 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Drought is one of the most important abiotic stressors that affect crop yield. Therefore, the aim of the present study was to investigate correlations between germination-stage drought tolerance and the microscopic testa (i.e., seed coat) characteristics (color and papilla morphology) and imbibition abilities of 35 rapeseed (Brassica napus L.) accessions. After 2 h imbibition, seed water uptake (fresh weight increase) was significantly positively correlated with testa hue (HHSB), brightness (BHSB,), blue (BRGB), and lightness (L*), with correlation coefficients of 0.38, 0.34, 0.53, and 0.36, respectively, and significantly negatively correlated with saturation (SHSB), greenness-redness (a*), blueness-yellowness (b*), magenta (M), and yellow components (Y), with correlation coefficients of −0.53, −0.40, −0.53, −0.39, and −0.55, respectively. Furthermore, 5-h seed water uptake was significantly positively correlated with number of papillae (No.P), mean papillae area (APA), the papillae area ratio (PAR), gray value of red channel of papillae, with correlation coefficients of 33, 0.36, 0.43, and 0.43, respectively. Under drought conditions, genotypes with more rapid water absorption exhibited higher germination rates and stronger drought tolerance, and the germination rate and drought tolerance of black-seeded accessions were highest, followed by red-seeded accessions and then yellow-seeded accessions, which exhibited the lowest germination rate and drought tolerance. Germination rate was significantly negatively correlated with BRGB, HHSB, L*, Dg, and Db and significantly positively correlated with SHSB and Y, regardless of drought conditions. At the germination stage, DbTP was negatively correlated with drought tolerance.
Introduction
The genus Brassica L. (Cruciferae) contains several economically important species, including Brassica campestris L. (low yield and poor resistance to stress), Brassica juncea L. (low yield and strong resistance), and Brassica napus L. (high yield and strong resistance). Rapeseed (i.e., B. napus) is one of the world’s most important oilseed crops [Citation1,Citation2]. China is one of the world’s largest rapeseed producers, with an annual cultivation area of ~7,000,000 ha and production of ~13,000,000 tons (in 2005), which accounts for about a third of total global production [Citation3,Citation4–9]. However, the total cultivation area allocated to rapeseed has been decreasing in China since 2016 and drought is one of the main reasons. In China, more than 85% of rapeseed production occurs in the Yangtze River Basin [Citation10].
Drought is one of many environmental factors that seriously affect plant growth and development. Drought can have serious consequences in both developed and developing countries, and their frequency, severity, and duration are increasing [Citation11]. The growing biophysical vulnerability contexts and intensity in Asian Least developed countries (LDCs) negatively impact food security, human health, biodiversity, water resources, hydroelectric power generation, streams, perennial springs, and livelihoods. In the monsoon climatic zone, South and Southeast Asian LDCs, such as Bhutan, Bangladesh, Nepal, Cambodia, and Lao PDR, have also experienced increasing droughts, which are caused by changes in the timing and distribution patterns of precipitation. South Asia has been recognized as among the world’s most drought-prone regions. Over the last five decades, drought has been reported at least once every three years in India, Pakistan, Afghanistan, Sri Lanka, Bangladesh, and Nepal [Citation12,Citation13].
Temperature, inadequate humidity, biological pollution, solar radiation, wind, chemical pollution, wildfires, and natural disturbances also affect the sensitivity of plants to stressors [Citation12,Citation14].
The negative effects of drought events are typically addressed by improving industrial structure and ecological environment, improving farming systems, changing crop composition, breeding drought-tolerant crop varieties, fully utilizing effective precipitation, improving irrigation facilities, and applying water-saving technologies [Citation11,Citation14]. In this area, seasonal precipitation is unevenly distributed, and 60–80% of the annual rainfall occurs during summer. Therefore, rapeseed, which is typically sown during autumn, often encounters drought stress, which ultimately results in resulting in reduced seedling emergence and vigor, and improving both the germination rate of rapeseed during drought conditions and germination-stage drought tolerance are important strategies for countering drought event during autumn. Seed germination is the most sensitive developmental stage in regard to drought stress [Citation15,Citation16], and drought is one of the main environmental factors that affect seed germination and plant growth [Citation16,Citation17].
Nitric oxide, cyanide, and karrikin1 have been reported to induce seed germination and stimulate the production of ethylene, 1-aminocyclopropane-1-carboxylic acid (ACC), ACC oxidase (ACO), and ACC synthase (ACS) [Citation1,Citation2,Citation16]. Melatonin has also been reported to increase seedling growth, catalase, peroxidase, ascorbate peroxidase, proline, chlorophyll, and anthocyanin content, as well as photosynthesis rate, under aluminum and cadmium stress [Citation16]. Under low temperature and drought conditions, appropriate concentration seed priming such as salicylic acid (SA), gibberellic acid (GA), sodium nitroprusside, calcium chloride(CaCl2) and abscisic acid (ABA) significantly improved germination potential, germination rate, germination index, fresh stem weight, dry stem weight, stem length, and seed vigor index [Citation14].
