ABSTRACT
Bushen Huoxue (BSHX) has been applied in clinical traditional Chinese medicine treatment, and has definitive clinical efficacy in the treatment of Premature Ovarian Insufficiency (POI) in China. However, little is known of the underlying mechanism of BSHX. The purpose of this paper is to study the pharmacological mechanisms of BSHX acting on POI based on a pharmacology and experimental validation. The pharmacological database of chinese medicine system and analysis platform (TCMSP) were used to search the effective active ingredients and potential action targets of BSHX. Drugbank, Online Mendelian Inheritance in Man (OMIM), Genecards, and Disgenet databases were used to obtain relevant targets of POI. Gene Ontology (GO) enrichment, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, and the visual network of protein-protein interaction network were constructed by FunRich3.1. Pymol software, and Auto Dock tools 1.5.6 were used for molecular docking. Murine model of POI was used to further investigate the mechanism of BSHX against on POI. Finally, 127 active compounds were collected from TCMSP database, and 215 active targets were identified. There were 1366 targets related to POI and 99 targets of BSHX for the treatment of POI. Quercetin, kaempferol, and stigmasterol were recognized as the most effective compounds corresponding to targets. The top three genes according to degree value are TP53, Akt1, and VEGFA. Further, the results of GO and KEGG enrichment analysis revealed that those core targets were mainly enriched on TRAIL and TGF-β receptor signaling. The results of molecular docking showed that stigmasterol had good binding ability to Akt1. Moreover, experimental validation suggests that BSHX significantly Increased the expression of TGF-β1 and Smad2/3, regulating the release of serum sex hormones, which include Follicular stimulating hormone (FSH), Estradiol (E2), and Antimullerin hormone (AMH).
HIGHLIGHTS
BSHX treats POI by regulating TGF-β1 and Smad2/3 signaling pathways; Quercetin, kaempferol, and stigmasterol were the most effective compounds in BSHX.
1. Introduction
Premature ovarian insufficiency (POI) refers to the clinical syndrome that women appear abnormal ovarian function decline before the age of 40. It is characterized by abnormal menstruation, accompanied by an increase in gonadotropin and a decrease in estrogen concentration. It not only seriously affects the reproductive health and quality of life in women under childbearing age, but also increases a series of life-threatening diseases such as cardiovascular disease. Due to the progress of society and the change in life style, the incidence of the POI is elevating and tends to be younger in recent years. At present, there is no effective method to restore ovarian function, and the most commonly used treatment is hormone replacement therapy. However, the United States Preventive Services Task Force reviewed the evidence that hormone therapy did not address or prevent menopausal symptoms effectively in 2017 [Citation1]. With hormone therapy, there is an increased risk of tumor development [Citation2]. And hormones should not be used if there is a history of cancer [Citation3].
Traditional Chinese medicine is the preferred alternative medicine for POI patients in China. The theory of traditional Chinese medicine holds that the basic pathogenesis of POI is kidney deficiency and blood stasis. According to the pathogenesis, tonifying the kidney and promoting blood circulation are the main methods of treatment. Bushen Huoxue (BSHX) treats POI by systematically balancing Yin and Yang of the human body and correcting the pathophysiological condition of kidney deficiency and blood stasis [Citation4]. Therefore, BSHX revolutionized the treatment of POI. Modern pharmacological studies have shown that BSHX has a strong estrogen-like effect and can promote the growth and maturation of follicles. The treatment of POI by BSHX is not only effective in regulating the female hypothalamus-pituitary-ovarian reproductive axis [Citation5], but also the immune system function can be adjusted by increasing mouse Treg cells [Citation6]. In addition, the results of previous clinical trials have shown that BSHX can effectively alleviate the clinical symptoms of patients with POI, and can increase the patient’s pregnancy rate [Citation7]. At the same time, it can improve the patient’s sex hormone levels, menstrual cycle and menstrual volume [Citation8]. BSHX has obvious clinical effect in treating POI and it is recommended as a high efficacy in treatment. However, the mechanism underlying the therapeutic effects of BSHX in POI is unclear. Traditional Chinese medicine has the characteristics of multiple components and targets. Besides, its therapeutic effects are systematic and integral. With the deepening of research, the interaction between the complex chemical system of Chinese medicine prescription and the complex biological system of human diseases is one of the basic question in the research of effective compounds in Chinese medicine [Citation9].
Network pharmacology is a multidisciplinary and systematic analysis of the interaction relationship between drugs and disease networks, which is based on the ‘drug-gene-target-drug’ interaction network [Citation10]. It requires the use of multiple technologies such as omics, high-throughput screening, network visualization, and network analysis [Citation11]. It can be applied to reveal active mechanisms and obtain scientific evidence for herbal formulae based on complex biological systems [Citation12]. Therefore, its holistic and systematic research methods have been widely used to study the relationship between traditional Chinese medicine compounds and diseases from the perspective of system and network. This is consistent with the modern development Chinese medicine.
Therefore, the present study was designed to determine the potential mechanisms of ingredients in BSHX in treating POI by utilizing the method of network pharmacology and molecular docking. Furthermore, experimental evidence shows that BSHX improved POI by modulating the TGF-β signaling pathway. Our research may provide an evidence to support the clinical use of BSHX for the treatment of POI, and help in BSHX drug screening, improve the formulation of BSHX, and may provide a reliable reference for the exploration the therapeutic mechanism of other traditional Chinese medicine.
