (1 mg/L) were added to albumin-contained or albumin-depleted monkey plasma and then incubated in 96-well plate for indicated time at 37°C and protected from light. At last, the remaining HEX peptides were determined by LC-MS/MS method.
2.5 Oral glucose tolerance test (OGTT)
Male db/db mice were randomly assigned into five groups and received single subcutaneous (s.c.) injection of saline, 10, 30, 90 and 150 nmol/kg HEX15 in single oral glucose tolerance test (OGTT), while received single s.c. injection of saline, 90 nmol/kg Semaglutide and 10, 30 and 90 nmol/kg HEX15 in multiple OGTTs. All animals were fasted for 12 h before operating OGTT. The blood glucose levels (BGLs) were detected immediately from tail blood with handheld one-touch UltraEasy glucometer (Johnson & Johnson, USA) before and 15, 30, 60 and 120 min after glucose loading (2 g/kg body weight). After first oral glucose loading, OGTT was performed another two times at a time interval of 70 hours (0–2, 70–72 and 144–146 h, respectively).
2.6 Hypoglycemic duration test
Male db/db mice were split into five groups and received single s.c. injection of saline, 90 nmol/kg Semaglutide, 10, 30 and 90 nmol/kg HEX15, respectively. All animals were allowed to move, eat and drink freely during the hypoglycemic duration test. The BGLs were detected from tail blood with handheld one-touch UltraEasy glucometer (Johnson & Johnson, USA) before and 1, 2, 4, 8, 12, 24, 36, 48, 72, 96, 120, 144, 168, 192 h after drug administration.
2.7 Pharmacokinetic study
Male SD rats were assigned into three groups and received s.c. injection of 10, 30 and 90 nmol/kg HEX15, respectively. Blood samples were obtained from tail at time points of 0, 2, 4, 8, 12, 24, 36, 48, 72, 96, 120, 144 and 168 hours after drug administration and then centrifuged at 4°C, 12,000 rpm for 10 min. Following centrifugation, the generated plasma was mixed with acetonitrile in a ratio of 1:3 and further determined by LC-MS/MS method.
2.8 Long-term efficacy study
Male diabetic mice were randomly assigned into five groups and received s.c. injection of saline, 90 nmol/kg Semaglutide, 10, 30 and 90 nmol/kg HEX15, respectively. All animals were weekly administrated with different agents for 8 consecutive weeks. Body weight gains and food intakes were monitored twice per week. Fat (% of body mass) was calculated with the following formula: (fat mass/(fat mass + lean mass)) × 100%. Following area under the curve (AUC) of BGLs was calculated, and the HbA1c values were detected by ELISA kits before and after the long-term treatment. Various lipid metabolic indicators were detected by using automatic biochemical analyzer.
The recombinant plasmid pcDNA(+)-ENST00000411554 were synthesized by Shanghai Sangon Biotechnology Co., Ltd. Briefly, total RNA was extracted in the skin of DFU rats and amplified by F: ATAAAGTTACTTTATACG and R: TACCAGGAGACATGAGA primer pair. PCR products were cloned into pcDNA 3.1+ backbone after being verified by sequencing.
Male SD rats were fed with high-fat diet for 4 weeks, and then single intraperitoneally injected with 1% streptozotocin (STZ) solution at a dose of 40 mg/kg after fasted for 12 hours. SD rats with fasting blood glucose ≥16.7 mmol/L were considered as eligible diabetic model animals. Subsequently, diabetic rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium solution, and the right groin was incised with routine sterile disinfection. Femoral artery and superficial circumflex iliac artery were separated, and a rectangular mark (3 mm × 7 mm) was made on the corresponding dorsum of the foot using a seal to remove the full thickness of the skin with the rectangular mark to prepare a DFU model of lower limb ischemia. DFU rats were assigned into five groups, received saline, lncRNA and HEX15 alone or in combination. All rats were administrated with saline, lncRNA (300 mg/kg), HEX15 (90 nmol/kg) or lncRNA (300 mg/kg) + HEX15 (90 nmol/kg) once-daily for 2 consecutive weeks.