As a result of frequent seasonal (autumn) drought and inadequate irrigation facilities, rapeseed production in the Yangtze River basin often suffers from low germination rates and poor seedling quality and, consequently, suffers from relatively low seed yield and reductions in seeded area [Citation12].
Seed germination is a fundamental process in plant growth and development. The germination process is initiated by the uptake of water (i.e., imbibition), followed by increases in respiratory and enzymatic activities, consequent reactivation of metabolism, and, ultimately, emergence of the radicle [Citation18]. Imbibition is a physical process that mainly involves the rapid uptake of water [Citation19,Citation20] and plays a critical role in seed germination since it is essential for the activation of enzymes, decomposition of starch into sugar, and transport of nutrients to the developing embryo [Citation21,Citation22].
Seeds are particularly stress-sensitive during seed imbibition [Citation23]. Because water restriction affects water uptake and seed germination ability, adequate water availability is a critical factor during the germination stage [Citation17]. These traits affect the effective absorption of water, directly or indirectly, on the germination and drought tolerance of rapeseed seeds. Water uptake rate is important for seed germination and is strongly affected by water permeability, which is influenced by seed shape, structure, composition, and initial water content [Citation24]. Genetic factors play a significant role in imbibition and germination [Citation24,Citation25], as do seed coat features.
The testa (i.e., seed coat) is the primary barrier to water penetration during imbibition, and both seed imbibition and germination are significantly affected by testa thickness, density, cell wall thickening, and chemical composition (e.g., presence of tannins, pigments, crystal substances) [Citation10,Citation26,Citation27]. For example, dark-colored seeds have been reported to exhibit slower water absorption [Citation10,Citation26–28], and yellow-seeded rapeseed accessions exhibit more rapid water uptake than red- or black-seeded accessions [Citation10], whereas black-seeded accessions exhibit higher germination rates, emergence percentages, and seedling establishment than dark and light brown-seeded accessions [Citation10]. Testa color determines seed composition, which, in turn, affects seed germination and seedling growth [Citation29].
Studies that have investigated relationships between testa color, water absorption rate, and seed germination have mainly focused on testa color classification [Citation10], and relationships between rapeseed testa microstructure and color, seed imbibition, and germination-stage drought tolerance have yet to be reported. The aims of the present study were (a) to analyze the imbibition ability and germination-stage drought tolerance of rapeseed accessions and (b) to assess the associations of these traits with testa microstructure and color.
Methods and materials
Materials
Seeds of 35 rapeseed (Brassica napus L.) germplasm accessions () were selected and collected from winter rapeseed cultivation areas in China, including Huang Huai and areas surrounding the upper, middle, and lower Yangtze River. After air-drying, the seeds were stored with silica gel.
Table 1. Sources and characteristics of rapeseed (Brassica napus L.) germplasm accessions used in the present study
Microstructure and color analysis
The testa was manually cut, and photomicrographs were collected using an DP70 digital camera (OLYMPUS, JAPAN) (aperture set to a maximum of 6 and magnification of 400 times; ).
Figure 1. Microscopic color and morphology of testa from 35 rapeseed (Brassica napus L.) accessions from China
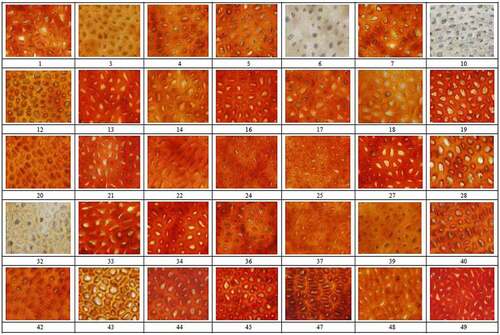
The microscopic color of the testa images was assessed using four color spaces: RGB (red, green, and blue) in which RRGB, GRGB, and BRGB were in the range 0–255, RGB gray values (gray values of the red, green, and blue channels [Dr, Dg, and Db, respectively]). Hue saturation and brightness (HSB), in which HHSB was defined as the spectral values of different wavelengths, ranging from 0 to 360, where 0 and 360 were red, and every 60 were successively yellow, green, cyan, blue, and magenta, SHSB was defined as color depth, ranging from 0% to 100%, BHSB was defined as color brightness, ranging from 0% to 100%, CIE L*a*b*(lightness greenness-redness and blueness-yellowness), in which L* values ranged from 0 (black) to 100% (white), a represents the range from red to green, b represents the range from blue to yellow, the value range of a and b were −120 to +120), and CMYK (C, cyan; M, magenta; Y, yellow; K, black). The HSB, L*a*b*, and CMYK values were measured using image analysis software (Photoshop CS v.6.0, Adobe USA) and the software (Image Pro-Plus v.6.0 Media Cybernetics, USA), was used to measure RGB gray values . The color values of each accession were calculated as the mean values of five individual images of five single seeds, which were repeated three times using appropriate image analysis software (Image Pro- Plus v.4.5.