2. Materials and methods
2.1. Screening of active ingredients and targets of BSHX
The active ingredients of BSHX and their target proteins were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://tcmspw.com/tcmsp.php). TCMSP is an efficient systems pharmacology platform designed for studying TCMs comprehensively, which represents ideal information convergence of pharmacochemistry (Absorption, Distribution, Metabolism, and Excretion), ADME properties, drug-likeness, drug targets, associated diseases and interaction networks [Citation13]. According to the characteristics of pharmacokinetics ADME, the two most important indexes, oral availability (OB) ≥30% and drug-like (DL) ≥0.18, were selected as screening conditions to obtain the active components. According to the published article, the known target of its active compound was added which was not predicted. After the screening, the protein targets acted by the compounds were standardized in the Uniport Protein Database (https://www.uniprot.org/) for the purpose of standardized protein target information. Finally, human target proteins of BSHX were obtained after integration.
2.2. The acquisition of gene targets for POI
All POI-related target genes were collected from DrugBank database (https://www.drugbank.ca), Online Mendelian Inheritance in Man (OMIM) database (https://omim.org/), GeneCards database (https://www.GeneCards.org/), and DisGenet database (https://www.disgenet.org).We searched the four databases with the keyword ‘Premature ovarian insufficiency’ and set the species to ‘Homo sapiens’.
2.3. Drug-compounds-genes-disease network construction
The overlapping genes were obtained from the target genes extracted from the active ingredients of BSHX and the target genes of POI. And then we integrate the ingredients, the drugs, the genetics, the disease. Finally, we used the Cytoscape software (https://cytoscape.org/) for visual analysis of these data to construct a three-dimensional model.
2.4. PPI networks construction
The common target genes of BSHX in the treatment of POI were imported into the String (https://string-db.org) database, which is the database of known and predicted protein–protein interactions, derived from genomic context predictions, high-throughput lab experiments, (conserved) co-expression, automated textmining and previous knowledge in databases. Search in the ‘Homo sapiens’ mode, and the analysis mode is ‘multiple protein’ to get the shared protein-protein interaction network of the target gene. Import the ‘.tsv’ format file containing the role node relationship information into Cytoscape builds software diagrams for more advanced protein interaction networks. Furthermore, the Network Analyzer plug-in is utilized to analyze and calculate topological parameters, such as the topological parameters associated with target degree and betweenness centrality (BC) points.
2.5. GO and KEGG pathway enrichment analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the POI target of BSHX were calculated and evaluated by FunRich software (http://www.funrich.org/). FunRich is a functional software used mainly for enrichment analysis of genes and proteins. Difference was considered to be statistically significant at p < 0.05. Gene Ontology (GO) is an international standard system used to classify gene functions. It divides gene functions into three aspects: molecular function (MF), cell composition (CC) and biological process (BP).
2.6. Molecular docking technology
Auto Dock Tools is used for molecular docking of the main active components of BSHX and the core target protein, which is a web server for network pharmacology-based prediction and analysis [Citation14]. The Auto Dock software is composed of the Auto Grid program responsible for calculating the relevant energy in the grid and the Auto Dock program responsible for conformation search and evaluation. It uses the Lamarckian Genetic Algorithm (LGA) to find the best binding position between the ligand and the receptor. First, we downloaded 3D structure of the protein in the Protein Database Bank (https://www.wwpdb.org/) database, and the 2D structure for the molecule ligands was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The PubChem Database is committed to serving a public repository for information on chemical substances and their biological activities [Citation15]. Then, the search results were imported into the Auto Dock software. The target protein is used as a receptor and the water molecule is added with nonpolar hydrogen, the active ingredient is used as a ligand, and the Grid box coordinates and size are set according to the target protein. Finally, the binding conformation with the lowest free binding energy is selected and exported as a ‘.pdbqt’ format file, and the Convert the file to ‘.pdb’ format and import it into PyMol software for visualization.
2.7. Experimental validation
2.7.1. Animals
Eighty 9-week-old healthy female ICR mice (weighing about 20 ± 2 g) were purchased from the Experimental Animal Center of Nantong University (license number: SCXK Su 2019–0001). Mice were raised in the SPF animal laboratory of Jiangsu Hospital of Integrated Traditional Chinese and Western Medicine and they were adapted for 1 week after entering the room. All experiments were approved by the Experimental Animal Ethical Committee of Jiangsu Province Hospital on Integration of Chinese and Western Medicine.
2.7.2 Preparation of BSHX lyophilized powder.
The Chinese herbal medicine of BSHX (Guizhou Tongde Pharmaceutical Co. LTD) () is mixed according to the ratio: SHU DI HUANG (Henan China 21050403), TU SI ZI (Neimengu China 2105008), CHUAN XIONG (Sichuan China 20210701–01), ZHI MU (Jilin China 210222), BAI SHAO (Anhui China 2012043), CHI SHAO (Sichuan China 20030521), DANG GUI (Gansu China 200903), CHAI HU (Shanxi China 2106002), HUANG BAI (Guizhou China 20210601–01), YIN YANG HUO (Zhejiang China 191008). The ratio is 5: 5: 5: 5: 5: 5: 3: 5: 5. The purity of BSHX herbal composition materials are presented in . Use 10 times the volume (v/w) of distilled water to reflux extract 344 g dry BSHX powder for 2 hrs. Following filtration, the residue was further extracted with 10 times the volume (v/w) of distilled water for 2 hrs. The filtered supernatants were combined and use a rotary evaporator (Greatwall) to evaporate under reduced pressure at 55°C for concentration. The concentrated decoction liquid is mixed and divided into sterile glass bottles to ensure the uniformity of each tube of liquid medicine and gradient cooling Freeze. First freeze in the refrigerator at −20°C for 24 hrs, and freeze in the refrigerator at −80°C for 24 hrs, then place in a vacuum freeze dryer and freeze-dry to obtain the lyophilized powder of BSHX, 344 g crude drug can obtain 114.5 g. BSHX Decoction Freeze-dried powder (33.3% yield) [Citation16,Citation17].