Serum levels of C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), endothelin-1 (ET-1) and thromboxane A2 (TXA2) were detected by ELISA method (the indicated ELISA kits were obtained from Biolegend, San Diego, CA). Nitric oxide synthase (NOS) activity was determined via an NOS activity assay kit (Biolegend, San Diego, CA).
2.9 Data analysis
One-way ANOVA and t-test procedures were used as appropriate in quantitative data analysis via Graphpad prism 8.4, and all the values were presented as means with error as standard deviations. P value less than 0.05 were considered significant.
3. Results
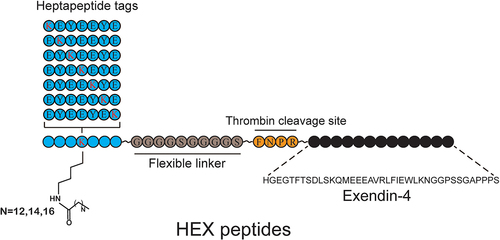
3.1 Design and preparation of HEX peptides
Previous studies have demonstrated that the heptapeptide tag can significantly improve the half-life of peptide drugs by serum albumin binding [Citation18]. Here, the strategy of thrombin-based sustained-release and heptapeptide tag were applied to design a series of Exendin-4 analogs with potent long-acting in vivo circulation (). Briefly, seven different heptapeptide tags (with different lysine positions and lengths of aliphatic side chains) were designed and further fused to Exendin-4 by a linker (GGGGSGGGGS) and a thrombin-cleaved sequence (FNPR) to generate 21 candidates, termed HEX01 to HEX21. The detailed peptide sequences of 21 HEX peptides are shown in Table S1, and the data of mass and purity of HEX peptides are shown in Table S2. These candidates predicted could bind to HSA with high affinity and slowly release active Exendin-4 in vivo. To validate the above hypothesis, in vitro albumin binding assay and thrombin cleavage assay were performed.
3.2 In vitro evaluation of HEX peptides
The association (ka) and dissociation (kd) constants of HEX peptides with albumin were further determined by SPR assay, and the albumin affinity constants (ka/kd, KD) were calculated. As shown in , different acylation sites and different lengths of fatty acid side chains in HEX peptides exhibited different albumin binding affinities. Specially, HEX13, HEX14 and HEX15 bound to HSA with higher affinities than other HEX peptides with the KD of 5.44 × 10−7, 9.35 × 10−7 and 3.20 × 10−7 M, respectively, which implies the conjugate with fifth acylation site modification exhibits the higher HSA-binding potency. As a result, HEX13 to HEX15 were selected for further protease cleavage assays.
Table 1. The binding affinity constants of HEX peptides for HSA
Subsequently, the kinetic profile of in vitro release of Exendin-4 in the presence of thrombin were evaluated. As evident from ), the intact fusion peptide was gradually digested accompanied by the persistently released Exendin-4 during the duration of hydrolysis, and the heptapeptide tags effectively affected the hydrolysis of thrombin. Interestingly, the transient concentration of Exendin-4 in the HEX15 group was obviously higher than that of HEX13 and HEX14 groups at day 7, which is possibly due to the increased in vitro stability of the larger side chain molecular weight. In addition, the LC-MS/MS analysis was performed to identify the transient presences of intact HEX peptide, heptapeptide tag-GGGGSGGGGS-FNPR fragment and Exendin-4 in the thrombin cleavage reaction ()).
Figure 2. In vitro activities of long-acting Exendin-4 analogs. (a) Concentration versus time plots of released Exendin-4 from HEX13-HEX15. Spectrometric profiles of (b) HEX13 (day 1), (c) HEX13 (day 7), (d) HEX14 (day 1), (e) HEX14 (day 7), (f) HEX15 (day 1) and (g) HEX15 (day 7) after TBN digestion; Peak 1: heptapeptide tag-GGGGSGGGGS-FNPR fragment; Peak 2: Released Exendin-4; Peak 3: HEX13-HEX15. Degradation profiles of HEX13-HEX15 in (h) albumin-depleted or (i) albumin-contained monkey plasma. All data are expressed as means with error bars as standard deviations (n = 8). *, ** or *** denotes P < 0.05, 0.01 or 0.001 vs. HEX13 group; # or ## denote P < 0.05 or 0.01 vs. HEX14 group.