For microstructure analysis, papillae area was calculated using the density tools in Quantity one (×400 calculates microscopic papillae area). The papillae area ratio (PAR) was defined as the proportional area of each micrograph represented by papillae, whereas average papillae area (APA) was calculated as the average of 50 papillae, and the number of papillae (No.P) in each micrograph was counted manually. Mean values for each of the seed characteristics were calculated using five individual seeds, with three replicates each. IOD, integrated optical density; DrP, red channel gray value of papilla; DgP green channel gray value of papilla; DbP, blue channel gray value of papilla; Dg, green channel gray value of; Db, blue channel gray value of testa. Average papillae area.
Water uptake analysis
For each accession, 500 seeds (with three replicates per accession) were weighed and then placed in 100-ml plastic centrifuge tubes with 50 ml room temperature (25°C) distilled water. The seeds were removed from the water after 1, 2, 3, 4, 5, and 24 h, blotted dry, and weighed. Water uptake was then calculated as mean percent weight increase (n = 3) [Citation30].
Conductivity analysis
For each accession, 1 g seeds were placed in plastic centrifuge tubes with 100 ml room temperature (25°C) deionized water. The seeds were removed after 1, 2, 3, 4, 5, and 24 h, and then electrical conductivity of seed imbibition was measured using a conductivity meter (DDS-12D, Hongyi Shanghai, China) as described previously [Citation31].
Evaluation of drought tolerance
For each accession, 100 randomly selected seeds were weighed, soaked in 1% sodium hypochlorite solution for 5 min, and rinsed three times using distilled water. The sterilized seeds were then dried at room temperature (25°C) until reaching their initial weight. Drought conditions were simulated using 10% PEG-6000, as described previously [Citation32], and controls were also included. Seed germination was evaluated by incubating seeds between wet filter paper in Petri dishes (25 × 25 cm) for 7 d and then employing the International Seed Testing Association (ISTA) vitality test method [Citation33]. Germination rate, seedling height, and fresh seedling weight were recorded, and relative germination rate, relative seedling height, and relative seedling fresh weight were calculated as percentages of the control seeds and seedlings. Finally, drought tolerance (drought tolerance index, DTI) was calculated as the product of relative germination rate and relative seedling height [Citation34].
Statistical analysis
The experiment was conducted using a completely randomized design, and all experiments were performed using at least three replicates per accession. Microsoft Office Excel (Microsoft Co., 2007) was used for primary data analysis. Percentage data were subjected to arcsine transformation, and the significance of differences between means was calculated using Duncan’s new multiple range test (P < 0.05). Analysis of variance (ANOVA) and correlation analyses were conducted using SPSS10. Principle component analysis (PCA) was performed using the FactoMineR package (version 2.1). Principal component analysis can extract less and uncorrelated comprehensive indices from measured variables and circumvent problems caused by collinearity among variables. The Pearson correlation analysis method was used to screen indices for significant correlations with germination rate, seedling height, fresh seedling weight, relative germination rate, relative seedling height, and DTI. Principal component analysis was applied to 12 of the 26 testa color and papilla trait indices.
Results
Seed characteristics
The 35 rapeseed accessions were categorized as black (21 accessions), red (11 accessions), or yellow (3 accessions) by eye (), and based on the number and size of papillae, were also categorized as MBP (more and bigger papilla, five accessions), LBP (less and bigger papilla, 10 accessions), MSP (more and smaller papilla, 11 accessions), or LSP (fewer and smaller papillae, 9 accessions; , ).
The microscopic color parameters and papillary indices varied significantly between varieties, and the calculated coefficient of variation values ranged from 1.05% to 152.85% (). For color, the greatest and smallest coefficient of variation values were observed for Db (152.85%) and Dr (1.05%), respectively, and for papilla traits, the greatest and smallest coefficient of variation values were observed for PAR (37.45%) and P (24.40%). In general, the coefficients of variation values were relatively large, which indicated significant differences in the color and papilla traits of the rapeseed accessions.