Table 1. Chinese name of component, source, pharmacological characteristics, and the purity
2.7.3. Induction of POI in mice with Tripterygium wilfordii
80 male mice were randomly divided into four groups: Model Group, Control Group, BSHX Group (25.8 g/kg/d) and Progynova Group (0.15 g/kg/d). Model group, BSHX Group and Progynova Group mice were given TG suspension 80 mg/kg by intragastric administration, once a day, for 3 weeks, while Control Group mice were given normal saline (Wuhan China) with the same volume by intragastric administration, once a day, for 3 weeks. After successful modeling, BSHX Group and Progynova Group were administered once daily for 4 consecutive weeks by intragastric administration, and the Model Group and Control Group were administered saline by intragastric administration. The dosage of mice was calculated according to the surface area conversion coefficient of mice and human body. After the last gavage, the body was weighed and executed after 12 hours fasting. Serum and ovaries tissues were collected.
2.7.4. ELISA assay for hormone in each group
After the treatment, blood was taken from the mice after which serum was obtained by centrifuging at 3500 rpm for 10 min at 4°C. Anti-Mullerian hormone (AMH), estradiol (E2), and FSH levels in serum were measured by evaluated by ELISA kit (Shanghai Yifang Biotechnology Co., Ltd, China) [Citation18]. According to the manufacturer’s instructions, the levels of serum levels were measured. Finally, we measured the optical density (OD) at a 450 nm wavelength with the Microplate reader (Thermo Fisher Scientific USA). The concentration of these hormones was calculated based on the standard curve.
2.7.5. Hematoxylin and eosin staining of ovary in each group
The protocol of Hematoxylin and eosin staining was similar to that used in a previous study [Citation19]. The ovarian tissue of mice was dissected, and fixed in 4% formalin. Subsequently, the ovarian tissue was dehydrated, and embedded in paraffin. The embedded ovaries were sectioned consecutively for 5 μm thickness by placing the sections in a parallel direction to the stab for staining 10 min using hematoxylin, and washed with running water. The 1% hydrochloric acid-alcohol was used to decolorize the excess hematoxylin dye for 30 s. The samples were immersed in water for 15 min, stained with eosin solution for 2 min, rinsed the excess dye with running water, dehydrated, and sealed with gum. Visualization and photography were performed under a microscope. The films were read under a light microscope. The nucleus presented blue and the cytoplasm red.
2.7.6. Immunohistochemical detection of TGF-β1 and Smad2/3 protein expression in ovarian tissues of mice in each group
The protocol of Immunohistochemical was described by a previous study [Citation19]. The ovaries was removed in 4% paraformaldehyde solution for fixation, followed by gradient alcohol dehydration, paraffin embedding, and sectioning. The sections were incubated with 3% H2O2 for 10 min and 0.1% trypsin for 20 min. Primary antibodies were subsequently incubated at 4°C overnight, using TGF-β1(Proteintech, USA, 21898-1-AP), Smad2/3(Cell Signaling Technology, USA,8685 T). The secondary antibodies used were HRP-conjugated and the section incubation was performed in a 37°C incubator for 30 min. Finally, the bound antibodies were stained with diaminobenzidine, and the sections were counterstained with hematoxylin. Images were photo-graphed by an inverted microscope. The semi-quantitative scores were evaluated based on the percentage of positive rates: (1) 0 (<5%); 1 (5%–25%); 2 (26%–50%); 3 (51%–75%), 4 (>75%); and staining intensity: (2) 0 (negative); 1 (weakly positive); 2 (moderately positive); 3 (strongly positive). The two scores were multiplied and then used as the final IHC score [Citation20].
2.7.7. Evaluation of transcript levels of TGF-β1 and Smad2/3 using qRT-PCR
The ovaries were removed in a sterile state, placed in a sterile EP tube, and quickly placed in a − 80℃ refrigerator for use. The total RNA was extracted using TRIzol method. Reverse transcription was performed using reverse transcriptase and 2 μg RNA to synthesize the cDNA. PCR amplification was carried out using gene-specific PCR primers provided by Jiangsu KeyGEN Biotechnology Company (Jiangsu, China). The primer sequences and the length of products are summarized in . The amplification was performed in a 20 μL reaction volume containing 1 μL cDNA, 0.5 μL 10 μmol/L forward and reverse primers, 10 μL qPCR Master Mix and 8 μL sterile DEPC H2O. Each reaction was carried out for 40 cycles of denaturation at 95°C for 10s and annealing at 60°C for 30 s. The mRNA expression levels were calculated by the 2−ΔΔCt (Quantitation-Comparative CT) method, which is calculated based on the ct value of β-Actin genes and the target genes [Citation21]. β-Actin was used as a control for normalization.