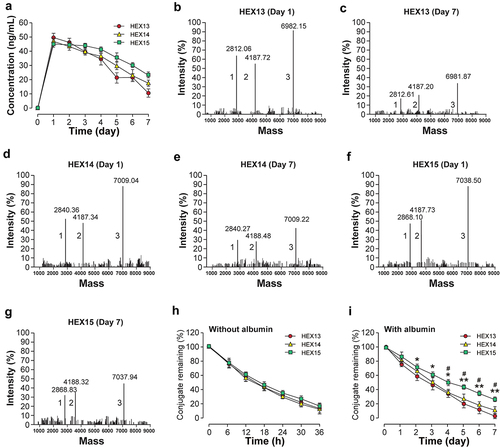
The stability of peptides in blood is often limited by the rapid cleavage of catalytic enzymes and the inducible short biological half-life. To verify whether the HEX peptides could be used for in vivo applications, the plasma stability assays in albumin-contained or albumin-depleted monkey plasma were further performed. As shown in , the elimination half-lives of all three HEX peptides were approximately 14 hours in plasma without albumin. Notably, the plasma stability of the three peptides was significantly improved in albumin-containing plasma, suggesting the high applicability of the heptapeptide tag strategy in improving peptide stability (). In particular, the elimination half-life of HEX15 was significantly prolonged compared to that of HEX13 or HEX14 (4.9 days vs. 3.1 or 3.4 days). Therefore, HEX15 was selected as candidate molecule for further pharmacodynamic evaluation in vivo.
3.3 Acute in vivo efficacy evaluation of HEX15
First, OGTT was used to examine the hypoglycemic effect of HEX15 in diabetic mice via measuring and calculating of the BGL-time curve and AUC within 120 mins. As the results shown in , single injection of HEX15 at different doses all exhibited the significantly enhanced glucose-lowering effects compared to that of the saline group. Moreover, the AUC were gradually decreased with the increase in the doses of HEX15, indicating that HEX15 exhibited a clear dose-efficacy relationship within the range of 10–90 nmol/kg. Nevertheless, the AUC of 150 nmol/kg HEX15-treated group was similar to that of 90 nmol/kg group, indicating that 90 nmol/kg is the more reasonable dose of HEX15 for the in vivo efficacy evaluation ().
Figure 3. In vivo activities of long-acting Exendin-4 analogs. (a)-(b) Dose–response relationships for hypoglycemic effect of HEX15 in fasted db/db mice; (A) Glycemic changes and (B) AUC value of OGTT integrated from 0 to 120 min in fasted db/db mice. (c)-(d) Sustained glucose-stabilizing effects of HEX15 in fasted db/db mice; (C) Glycemic changes and (D) AUC value integrated from 0 to 120 min of each OGTT in fasted db/db mice. (e)-(f) Hypoglycemic durations of HEX15 in non-fasted db/db mice; (E) Glycemic changes and (F) AUC value during 0–192 hours in db/db mice. (g)-(h) Pharmacokinetic profiles of HEX15 in SD rats; The time-dependent plasma levels of (G) intact HEX15 and (H) released Exendin-4. All data are expressed as means with error bars as standard deviations (n = 8). *, ** or *** denote P < 0.05, 0.01 or 0.001 vs. saline group; # denotes P < 0.05 vs. Semaglutide group.