Table 2. Micromorphological testa traits of 35 rapeseed (Brassica napus L.) germplasm accessions from China
Water uptake
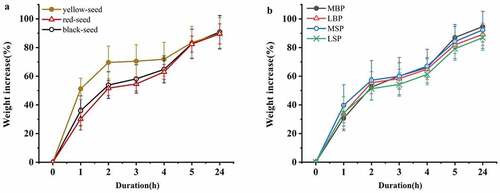
Most accessions exhibited rapid water uptake, with fresh weights increasing by >80% within 5 h (). Over the full 24 h, the imbibition process could be separated into four distinct stages: fastest period (phase I, 0–2 h), steady period (phase II, 2–4 h), sub-fast period (phase III, 4–5 h), and slowest period (phase IV, 5–24 h). In phases I and II, the imbibition rates of the yellow-seeded accessions were significantly greater than those of either the red- or black-seeded accessions, and in phases III and IV, the imbibition rates of the yellow- and black-seeded accessions were greater, although not significantly (). Furthermore, the imbibition rates of the MSP and MBP accessions were greatest during phases I and II and phases III and IV, respectively, whereas the rates of the LSP accessions were lowest during all four phases and were significantly lower than those of the MSP and MBP accessions during phases I and II and phases III and IV, respectively ().
Table 3. Effect of imbibition duration on the water uptake and electrical conductivity of seed from rapeseed (Brassica napus L.) germplasm accessions from China
Figure 2. Effect of testa color and morphology on the water uptake rate of rapeseed accessions from China. (a) Effect of testa color. (b) Effect of testa morphology
Correlation analysis indicated that 2-h water uptake (weight increase after 2 h imbibition) was significantly positively correlated with HHSB, BHSB, BRGB, and L*, with correlation coefficients of 0.38, 0.34, 0.53, and 0.36, respectively, and significantly negatively correlated with SHSB, a*, b*, M, and Y, with correlation coefficients of −0.53, −0.40, −0.53, −0.39, and −0.55. Meanwhile, 5-h water uptake was significantly positively correlated with the indices of papillae (No.P, DrP, APA, and PAR), and the correlation coefficients were 0.33, 0.43, 0.36,, and 0.43, respectively.
Electrical conductivity
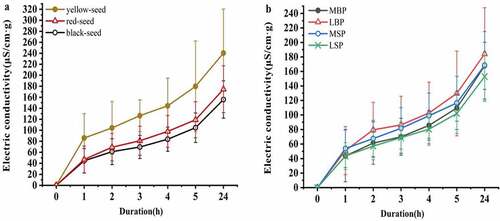
After 1 h imbibition, the three yellow-seeded accessions (Huahuang No.1, Yuhuang No.1, and Youyan No.10) exhibited the greatest electrical conductivity (>110 µSg−1cm−1), and except for Huayouza No.9 and Huayouza No.10, the red- and black-seeded accessions exhibited low electric conductivity (>70 µSg−1cm−1; ). The mean electrical conductivity of the yellow-seeded accessions was significantly greater than that of either the red- or black-seeded accessions, regardless of soaking time. The mean electric conductivity of the black-seeded accessions was consistently lowest but not significantly lower than that of the red-seeded accessions ()). After 2, 3, 4, 5, and 24 h imbibition, the mean electric conductivity of the LBP accessions was greatest, whereas that of the LSP accessions was lowest, and there were significant differences between the two groups ()).
Figure 3. Effect of testa color and morphology on the rate of electric conductivity increase for rapeseed accessions from China. (a) Effect of testa color. (b) Effect of testa morphology
Correlation analysis showed that SHSB, a*, b*, M and Y were significantly negatively correlated with electrical conductivity after 3 h imbibition (P < 0.01), while HHSB, BHSB, Db, IOD, and the electric conductivity after 5 h imbibition was significantly (P < 0.05) or extremely significantly (P < 0.01) negatively correlated, and the correlation coefficient was the highest. With the exceptions of APA and electric conductivity after 24 h imbibition, electrical conductivity was significantly positively correlated with all the papilla indices (P < 0.05), and associations with the other indices were not significant.
Drought tolerance
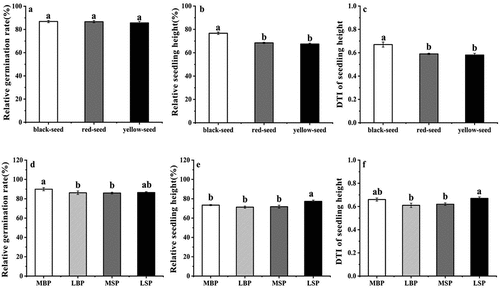
The mean drought tolerance index (DTI) was 0.63, and the accessions with the highest and lowest drought tolerance indices were Huyou No.18 (0.92) and Huyou No.15 (0.45), respectively (Supplementary Table S1). There was no significant difference in the relative germination rates of the black-, red-, and yellow-seeded accessions (). The relative seedling height and DTI values of the black-seeded accessions were considerably higher than those of the red- and yellow-seeded cultivars, and there was no significant difference between values for the red- and yellow-seeded accessions (). The relative germination rate values of the MBP accessions were significantly greater than those of the LBP and MSP accessions but not significantly different than those of the LSP accessions (). The relative seedling height values of the LSP accessions were greater than those of the MBP, LBP, and MSP accessions (), and the DTI values of the LSP accessions were significantly higher than those of the LBP and MSP accessions. However, there was no significant difference between the drought index tolerance of the LSP and MBP accessions or between the LBP and MSP accessions ().