Table 2. Specific primer sequence, length of products, accession number, and annealing temperatures for genes
2.8 Statistical analysis
SPSS 19.0 and GraphPad Prism 8.0 statistical software were used for data analysis. All data are expressed as the mean ± SEM, and the levels of significance was determined using a repeated-measures analysis of variance (ANOVA) test followed by Dunnett’s multiple comparison test. P-values were analyzed using Student’s t-test, and the significant difference was regarded as P < 0.05.
3. Results
The purpose of this paper is to explore the pharmacological mechanisms of BSHX acting on POI based on a pharmacology and experimental validation. This strategy contained three-parts. First, the effective active ingredients and potential targets of BSHX were searched. Meanwhile, Drugbank, OMIM, Genecards, and Disgenet databases were used to obtain relevant targets of POI. Second, the target genes of BSHX treatment for POI were evaluated, followed by enrichment analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Third, Auto Dock tools 1.5.6 were used for molecular docking. Last, murine model of POI was applied to further investigate the mechanism of BSHX against on POI. Experimental validation suggests that BSHX significantly Increased the expression of TGF-β1 and Smad2/3, regulating the release of serum sex hormones (E2, FSH, and AMH). In this work, this strategy may provide an evidence to support the clinical use of BSHX for the treatment of POI, and help in BSHX drug screening, and may provide a useful reference for the exploration the therapeutic mechanism of other traditional Chinese medicine.
3.1. Candidate compounds and potential targets
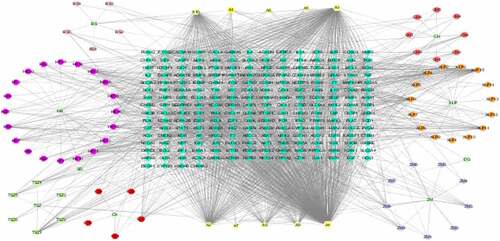
A total of 127 herbal active compounds of BSHX () were collected and screened from TCMSP database, which conformed to the characteristics of various ingredients for TCM. Ten compounds were common to more than two traditional Chinese medicines, including quercetin (MOL000098), kaempferol (MOL000422), stigmasterol (MOL000449) and so on. A total of 2168 compounds-related targets genes were obtained, and 215 (Supplementary Table 1) target genes were determined after the integration of UniProt database. shows the network of interaction between active ingredients and action targets, in which the circle represents the prescriptions of BSHX, the hexagon represents the active ingredients of each traditional Chinese medicine, and the diamond represents the potential action targets of active ingredients. A total of 3694 POI disease-related targets were retrieved from GeneCards database, and 919 relevant targets were obtained with Relevance score greater than 9.06. A total of 267 disease-related targets of POI were retrieved from OMIM database. 299 disease-related targets of POI were retrieved by DisgeNet. Drugbank retrieved 4 POI disease-related targets, and integrated and reprocessed the collected targets to obtain a total of 1366 POI disease-related target genes.
Table 3. Molecular ID, molecule name, oral availability, AND drug-Like Properties Of 127 ingredients Of BSHX
3.2. Construction of BSHX-target-POI and protein-protein interaction networks analysis
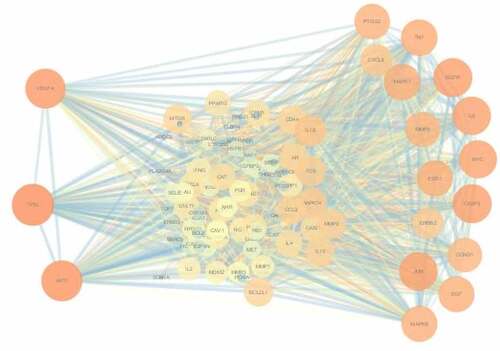
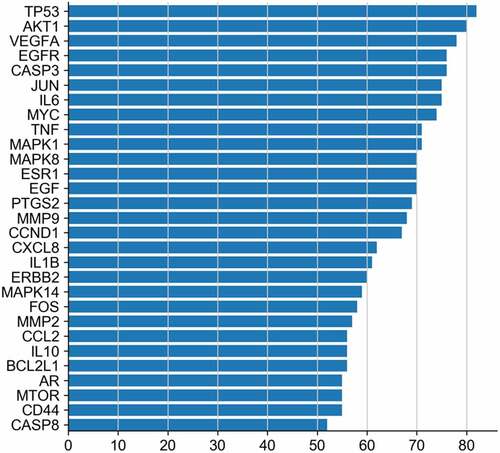
After analyzing the target genes related to BSHX and POI, 99 common matching targets were obtained. (Supplementary Table 2). 99 intersection targets were input into the String database, the species was set as ‘Homo sapiens’, medium confidence > 0.900, and free and unrelated partial nodes were excluded to obtain the interaction network diagram of ‘drug-disease’ proteins. The obtained protein interaction information was imported into Cytoscape 3.7.2 software in the form of ‘.tsv’ to produce Protein-Protein Interaction network map. As shown in , the Protein-Protein Interaction network contained candidate targets of BSHX remedying POI and their inter-acting proteins. Among them, nodes of different colors have different functions. The deeper the red color of the node is always companied with the higher the corresponding degree value, which means the larger the size of the node. The thicker the connected edges are, the greater the interaction between two nodes. The network comprehensively summarized the internal net of BSHX in healing POI. Finally, the net-work Analyzer and CytoNCA in Cytpscape software were used to screen the core targets by analyzing degree and betweenness centrality (BC), etc. According to the concept that the greater the degree, the greater the role in the network, the maximum first three nodes are TP53, AKT1 and VEGFA. The first 30 nodes of degree value are shown in . It is believed that these core targets play a major role in the treatment of POI by BSHX. The abscissa represents degree values and the ordinate represents gene names.