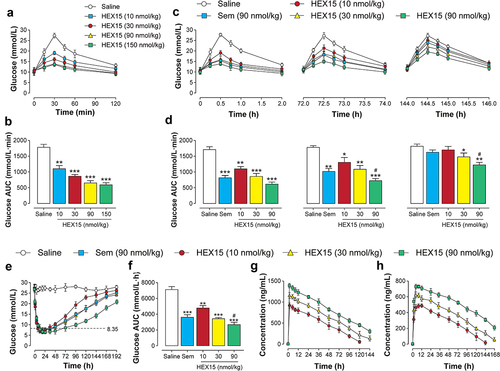
Subsequently, multiple OGTTs were performed to evaluate the sustained glycemic control efficacy of HEX15 in fasted db/db mice. As evident from the , BGLs in saline-treated mice peaked 30 mins after each oral glucose administration throughout the experimental period (0–144 h) and then slowly decreased to baseline levels over 120 mins. As expected, dose-dependent hypoglycemic effects were observed in three doses of HEX15-treated groups. In particular, the hypoglycemic effect of HEX15 at the maximum dose lasted at least for 146 hours, and the same dose of Semaglutide, in contrast, was almost ineffective during the period of 144–146 h (). Above results suggested that treatment of HEX15 at all three doses significantly improved the systemic glucose intolerance in the diabetic db/db mice.
Hypoglycemic efficacy of HEX15 was further verified in non-fasted db/db mice, and Semaglutide (90 nmol/kg) was used as a positive control. As evident from the , the lowest glucose levels were similar in different doses of HEX15-treated group, while the hypoglycemic duration (time period with blood glucose values ranging from 0 to 8.35 mmol/L) was approximately 34, 42 or 68 hours for 10, 30 or 90 nmol/kg dosage, respectively. In contrast, the hypoglycemic duration was much greater in mice treated with HEX15 than with same dose Semaglutide (only ~41 h). Moreover, three doses of HEX15 (10, 30 and 90 nmol/kg) remarkably decreased the AUC values integrated from 0 to 192 h by 36.2%, 51.6% and 62.9%, respectively, when compared to the saline group (). Above results collectively demonstrated that the HEX15 treatment could stabilize the BGL of db/db mice for nearly 60 h at the dose of 90 nmol/kg.
Pharmacokinetic profiles of HEX15 were characterized in SD rats. As evident from the and , the peak plasma concentration and the area under the drug concentration-time curve were increased along with the increasing dose of HEX15. Of these, the elimination half-life of intact HEX15 at 10, 30, or 90 nmol/kg was 42.14, 50.41 or 87.22 hours, respectively, while the elimination half-life of released Exendin-4 was 46.30, 60.55 or 142.74 hours, respectively. Combined with the above acute pharmacodynamic results, HEX15 holds potential to be developed as a weekly anti-diabetic drug.
Table 2. Pharmacokinetic parameters of HEX15 in SD rats. All data are expressed as means with error bars as standard deviations (n = 8)
3.4 Chronic in vivo efficacy evaluation of HEX15
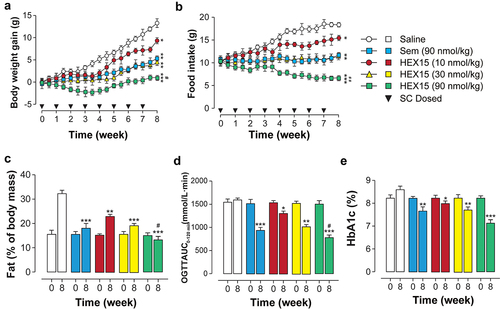
The antidiabetic and anti-obesity potential of HEX15 was further evaluated under chronic conditions. Db/db mice were subcutaneously injected with 90 nmol/kg Semaglutide (positive control) or three different doses of HEX15 (10, 30 or 90 nmol/kg) once a week for 8 weeks. The data observed from , b, c) revealed that the body weight gain and food intake of saline-treated group remained continuously elevated accompanied by an increase in fat mass. On the contrary, three doses of HEX15 treatment clearly reversed these changes in a dose-dependent manner. Interestingly, HEX15 treatment demonstrated superior reduction of body weight and fat mass compared to same dose Semaglutide. Moreover, 8-week HEX15 treatment displayed significant improvement on glucose tolerance in db/db mice, and the degree was superior to Semaglutide treatment (). Next, the %HbA1c values were measured by ELISA in db/db mice, which is a reliable indicator for chronic glycemic control in diabetes. As evidenced in , HEX15 treatment exerts an obvious reduction on the %HbA1c value, suggesting the significant improvement of blood glucose metabolism in diabetic mice.