Figure 4. (a and d) Relative germination rate. (b and e) Relative seedling height. (c and f) DTI of seedling height
Germination rates under normal and drought conditions were significantly (P < 0.05) or extremely significantly (P < 0.01) negatively correlated with the testa micro-color parameters BRGB, HHSB, L*, Dg, and Db and significantly (P < 0.05) or extremely significantly (P < 0.01) positively correlated with SHSB and Y. The DTI and DbTP were significantly negatively correlated (P < 0.05).
Relative seedling height, relative fresh weight, and DTI were significantly positively correlated with 4-h water uptake (r = 0.61, P < 0.01). Electric conductivity after 1, 2, 3, 4, 5, and 24 h of imbibition was significantly correlated with the germination rate and relative germination rate under drought stress (P < 0.05) but not with DTI.
Germination rates under normal and drought conditions were negatively correlated with BRGB, HHSB, L*, Dg, and Db (P < 0.05) and significantly positively correlated with SHSB and Y (P < 0.05). Seedling height under normal water availability conditions was significantly negatively correlated with testa BRGB (P < 0.05) and significantly positively correlated with SHSB and Y (P < 0.05), whereas seedling height under drought conditions was significantly negatively correlated with HHSB, SHSB, and L*(P < 0.05). In addition, fresh seedling weight under drought conditions was significantly negatively correlated with b*and Y (P < 0.05). Furthermore, DgP was significantly negatively correlated with germination rate under normal water availability (P < 0.05), and DbTP was significantly negatively correlated with seedling height, relative seedling height, and DTI under drought conditions (P < 0.05).
Principal component analysis (PCA)
Principal component analysis of the 12 parameters of testas showed that the contribution rates of the first three principal components were 61.884%, 15.723%, and 8.820%, respectively, and the cumulative contribution rate was 86.428%, which basically included most of the information of the seed coat (supplementary Table S2). The feature vectors of SHSB, HHSB and L* in the first principal component are relatively high, indicating that the three parameters of SHSB, HHSB and L* in the first principal component account for the main factors; In the second principal component, the feature vectors of DgP and DbTP were higher, indicating that the two micro-color parameters were the main factors, and the feature vector of the blue channel gray value (Db) in the third principal component was higher, indicating that the blue channel gray value (Db) was the main factor in the third principal component.
Positive correlations were found for the following indices: HHSB and BHSB; L* and BRGB; DgP and Dg, Db; SHSB and b*,M,Y since the angle and directions between vectors is below 90° ()). Negative correlations were observed between HHSB,BHSB, BRGB, and SHSB, b*,M,Y parameters. Negative correlations were also recorded between SHSB and L*, Dg, Db; L* and b*, M, Y parameters since the angle is higher than 90°and directions between vectors is below 90º. There was no correlation between DbP and all other parameters since the angle between the vectors was 90º.
Figure 5. Principal component analysis of relationships among micromorphological testa characteristics. (a) Contribution of testa characteristics to two main principal components. (b) Grouping of accessions based on two main principal components
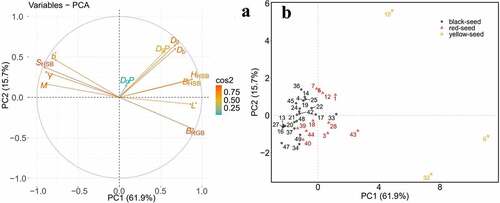
Principal component analysis can distinguish rapeseed of yellow-seed, red-seed and black-seed. It was possible to select three groups of all lines (I and II) ()). Group I with the higher PC1 comprised three yellow seed lines (6, 10 and 32), most of the red-seed rapeseed accessions (1,3,7,28,43, and 44) and four black-seed accessions (17,22,33 and 43). The accessions in group I had lower mean values of relative germination rate (with 85.62%), relative seedling height (with 67.02%) and DTI of seeding height (with 0.57) (supplementary Table S2), which could be recorded as more sensitive to drought stress. Group II with the lower PC1 consisted of other 22 accessions with the higher mean values of relative germination rate (with 87.34%), relative seedling height (with 77.02%) and DTI of seeding height (with 0.67) (supplementary Table S2) could be named as higher resistance to drought stress ()).