3.3. GO, KEGG pathway enrichment analysis
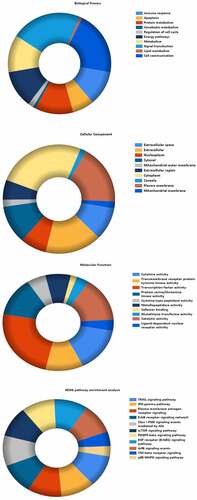
To further investigate the multiple mechanisms of BSHX on POI from a systematic level, GO-enrichment analysis for the Cellular Component (CC), Molecular Function (MF) and Biological Process (BP) of the 99 selected targets were performed using Funrich software, which respectively reflect the action sites, biological processes, as well as the functions at the molecular level of BSHX for POI treatment. The results of each type were selected according to the p value and the visual analysis was performed. The results showed that the biological processes of the target of BSHX and POI mainly including, Immune response, Apoptosis, Protein metabolism, etc. The cell components mainly concentrated in the Extracellular space, Extracellular, Nucleoplase, etc. Molecular functions mainly focus on Cytokine activity, Transmembrane receptor protein tyrosine kinase activity, transcription factor activity. Similarly, biological pathway enrichment analysis of the target genes was also performed by FunRich. The enriched pathways contained signaling events mediated by TRAIL signaling pathway, IFN-gama pathway, Plasma membrane estrogen receptor signaling, ErbB receptor signaling pathway, PI3k-Akt signaling pathway, mTOR signaling pathway, PDGFR-beta signaling pathway, Arf6 signaling pathway, TGF-β receptor signaling, p38-MAPK signaling pathway ().
3.4. Molecular docking results and analysis of core active ingredients and targets
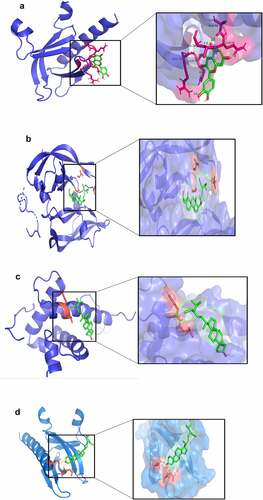
In this study, the top 3 core targets of BSHX for POI treatment TP53, Akt1 and VEGFA, were selected for small-molecule-protein molecular docking with the active ingredients of quercetin, kaempferol and stigmasterol, whose number of effect targets is currently three. Binding energy refers to the interaction between ligand and receptor [Citation22]. When the binding energy is less than 0, it is considered that ligand and receptor can bind freely, and the lower the binding energy, the stronger the affinity between the two. The docking results are shown in , in which stigmasterol and Akt1 encoded protein have the strongest affinity. The schematic diagrams from present the molecular docking of TP53 protein-stigmasterol, VEGFa protein-stigmasterol interactions, Akt1 protein-quercetin, Akt1 protein-stigmasterol, respectively.
Table 4. Binding energy of active ingredients and targets
3.5. Investigation of the mechanism of action of BSHX against POI by experimental validation
3.5.1. Effect of BSHX on serum sex hormones of POI
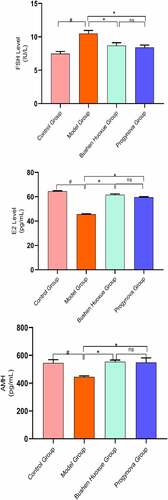
The results showed the effects of BSHX on Tripterygium wilfordii-induced POI. The E2 and AMH in the model group was significantly decreased as compared with that in the Control Group (P < 0.05), and the FSH in the model group was significantly increased as compared with that in the Control Group (P < 0.05). Meanwhile, the serum sex hormones of mice in the BSHX groups had significantly changes ().
3.5.2. Effect of BSHX on histopathological changes
3.5.2.1. Hematoxylin and Eosin staining results.
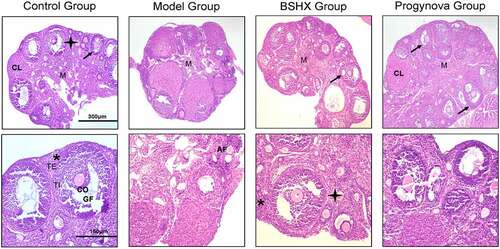
The histopathological changes in each group are shown in . The Control Group HE staining microscopic examination of the ovaries results showed that follicles in different stages of development were observed. These included several primordial follicles with central oocytes and nuclei, primary follicles with larger follicles, and secondary follicles with larger follicles surrounded by a layer of cubical cells overlying a zona pellucida, multiple layers of granule cells and cavity. All follicles were surrounded by flat stromal cells. The cumulus oophorus comprised one big oocyte with a prominent nucleus, surrounded by a zona pellucida, corona radiata and granule cells. The corpus luteum exhibited large faintly stained acidophilic cells. The number of atretic follicles is few, the general shape of growing follicles is normal, and the mature follicles may be elliptic due to the surge of follicular fluid. The granulosa cells are round with regular and dense arrangement. In the Model Group, the primary and secondary follicles count was significant decreased. There was a remarkable increase in the number of atretic follicles compared to other groups. The ovarian interstitium was also destructed with massive edema and fibrosis. The granulosa cells shrink and have a representative pyknotic nuclei, while the cells are loosely arranged and disorganized. These changes demonstrated the induction of POI in mouse received Tripterygium wilfordii. The total follicle count in BSHX Group and Progynova Group significantly increased compared with Model Group, and the number of follicles in both groups were similar. These confirms that BSHX prevented the Tripterygium wilfordii-induced injury in the ovarian.