Figure 4. Long-term study of HEX15 in db/db mice. (a) Body weight gain, (b) food consumption, (c) fat mass, (d) OGTT AUC value integrated from 0 to 120 min and (e) %HbA1c value after chronic dosing of HEX15 in db/db mice. All data are expressed as means with error bars as standard deviations (n = 8). *, ** or *** denote P < 0.05, 0.01 or 0.001 vs. saline group; # or ## denote P < 0.05 or 0.01 vs. Semaglutide group.
Finally, the blood biochemical parameters in db/db mice after long-term HEX15 treatment were examined. As shown by the data in , the serum triglycerides (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL) levels of saline-treated were maintained at a high level. This is clearly the result of the disturbed glucose and lipid metabolism that accompanies diabetes. In addition, chronic HEX15 treatment dose-dependently decreased serum levels of TG, TC and LDL and increased high-density lipoprotein cholesterol (HDL) levels in db/db mice. Interestingly, HEX15 exhibited a significant efficacy on lipid metabolism regulation than the same dose of Semaglutide. The above results indicate that HEX15 treatment is able to normalize hyperlipidemia.
Table 3. Lipid metabolic indicators in 8-week HEX15 treated db/db mice. All data are expressed as means with error bars as standard deviations (n = 8). *, ** or *** denote P < 0.05, 0.01 or 0.001 vs. saline group; # denotes P < 0.05 vs. Semaglutide group
Previous studies have identified the long non-coding RNA-ENST00000411554 as critically involved in the pathogenesis of DFU [Citation16]. LncRNA can reduce the expression of pro-inflammatory factors by inhibiting the MAPK signal transduction pathway. Here, the ameliorative effects of combination therapy of HEX15 and LncRNA in established DFU model were investigated. The results shown in revealed that once-daily treatment with HEX15 or IncRNA alone or in combination all significantly promoted wound healing compared with the control group. In addition, after 2 weeks of continuous treatment, the highest wound healing rate (more than 80%) was observed in the group treated with HEX15 combined with LncRNA.
Figure 5. Combination therapy of HEX15 and LncRNA in DFU rats. The changes of (a) wound healing rate, (b) pro-inflammatory factors and (c-d) vascular regulator after 2-week combined treatment of HEX15 and lncRNA in DFU rats. All data are expressed as means with error bars as standard deviations (n = 8). *, ** or *** denote P < 0.05, 0.01 or 0.001 vs. saline treated group.
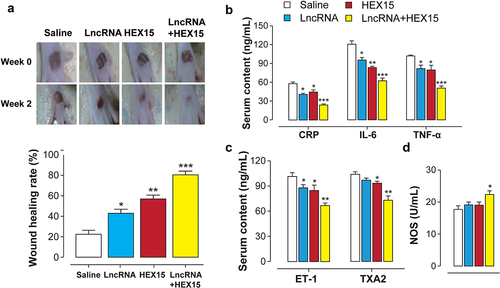
The serum levels of inflammatory factors in DFU mice were also detected by ELISA method, and the results show that the combination treatment with HEX15 and lncRNA significantly reduced pro-inflammatory factor levels in DFU mice (). Moreover, compared with the control group and the monotherapy group, the serum levels of endothelin-1 (ET-1) and thromboxane 2 TAX2 were significantly decreased, while the activity of NOS was significantly increased in the combination therapy group, indicating that the combination therapy of HEX15 and lncRNA could significantly improve the functions of vasoconstriction and vasorelaxation as well as promote the vascular endothelium repair (, d)).
4. Discussion
In the current study, the joint strategy of heptapeptide tag and thrombin-cleavable linker with the capacity of albumin-binding and sustained release, respectively, was applied in developing a novel Exendin-4 analog with the prolonged in vivo half-life and favorable antidiabetic efficacy. The aim of current research was to select the optimal fusion peptide via in vitro and in vivo evaluation and then subjected to preclinical pharmacodynamic evaluation in diabetic model animals. Hence, the potent improved efficacies of combination therapy with HEX15 and LncRNA on the DFU were also investigated. Current main experimental hypothesis is that the heptapeptide tag and thrombin-cleavable linker significantly improved the in vivo antidiabetic efficacies of Exendin-4, and the combination therapy may exhibit protective efficacies on DFU in animal model.