Discussion
Seed characteristics
Testa color is one of the main contributing factors of imbibition rate in a variety of crop plants, including soybean, rapeseed, wheat, and chickpea [Citation11,Citation16,Citation35,Citation36]. Indeed, as in the present study, previous studies have reported that the imbibition rates of black-seeded accessions are higher than those of yellow-seeded accessions [Citation26–28,Citation37]. The present study’s finding that 2-h water uptake and electric conductivity were significantly positively correlated with both testa hue and brightness has also been reported previously [Citation10].
In beans, black color has been reported to improve seed vigor and germination [Citation30,Citation38]. In the present study, the germination rates and seedling heights of black- and red-seeded accessions were significantly greater than those of yellow-seeded accessions, regardless of water availability, and black-seeded accessions exhibited significantly greater germination-stage drought tolerance than either the red- or yellow-seeded accessions. In the present study, the yellow-seed accession 6 exhibited relatively high drought tolerance during germination, which indicated that the germination-stage drought tolerance of yellow-seeded rape was relatively weak. However, yellow-seeded germplasm with strong germination-stage drought tolerance could still be selected and used to generate more drought-tolerant yellow-seeded varieties [Citation39,Citation40]. Drought resistance should be established to ensure yield stability under water scarcity. The resistance indices investigated in the present study is considered the most appropriate criteria for the assortment of high-yielding genotypes under drought stress [Citation41].
Seed imbibition and papillae
During imbibition, water is distributed into the crevices, cracks, and flaws of the testa and is then absorbed by seed tissues. Previous studies have demonstrated that the rate of water uptake during this phase is (1) temperature-dependent and (2) accompanied by increases in respiration rate and, in some species, light sensitivity (Supplementary Table S3). Li et al. [Citation35] reported a correlation between imbibition rate and germination rate, as in the present study, which also found a correlation between imbibition rate and germination-stage drought tolerance, as reported previously ([Citation29,Citation41], Supplementary Table S3).
Papilla shape, length, and density can be used as distinguishing taxonomic characteristics. However, the observation of these traits using light microscopy is nearly impossible. In most cases, individual cells possess four to five papillae, which are distributed in a regular pattern along the axis of the cell. Young papillae are wider than mature papillae but are also shorter and undergo morphological changes as they mature, eventually developing into three-m to five-branched structures. Rashid et al. [Citation42] suggested that testa papillae contribute to moisture retention, especially in the soil. In the present study, the No.P, APA, and PAR were all significantly positively correlated with 5-h water uptake rate, and DbTP was significantly negatively correlated with germination-stage drought tolerance ().
Table 4. Drought tolerance parameters of 35 rapeseed (Brassica napus L.) germplasm accessions from China
Table 5. Eigenvalues and contribution rate of principal components
Previous studies of other crop species have demonstrated that testa color affects seed germination rate, emergence part, seedling dry weight, and water uptake percentage. For example, black guar (Cyamopsis tetragonoloba L. Taub) seeds exhibit higher water uptake and germination rates than light-colored guar seeds [Citation43]. In Atriplex cordobensis, reddish and light-brown seeds exhibit higher germination ratios and seedling dry weights than dark-brown seeds [Citation44], and in red clover, yellow seed lots exhibit the highest germination and emergence ratios and most rapid germination [Citation44].
Principal component analysis and electric conductivity
Principal component analysis of the 12 microscopic color and morphology traits indicated that the 35 rapeseed accessions could be classified as drought tolerant or drought resistant. However, the PCA results of some accessions (e.g., 1, 07, 13, and 21) were inconsistent with the DTI [Citation41], possibly owing to the selection of evaluation indices or to the selection of indices for PCA. Plant breeders around the world have been trying to develop yellow-seeded B. napus genotypes using crosses that involve naturally occurring yellow-seeded Brassica species [Citation45]. Consequently, most research efforts have focused on the characteristics of different genotypes with testa colors in B. napus and molecular markers associated with seed color traits
As previously reported by Mandizvo and Odindo [Citation13], the present study found that black, red, or LSP seeds exhibited lower rates of exudate leakage, whereas yellow and LBP seeds are more conducive to water entry, resulting in more rapid water update and, possibly, imbibition damage [Citation13]. Previous studies have associated reductions in germination ability with membrane deterioration, as measured by electrical conductivity and electrolyte leakage [Citation16]. Landraces differed significantly (P < 0.001) in testa color, testa thickness, electrical conductivity, hydration rate, germination rate, and seedling length. The electrical conductivity of the seed leachate increased with increased duration.
Conclusion
The aim of the present study was to investigate the relationship between microscopic testa color and papilla characteristics, imbibition, and germination-stage drought tolerance in rapeseed. Analysis indicated that both microscopic color and papilla traits were correlated with seed imbibition, seed germination, seedling growth, and germination-stage drought tolerance. However, additional research is needed to determine the composition of testa color and mechanism(s) by which papilla structure affects seed imbibition, seed germination, seedling growth, and germination-stage drought tolerance.