Figure 7. H&E × 40 and ×100 photomicrograph from each group ovary. Mature Graafian Follicle (GF) containing Cumulus Oophorus (CO) and surrounded with Theca Interna (TI) and Theca Externa (TE) layers. Primordial follicles (*), primary follicles (), and secondary follicle (↑) with Corpora Lutea (CL) had large faintly stained acidophilic cells, Atretic Follicles (AF), and Medulla (M) are identified in the figure
3.5.2.2. Results of TGF-β1 and Smad2/3 immunostaining.
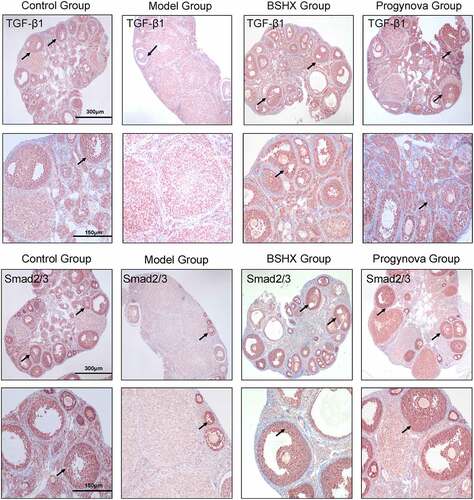
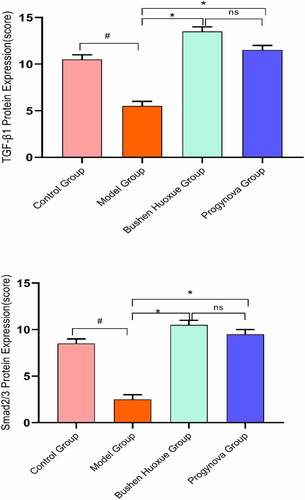
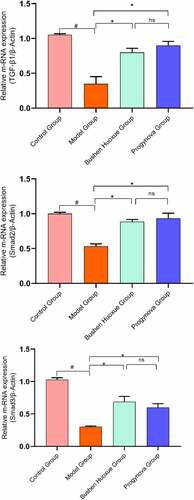
Immunohistochemistry staining for TGF-β1 and Smad2/3 in ovarian sections () from the Model Group had significant decreases in the score compared to the Control group. Moreover, the score of was significantly increased in the BSHX Group and Progynova Group compared to the Model Group. As shown in .
3.5.3. Real-time quantitative PCR of TGF-β1 and Smad2/3
To better characterize the effects of BSHX in ovarian function, the mRNA expression of TGF-β1 and Smad2/3 were performed (). In comparison with the Control Group, the mRNA levels of TGF-β1 and Smad2/3 were markedly decreased in the ovarian tissue of the Model Group (P < 0.05). Meanwhile, the BSHX Group and Progynova Group mRNA expression of TGF-β1 and Smad2/3 had marked elevation. Besides, treatment with BSHX resulted in no significant change as compared with the Progynova Group (P > 0.05).
4. Discussion
In this study, a network pharmacology approach combined with experimental validation were conducted to clarify the underlying mechanism of action of BSHX against POI. There are 127 active ingredients of BSHX and their corresponding 215 potential targets. Among them, quercetin, kaempferol, isorhamnetin, and stigmasterol are the most corresponding chemical ingredients. Through topological analysis and pathway enrichment analysis, it was found that BSHX acts on TP53, Akt1, VEGFA and other core targets, involving biological processes and signaling pathway. POI is treated by TGF-β receptor signaling regulating TRAIL signaling pathway, PI3K/AKT signaling pathway, mTOR signaling pathway, etc. Further, via experimental evidence, we show that BSHX against POI by modulating the TGF-β1 and Smad2/3 signaling pathway. The present research provides evidence to support the clinical use of BSHX for the treatment of POI. The network of active ingredients and targets directly reflects the characteristics of BSHX in the treatment of POI through multi-component and multi-target, Chinese medicine treats diseases from a holistic perspective.
Kaempferol is a selective estrogen receptor and related receptor modulators, which plays some biological roles through estrogen receptor and estrogen-associated receptor [Citation23]. Quercetin has antiproliferative effects by regulating EGFR or estrogen receptor-mediated signal transduction pathways. TP53is involved in DNA repair and regulates the expression of apoptotic genes [Citation24]. It has been proved by experiments that when the cell cycle regulation dysfunction of cultured oocytes in vitro, the expressions of TP53 and caspase-3 are up-regulated, and the mechanism of DNA damage repair improved, leading to cell cycle stagnation or apoptosis [Citation25]. The level of Akt1 plays an important role in the degree of apoptosis of follicular granulosa cells and the meiosis cell cycle of oocytes [Citation25,Citation26]. Phosphorylated Akt1 can phosphorylate a series of downstream target proteins that inhibit proliferation and promote apoptosis, including forkhead box O3a (FOXO3a), Bcl-xL/Bcl-2 associated death promoter (BAD), and mammalian target of rapamycin (mTOR). Vascular endothelial growth factor A (VEGFA) can regulate mammalian follicle development and ovarian vascular distribution [Citation27]. Therefore, inhibiting the granulosa cells apoptosis may prevent atresia of follicles in some certain extent.