Based on the biodegradable, nontoxic, non-antigenic and high bioavailability, HSA has become a research hotspot in the field of biomedicine [Citation19–21]. Nowadays, albumin carrier system has also become a very viable research direction in pharmaceutical research due to the long half-life and high abundance of HSA [Citation22]. According to previous studies, the heptapeptide tag strategy applied to bioactive short peptides presents a high albumin affinity and significantly prolonged in vivo stability, while its effectiveness has also been demonstrated in mouse models [Citation18].
A series of Exendin-4 conjugates, termed HEX01-HEX21, were designed () and further focused on the affinity of the HEX peptide to albumin as well as the sustained release properties in vitro. SPR measurement showed that all HEX peptides exhibited the high affinity for HSA, especially for the HEX13, HEX14 and HEX15, indicating that the fatty acid chain coupling at the fifth lysine of the heptapeptide tag sequence exhibited more favorable albumin binding ability. In addition, in vitro protease cleavage assays showed that HEX13-HEX15 could slowly release Exendin-4 under TBN hydrolysis for more than 7 days. It is worth noting that HEX15 showed a better sustained-release effect compared with HEX13 and HEX14. Not only that, in vitro plasma stability test also obtained similar results, indicating that the heptapeptide tag coupled with longer fatty chain shows better in vitro stability than that with shorter fatty chain. In summary, HEX15 was chosen for further pharmacodynamic evaluation in vivo.
The results of OGTT demonstrated that the single treatment of HEX15 significantly reduce the BGLs of diabetic mice in a dose-dependent manner within the range of 10~90 nmol/kg. Long-acting hypoglycemic profiles of HEX15 were then evaluated by multiple OGTTs, and HEX15 showed not only significantly better glucose-lowering ability but also more long-acting property compared to the Semaglutide at same dose, which was probably due to significantly reduced renal clearance resulting from the high albumin binding ability and persistent existence of Exendin-4 via sustained release, respectively. Importantly, the balanced glycemic effect of HEX15 is exerted through a glucose-dependent mode without the risk of hypoglycemia, which is an outstanding advantage for future clinical application. Longer hypoglycemic duration was also observed in HEX15-treated group compared to Semaglutide group. Additionally, the initial pharmacokinetic tests in SD rats were further carried out to determine the exposure and plasma-eliminated half-life of HEX15, and the results showed that the presence of Exendin-4 released from HEX15 lasted for more than 168 hours.
According to previous literatures [Citation23,Citation24], the hypoglycemic duration in db/db mice that received single injection of native Exendin-4 was only ~5.6 hours, while that in single HEX15-treated db/db mice at similar doses were nearly 40 hours. In addition, the elimination half-life of wild-type Exendin-4 in SD rats was only about 2 hours, while that of HEX15 (10 nmol/kg) could reach about 42 hours. The above results indicate that the heptapeptide-based and thrombin-based strategy greatly improves the stability and efficacy of Exendin-4 in vivo, suggesting that HEX15 holds potential to be developed as a weekly anti-diabetic agent.
The therapeutic effects of chronic treatment of HEX15 at different doses in age-matched db/db mice were further assessed. Chronic 8-week treatment of HEX15 effectively controlled the body weights and food intakes of db/db mice compared to negative control group. In addition, current findings also suggested that the loss of body weight is primarily a result of reduced fat mass, although a minimal reduction in lean mass was also observed. Meanwhile, chronic treatment of HEX15 could also improve glucose tolerance and %HbA1c levels in diabetic mice. In addition to the effects on body weight and fat mass, HEX15 treatment also improved the diabetes-induced lipid metabolism imbalance, including the significantly decreased TC, TG, and LDL-C levels and significantly increased HDL-C levels.