Authors’ contributions
ZHZ, MF, and AS designed and performed the experiment and wrote the manuscript; WYZ, ZPC, XZJ, HZ, YL, and YY analyzed the data; and ZHZ and KJZ provided guidance during all experiments. All authors have read and approved the manuscript.
Supplemental Material
Download MS Word (26.8 KB)Acknowledgements
We are grateful to Prof. Xue kun Zhang for his advice and suggestions.
Disclosure statement
The authors declare that they have no conflict of interest that could be perceived to influence the results or discussion of this manuscript.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Nakai J. Food and Agriculture Organization of the United Nations and the sustainable development goals. Sustain Dev. 2018;22.
- Sami A, Riaz MW, Zhou X, et al. Alleviating dormancy in Brassica oleracea seeds using NO and KAR1 with ethylene biosynthetic pathway, ROS and antioxidant enzymes modifications. BMC Plant Biol. 2019;19(1):577.
- FAOSTAT Food and agriculture data. 2019. Food and Agriculture Organisation of the United Nations, Rome. cited 2019 Jan 8. Available from: http://www.fao.org/faostat/en.
- Razzaq H, Nadeem Tahir MH, Ahmad Sadaqat H, et al. Screening of sunflower (Helianthus annus L.) accessions under drought stress conditions, an experimental assay. J Soil Sci Plant Nutr. 2017;17(3):662–671.
- Song F, Meng Z, Luo T, et al. Cytological identification of new-type Brassica napus materials and their physiological response to drought. Crop Pasture Sci. 2019;70(10):876–889.
- Tula S, Ansari MW, Babu AP, et al. Physiological assessment and allele mining in rice cultivars for salinity and drought stress tolerance. Vegetos. 2013;26(2s). 10.1016/j.heliyon.2019.e01249.
- Wei B, Yang J, Li D, et al. Physiological responses of safflower (Carthamus tinctorius) to osmotic stress. Int J Agric Biol. 2019;21(5):1106–1116.
- Xie X, Zhang X, He Q. Identification of drought resistance of rapeseed (Brassica napus L.) during germination stage under PEG stress. J Food Agric Environ. 2013;11(2 Part 2):751–756.
- Zhang CX, Li SF, Liu XY, et al. Establishment of evaluation system for drought tolerance at maize germination stage under soil stress. Sci Agric Sin. 2020;53(19):3867–3877.
- Zhang XK, Chen J, Chen L, et al. Imbibition behavior and flooding tolerance of rapeseed seed (Brassica napus L.) with different testa color. Genet Resour Crop Evol. 2008;55(8):1175–1184.
- Sami A, Zhu ZH, Zhu TX, et al. Influence of KAR1 on the plant growth and development of dormant seeds by balancing different factors. Int J Environ Sci Technol. 2021;1–10. doi:10.1007/s11738-014-1503-2
- Sami A, Xue Z, Tazein S, et al. CRISPR-Cas9-based genetic engineering for crop improvement under drought stress. Bioengineered. 2021;12(1):5814–5829.
- Miyan MA. Droughts in Asian least developed countries: vulnerability and sustainability. Weather Clim Extremes. 2015;7:8–23.
- Mandizvo T, Odindo AO. Seed coat structural and imbibitional characteristics of dark and light coloured Bambara groundnut (Vigna subterranea L.) landraces. Heliyon. 2019;5(2):e01249.
- Zhu ZH, Sami A, Xu QQ, et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS One. 2021;16(9):e0257236.
- Aliyev RT, Coskunçelebi KAMİL, Beyazoğlu O, et al. Alterations in the genome of wheat seedlings grown under drought stress and the effect of gibberellic acid on these alterations. Riv Biol. 2000;93(1):183–189.
- Sami A, Rehman S, Tanvir MA, et al. Assessment of the germination potential of Brassica oleracea seeds treated with Karrikins 1 and cyanide, which modify the ethylene biosynthetic pathway. J Plant Growth Regul. 2021;40(3):1257–1269.
- Sami A, Shah FA, Abdullah M, et al. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol (Stuttg). 2020;22(4):679–690.
- Pereira EP, Ribeiro PR, Loureiro MB, et al. Effect of water restriction on total phenolics and antioxidant properties of Amburana cearensis (Fr. Allem) AC Smith cotyledons during seed imbibition. Acta Physiol Plant. 2014;36(5):1293–1297.