Some scholars have retrospectively studied the effects of ovarian tissue transplantation on ovarian function, fertility and reproductive outcome after ovarian tissue transplantation. Existing evidence indicates that ovarian tissue transplantation is a useful method [Citation28]. However, surgery will bring trauma to people, and the probable adverse effects after treatment is indispensable, such as the invasion of follicles and oocytes after ovarian tissue transplantation. Oral BSHX can avoid this side effect.
Some scholars reviewed the trial literature of BSHX in the treatment of POI, and conducted a meta-analysis of the clinical total effective rate, symptom score, menstrual recurrence rate, and serum sex hormone improvement [Citation29]. The results showed that BSHX is a safe and effective drug for the treatment of POI, and is better than hormone replacement therapy. Through research, it is found that the transforming growth factor β (TGF-β) signaling pathway is closely related to the occurrence and development of POI. It is involved in many basic functions of germ cells, such as proliferation and differentiation, growth and development, apoptosis, steroid hormone synthesis, oocyte maturation, ovulation and luteinization [Citation30]. Abnormal TGF-β1 expression leads to follicular dysplasia and anovulation. TGF-β1 participates in the regulation of the production of estrogen in signal transduction by affecting the direct acting substrate smad protein, and has an important influence on maintaining the homeostasis of the ovarian environment. Studies have shown that the number of granular layers in the rat model of POI induced by Tripterygium wilfordii decreases and the number of atretic follicles increases, leading to a series of declines in ovarian function. The follicular dysplasia is causally related to the expression of TGF-β1 in granulosa cells, and its mechanism is closely related to TGF-β1 mediated apoptosis of sinus follicular granulosa cells [Citation31,Citation32]. At present, several studies have found that BSHX can significantly improve the protein expression of TGF-β1 and Smad2/3 in granulosa cells of mice follicle wall and ovarian tissue, and can significantly promote the conduction of TGF-β1/Smads signaling pathway in mice with POI, thus improving the ovarian function of mice. It also promotes the proliferation and differentiation of granulosa cells and reduces follicular atresia [Citation33].
To sum up, this study adopted network pharmacology and molecular docking methods to systematically explore and verify the potential mechanism of BSHX in the treatment of POI from multiple perspectives. Finally, the experimental verification results show that this method can better explain the network characteristics and the principle of synergistic action of traditional chinese medicine compound, indicating that BSHX is a multi-component and multi-pathway regulation in the treatment of POI.
5. Conclusion
In this study, a network pharmacology approach was combined with experimental validation to clarify the underlying mechanism of action of BSHX in the treatment of POI. These findings demonstrate that the main active compounds of BSHX, the core targets, and pathways maybe provide new insights into the development of natural herb-related therapy for the prevention and treatment of POI. Further, via experimental evidence, we show that BSHX treats POI by modulating the TGF-β1 and Smad2/3 signaling pathway. The present research provides evidence to support the clinical use of BSHX for the treatment of POI.
Author contributors
Ying Cao was responsible for conception and design. Ying Cao and Yue Chen has written the initial manuscript. Ying Cao, Yue Chen, Peijuan Wang were responsible for development of the methodology. Ying Cao, Yue Chen, Jialin Lu, Xuan Han Jingyao She did the experimental work. Ying Cao, Yue Chen, Peijuan Wang, Xuan Han analyzed the experimental data. Ying Cao, Yue Chen, Peijuan Wang revised the important intellectual content of the manuscript. Ying Cao, Yue Chen, Peijuan Wang, Jialin Lu, Xuan Han, Jingyao She have been critically discussed. All authors have read and approved the final manuscript.
Supplemental Material
Download Zip (65.3 KB)Acknowledgements
We thank all staff who devoted their time and efforts to the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
All the data was downloaded from Traditional Chinese Medicine Systems Pharmacoloy Database and Analysis Platform (TCMSP, https://tcmspw.com/tcmsp.php), DrugBank (https://www.drugbank.ca), Online Mendelian Inheritance in Man (OMIM) (https://omim.org/), GeneCards database (https://www.GeneCards.org/), and DisGenet database (https://www.disgenet.org). The data related to this research can be obtained from the corresponding author upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women. JAMA. 2017;318:2224.
- Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045.
- Deniz G, Antoine C, Liebens F, et al. Treatment of premature menopause in breast cancer patients. Acta Chir Belg. 2016;3:263–266.
- Cao Y, Wang P, Lu Y, et al. Effectiveness and safety of Bushen Huoxue in treatment of premature ovarian Insu ciency: study protocol for a randomised, double-blinded, placebo- controlled and multicentre clinical trial. 2021.
- Zhen-Bin Z, Shuang-Kui G, Lin K. Effect of traditional Chinese medicine tonifying kidney on hypothalamus-pituitary system. J Hebei Med Univ. 2004;25:185–187.
- Wang P, Lu Y, Chen S, et al. Protective function of Bu Shen Huo Xue formula on the immunity of B6AF1 mice with experimental autoimmune premature ovarian failure. Exp Ther Med. 2018;15:3302–3310.