Above results collectively indicated that HEX15 performed more effective anti-diabetic efficacies than Semaglutide in both acute and chronic efficacy tests. Previous studies revealed that Semaglutide could exert the optimal pharmacotherapeutic effect in T2D treatment because of its excellent in vivo stability [Citation25,Citation26]. Similarly, HEX15 also showed the prolonged in vitro stability in the presence of albumin and the favorable pharmacokinetic profile. Moreover, HEX15 can also slowly release the active Exendin-4 in a sustained manner through the digestion of thrombin, so as to achieve the purpose of continuous efficacy. Therefore, it was hypothesized that HEX15 has an enormous potential to be an anti-diabetic drug candidate.
It is unanticipated to noting that HEX15 could significantly improve DFU in diabetic rats. In a previous study, the lncRNA-ENST00000411554 involved in the progression of DFU [Citation16] was demonstrated. In the present study, the combination treatment of HEX15 and lncRNA significantly accelerated wound healing in DFU rats. In addition, the HEX15 and lncRNA combined treatment also significantly decreased the serum levels of pro-inflammatory factors (CRP, IL-6 and TNF-α) and vasoconstrictor factors (ET-1 and TXA2) as well as increased the NOS activity in diabetic rats. The above results indicated that the combined treatment of HEX15 and lncRNA can significantly promote the repair of vascular endothelial injury in the foot, maintain the balance of vasoconstriction and vasorelaxation functions, reduce the inflammatory response and accelerate the recovery of foot ulcers.
The current study still holds several limitations. First, the anti-diabetic effects of HEX15 in diabetic rat model were not assessed, while the protective efficacies of HEX15 combined with lncRNA in DFU rat model were directly explored, which may have some adventures. Second, we did not fully explain the synergistic mechanism of combination in improving DFU, which need further systematic study. Finally, the results of current research can also provide some scientific suggestions for potential clinical needs, although the experiments were performed based on rodent models. However, HEX-15, as a candidate molecule that is still preclinical, is not highly likely to be used in combination with lncRNA-ENST00000411554 in clinical practice. However, so far, there have been several GLP-1RAs on the market, especially benchmark drugs such as Semaglutide, which can have the opportunity to expand toward combination therapy for DFU. Of course, detailed therapeutic schedule also requires strict clinical trials to test its safety.
5. Conclusion
In summary, current study reported a strategy linking heptapeptide tag and thrombin-based sustained release and then designed a long-lasting Exendin-4 analog with significantly improved plasma stability and in vivo efficacy for treating diabetes. Current findings shed new light on the applicability of short-acting GLP-1 receptor agonists as a treatment for diabetes and diabetic complications. Future studies, including the application of other short-acting molecules, will allow us to further broaden the scope of application of this strategy and may provide additional therapeutic avenues for other metabolic diseases, such as obesity and hyperlipidemia.
Highlight
HEX15 exhibited either highest affinity for albumin or optimal in vitro stability.
A single dose of HEX15 exerted the long-lasting antidiabetic activity in db/db mice.
Chronic HEX15 treatment exerted beneficial metabolic effects in diabetic mice.
Chronic HEX15 treatment accelerated the recovery of diabetic foot ulcers.
Authors’ Contributions
Conceptualization: Shujuan Xu; Data curation: Shujuan Xu, Fang Wang; Methodology: Shujuan Xu, Fang Wang, Hui Li, Ya Wang, Dongzhong Fang; Resources: Shujuan Xu; Software: Shujuan Xu, Fang Wang; Supervision: Fang Wang; Validation: Fang Wang, Hui Li, Ya Wang, Dongzhong Fang; Writing-original draft: Shujuan Xu; Writing - review & editing: Fang Wang, Hui Li, Ya Wang, Dongzhong Fang.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability
All data generated or analyzed during this study are included in this article.
Additional information
Funding
References
- Lau JL, Dunn MK. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioor Med Chem. 2018;26(10):2700–2707
- Craik DJ, Fairlie DP, Liras S, et al. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81(1):136–147.
- Zaman R, Islam RA, Ibnat N, et al. Current strategies in extending half-lives of therapeutic proteins. J Control Release. 2019;301:176–189.