- Bewley JD, Bradford K, Hilhorst H. Seeds: physiology of development, germination and dormancy. Springer Science and Business Media; 2012. doi:10.2135/cropsci1981.0011183X002100020026x
- Kranner I, Kastberger G, Hartbauer M, et al. Noninvasive diagnosis of seed viability using infrared thermography. Proc Natl Acad Sci U S A. 2010;107(8):3912–3917.
- Rajjou L, Duval M, Gallardo K, et al. Seed germination and vigor. Annu Rev Plant Biol. 2012;63:507–533.
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9(7):1055–1066.
- Macovei A, Pagano A, Cappuccio M, et al. A snapshot of the trehalose pathway during seed imbibition in Medicago truncatula reveals temporal- and stress-dependent shifts in gene expression patterns associated with metabolite changes. Front Plant Sci. 2019;10:1590.
- Vertucci CW, Leopold AC. Dynamics of imbibition by soybean embryos. Plant Physiol. 1983;72(1):190–193.
- Miller ND, Stelpflug SC, Kaeppler SM, et al. A machine vision platform for measuring imbibition of maize kernels: quantification of genetic effects and correlations with germination. Plant Methods. 2018;14(1):115.
- Tully RE, Musgrave ME, Leopold AC. The seed coat as a control of imbibitional chilling injury. Crop Sci. 1981;21(2):312–317.
- Copeland LO, McDonald MB. Seed science and technology. 3rd ed ed. 1995. doi:10.22438/jeb/38/5/MRN-340
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000;122(2):403–414.
- Zhang J, Cui Y, Zhang L, et al. Seed coat color determines seed germination, seedling growth and seed composition of canola (Brassica napus). Int J Agric Biol. 2013;40(6):2345-2354.
- Chachalis D, Smith ML. Imbibition behavior of soybean (Glycine max (L.) Merrill) accessions with different testa characteristics. Seed Sci Technol. 2000;28(2):321–331.
- Goel A, Goel AK, Sheoran IS. Changes in oxidative stress enzymes during artificial ageing in cotton (Gossypium hirsutum L.) seeds. J Plant Physiol. 2003;160(9):1093–1100.
- Yang CJ, Zhang XK, Zou CS, et al. Effects of drought simulated by PEG-6000 on germination and seedling growth of rapeseed (Brassica napus L.). Chin J Oil Crop Sci. 2007;29(4):425.
- Hampton JG, TeKrony DM. Handbook of vigour test methods. Zurich Switzerland: The International Seed Testing Association; 1995.
- Li Z, Lu GY, Zhang XK, et al. Improving drought tolerance of germinating seeds by exogenous application of gibberellic acid (GA3) in rapeseed (Brassica napus L.). Seed Sci Technol. 2010;38(2):432–440.
- Lamichaney A, Kudekallu S, Kamble U, et al. Differences in seed vigour traits between desi (pigmented) and Kabuli (non-pigmented) ecotypes of chickpea (Cicer arietinum) and its association with field emergence. JEB. 2017;38(5):735–742.
- Shin OH, Kim DY, Seo YW. Effects of different depth of grain colour on antioxidant capacity during water imbibition in wheat (Triticum aestivum L.). J Sci Food Agric. 2017;97(9):2750–2758.
- Atak M, Kaya MD, Kaya G, et al. Dark green colored seeds increase the seed vigor and germination ability in dry green pea (Pisum sativum L.). Pak J Bot. 2008;40(6):2345–2354.
- Marler TE. Temperature and imbibition influence Serianthes seed germination behavior. Plants (Basel). 2019;8(4):107.
- Ahmadizadeh M, Valizadeh M, Shahbazi H, et al. Behavior of durum wheat genotypes under normal irrigation and drought stress conditions in the greenhouse. Afr J Biotechnol. 2012;11(8):1912–1923.
- Drikvand R, Doosty B, Hosseinpour T. Response of rainfed wheat genotypes to drought stress using drought tolerance indices. J Agric Sci (Toronto). 2012;4(7):126–131.
- Marcińska I, Czyczyło-Mysza I, Skrzypek E, et al. Application of photochemical parameters and several indices based on phenotypical traits to assess intraspecific variation of oat (Avena sativa L.) tolerance to drought. Acta Physiol Plant. 2017;39(7):1–13.
- Rashid N, Zafar M, Ahmad M, et al. Intraspecific variation in seed morphology of tribe Vicieae (Papilionoidae) using scanning electron microscopy techniques. Microsc Res Tech. 2018;81(3):298–307.
- Liu W, Peffley EB, Powell RJ, et al. Association of seedcoat color with seed water uptake, germination, and seed components in guar (Cyamopsis tetragonoloba (L.) Taub). J Arid Environ. 2007;70(1):29–38.
- Atis I, Atak M, Can E, et al. Seed coat color effects on seed quality and salt tolerance of red clover (Trifolium pratense). Int J Agric Biol. 2011;13:3.