- Xiao-Wei W, Hui-Fang Z. Recent research on the intervention of ovarian reserve function by BuShen HuoXue. J Zhejiang Trad Chin Med Univ. 2014;38:1245–1248.
- Bi-Rong Y, Hua Z. Clinical observation on treating the decline of ovarian reserve function with Bushen Huoxue prescription. Hebei J Trad Chin Med. 2019;41:698–701.
- Bo-Li Z. Theory and method of developing modern Chinese medicine by component compatibility. Cont Med Educ. 2006;20:89-91.
- Hai-Bin C, Hong-Guang Z, Wen-Ting L, et al. Network pharmacology: a new perspective of mechanism research of traditional Chinese medicine formula. Chin J Trad Chin Med. 2019;34:2873–2876.
- Shao L. Network target: an entry point in the research of network pharmacology of TCM prescriptions. Chin J Trad Chin Med. 2011;36:2017–2020.
- Xin-Rui X, Di-Ya L, Yi-Feng C, et al. Research progress of network pharmacology in mechanism of action of traditional Chinese medicine. J Pharma Pract. 2018;36:97–102.
- Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:1–6.
- Hsin K, Matsuoka Y, Asai Y, et al. systemsDock: a web server for network pharmacology-based prediction and analysis. Nucleic Acids Res. 2016;44:W507–W513.
- Kim S, Thiessen PA, Bolton EE, et al. PubChem substance and compound databases. Nucleic Acids Res. 2016;44:D1202–D1213.
- Honghao X, Zhenhui L, Chenghua L, et al. Effection of Huaxian decoction lyophilized powder solution on proliferation and differentiation of human embryonic lung fibroblasts induced by TGF-β1. Chin Acad J. 2020;26:11–15.
- Yuefeng L, Xinlei S, Jiangtao N, et al. Study on sleep improving mechanism of Sini San Freeze-dry powder in rats. Chin J Clin Pharmacol. 2018;34:2851–2853+2888.
- Li D, Jia Y, Hou Y, et al. Qilin Pill exerts therapeutic effect on resection-induced premature ovarian insufficiency rats by inhibiting the MAPK and PI3K-AKT signaling pathways. Drug Des Devel Ther. 2021;15:3331–3345.
- He M, Pang J, Sun H, et al. P14ARF inhibits regional inflammation and vascularization in intervertebral disc degeneration by upregulating TIMP3. Am J Physiol Cell Physiol. 2020;318:C751–C761.
- Fromowilz FB, Viola MV, Chao S, et al. ras p21 expression in the progression of. Hum Pathol. 1987;12:1268–1275.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408.
- Sen-nan XU, Li Z, Yuan-yuan Z, et al. Material basis and mechanism of Erzhi Pill for preventing osteoporosis based on network pharmacology. Chin Pharm J. 2018;53:1913–1920.
- Wang J, Fang F, Huang Z, et al. Kaempferol is an estrogen-related receptor α and γ inverse agonist. FEBS Lett. 2009;583:643–647.
- Zheng P, Schramm RD, Latham KE. Developmental regulation and in vitro culture effects on expression of DNA repair and cell cycle checkpoint control genes in rhesus monkey oocytes and embryos1. Biol Reprod. 2005;72:1359–1369.
- Arias-Álvarez M, García-García RM, López-Tello J, et al. In vivo and in vitro maturation of rabbit oocytes differently affects the gene expression profile, mitochondrial distribution, apoptosis and early embryo development. Reprod Fertil Develop. 2017;29:1667.
- Kui YX, Rui S, Wei JC, et al. The effect of akt phosphorylation on apoptosis in granulosa cells of rat antral follicle. Chin J Healthy Birth Gene. 2010;18:14–15+2.
- Shimizu T. Induction of follicular development by direct single injection of vascular endothelial growth factor gene fragments into the ovary of miniature gilts. Biol Reprod. 2003;69:1388–1393.
- Sheshpari S, Shahnazi M, Mobarak H, et al. Ovarian function and reproductive outcome after ovarian tissue transplantation: a systematic review. J Transl Med. 2019;17. DOI:10.1186/s12967-019-02149-2
- Li H, Shen Q, Chen W, et al. Efficacy of traditional chinese medicine tonifying kidney (Bushen) and activating blood (Huoxue) prescription for premature ovarian insufficiency: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:1789304–1789313.
- Li X, Tripurani SK, James R, et al. Minimal fertility defects in mice deficient in oocyte-expressed Smad41. Biol Reprod. 2012 86:1-6.
- Aidong L, Huijun Y, Fan W, et al. The expression of IGF- Ι,TGF β and Fas-L in granulosa cells of ovarian follicle with relation to the onset of A -tresia in rats. J West China Univ Med Sci. 1999;30:158–161.
- Shuai L. Changes of TGF 1 and TGF R 1 in ovaries of rats with ovulation disorder induced by Tripterygium wilfordii. Proceedings of the 10th National Conference of Traditional Chinese Medicine Gynecology; 2010. pHarbin,China. 279–281.
- Ping LH, Ting ZL, Juan HL, et al. Effects of Bushen Huoxue Recipe on the expressions of TGF- β1, TGF- βRII and Smad2/3 in granulosa cells in mice with premature ovarian failure. Chin Trad Pat Med. 2017;39:1782–1788.