- Diao L, Meibohm B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin Pharmacokinet. 2013;52(10):855–868.
- Mcgregor DP. Discovering and improving novel peptide therapeutics. Curr Opin Pharm. 2008;8(5):0–619.
- Yao JF, Yang H, Zhao YZ, et al. Metabolism of peptide drugs and strategies to improve their metabolic stability. Curr Drug Metab. 2018;19(11):892–901.
- Böttger R, Hoffmann R, Knappe D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS One. 2017;12(6):e0178943.
- Gentilucci L, De Marco R, Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des. 2010;16(28):3185–3203.
- Veronese FM, Mero A, Pasut G. Protein PEGylation, Basic science and biological applications. 2009, 11–31.
- Pollaro L, Heinis C. Strategies to prolong the plasma residence time of peptide drugs. Med Chem Commun. 2010;1(5):319–324.
- Fazavana J, Brophy TM, Chion A, et al. Investigating the clearance of VWF A-domains using site-directed PEGylation and novel N-linked glycosylation. J Thromb Haemost. 2020;18(6):1278–1290.
- Tian R, Zhu S, Zeng Q, et al. An albumin sandwich enhances in vivo circulation and stability of metabolically labile peptides. Bioconj Chem. 2019;30(6):1711–1723.
- Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. AcBB. 2013;1830(12):5526–5534.
- Zhong X, Yang S, Liu T, et al. Engineering a novel protease-based Exendin-4 derivative for type 2 antidiabetic therapeutics. Eur J Med Chem. 2018;S0223523418302952: 841-850.
- Kim TH, Jiang HH, Lee S, et al. Mono-PEGylated dimeric exendin-4 as high receptor binding and long-acting conjugates for type 2 anti-diabetes therapeutics. Bioconj Chem. 2011;22(4):625–632.
- Xu S, Weng X, Wang Y, et al. Screening and preliminary validation of T lymphocyte immunoregulation‑associated long non‑coding RNAs in diabetic foot ulcers. Mol Med Rep. 2019;19(3):2368–2376.
- Liang YH, Chang CC, Chen CC, et al. Development of an Au/ZnO thin film surface plasmon resonance-based biosensor immunoassay for the detection of carbohydrate antigen 15-3 in human saliva. Clin Biochem. 2012;45(18):1689–1693.
- Zorzi A, Middendorp SJ, Wilbs J, et al. Acylated heptapeptide binds albumin with high affinity and application as tag furnishes long-acting peptides. Nat Commun. 2017;8(1):16092.
- Rabbani G, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: a natural cargo. Int J Biol Macromol. 2019;123:979–990.
- Maciążek-Jurczyk M, Szkudlarek A, Chudzik M, et al. Alteration of human serum albumin binding properties induced by modifications: a review. Spectrochim Acta A Mol Biomol Spectrosc. 2018;188:675–683.
- Wan A, Miao Y, Peng L, et al. Binding and biologic characterization of recombinant human serum albumin-eTGFBR2 fusion protein expressed in CHO cells. Bioengineered. 2017;8(5):600–612.
- Ishima Y, Maruyama T. Human serum albumin as carrier in drug delivery systems. Yakugaku Zasshi Journal of the Pharmaceutical Society of Japan. 2016;136(1):39–47.
- Han J, Huang X, Sun L, et al. Novel fatty chain-modified glucagon-like peptide-1 conjugates with enhanced stability and prolonged in vivo activity. Biochem Pharmacol. 2013;86(2):297–308
- Han J, Sun L, Huang X, et al. Novel coumarin modified GLP-1 derivatives with enhanced plasma stability and prolonged in vivo glucose-lowering ability. Br J Pharmacol. 2014;171(23):5252–5264.
- Lau J, Bloch P, Schaffer L, et al. The discovery of the once weekly glucagon like peptide 1 (GLP-1) analog semaglutide. J Med Chem. 2015;58(18): 7370-7380.
- Holst JJ, Madsbad S. Semaglutide seems to be more effective the other GLP-1Ras. Ann Transl Med. 2017;5(24):505.