ABSTRACT
Macrophage infiltration is a hallmark pathological change observed in early stage myocardial ischemia/reperfusion (MI/R) injury and one of the main causes of myocardial damage. Here, we investigated the effects of p-Coumaric acid (p-CA) on macrophage polarization following MI/R injury and its mechanisms. In vitro, p-CA decreases the expression of LPS/IFN-γ-induced M1 macrophage markers (TNF-α, IL-6, iNOS and CCL2) and increases IL-4-induced M2 macrophage markers (IL-10, CD206, Arg1 and Mrc) in mouse bone marrow-derived macrophages (BMDMs). Additionally, p-CA elevated indoleamine 2, 3-dioxygenase (IDO) protein expression levels, M2 macrophage polarization and M2 macrophage markers through IL-4. In contrast, repression of IDO attenuated p-CA functions regulating BMDMs through IL-4. In vivo, IDO expression was downregulated in mouse hearts subjected to MI/R injury. Treatment of p-CA increased IDO expression in the hearts of MI/R mice. Functionally, p-CA decreases M1 macrophage markers, the number of M1 macrophages and inflammation around heart tissue following MI/R injury. Importantly, p-CA reduces cardiomyocyte apoptosis caused by MI/R. Altogether, our study identified that p-CA modulates macrophage polarization by promoting IDO expression and that p-CA attenuates macrophage-mediated inflammation following MI/R by promoting M2 macrophage polarization through IDO.
1. Introduction
Acute myocardial infarction (AMI) is one of the main causes of death. However, with the use of percutaneous coronary intervention (PCI), the mortality rate of AMI patients is decreasing yearly [Citation1,Citation2]. However, coronary artery reflow reconstructed by PCI treatment also leads to new injuries, including myocardial ischemia reperfusion (MI/R) injuries (MI/R) [Citation3]. Immune activation plays an important role in MI/R injury. When the blood flow of the ischemic myocardium is restored, damaged cardiomyocytes release endogenous danger signals to recruit inflammatory cells, initiate/amplify the local inflammatory response and cause secondary damage to cardiomyocytes [Citation4,Citation5].
Monocytes and macrophages play an important role in the inflammatory response induced by MI/R [Citation4,Citation5]. In the perfused heart tissue, macrophages are mainly classified as M1 and release a variety of pro-inflammatory cytokines and chemokines that induce inflammation and promote the phagocytosis of dead cells [Citation6,Citation7]. With prolonged reperfusion time, M1 macrophages aggravate tissue damage and myocardial apoptosis [Citation8]. In addition, the macrophages gradually become polarized toward M2, and M2 macrophages synthesize and release anti-inflammatory cytokines, immunosuppressive factors and a variety of growth factors that inhibit inflammation and promote myocardial tissue repair [Citation9,Citation10]. Therefore, it is important to suppress MI/R injury caused by excessive inflammation through promoting the conversion of macrophages from M1 to M2.
p-Coumaric acid (p-CA) is a derivative of cinnamic acid found in free form in fruits, vegetables and traditional Chinese medicines. It has many pharmacological functions, such as scavenging free radicals [Citation11], inhibiting lipid peroxidation [Citation12,Citation13], anti-inflammatory responses [Citation14,Citation15], immune regulation [Citation16] and plays a key role in neuroprotection [Citation17]. Based on previous studies, we assumed that p-CA attenuates MI/R injury since p-CA reduces doxorubicin-induced oxidative stress [Citation18], inhibits brain injury caused by ischemia-reperfusion [Citation17] and regulates indoleamine 2,3-dioxygenase (IDO) expression in mouse immune cells [Citation19]. Overexpression of IDO promotes polarization of dTHP-1 cells into M2 macrophages [Citation20]. Therefore, p-CA regulates the polarization of macrophages by regulating the IDO expression, thereby reducing MI/R damage caused by macrophage-mediated inflammation. In this study, we confirmed this hypothesis to show that p-CA promotes the polarization of M2 macrophages in MI/R mouse heart tissues by promoting IDO expression.
2. Materials and methods
2.1 MI/R mouse model
All animal studies were performed according to the National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. C57BL/6 J male (n = 70, 8–10 weeks old) mice were maintained in pathogen-free facilities (24 ± 2°C and 45 ± 15% humidity with a 12/12 h light-dark cycle). After a week of acclimatization, mice were utilized for developing the MI/R model as previously described [Citation21,Citation22]. In brief, the mice were anesthetized by intraperitoneal injection of sodium pentobarbital (45 mg/kg), and the heart was exposed through an incision in the left chest. Next, a 6.0 silk suture slipknot ligated around the left anterior descending (LAD) of the coronary artery was generated to induce myocardial infarction for 30 minutes. Mice were euthanized after perfusion for 12, 24 or 48 hours. Mice in the sham group experienced surgery that exposed the heart, but LAD ligation was not performed. Two weeks before establishing MI/R model, mice were treated orally with 100 mg/kg of p-CA (C9008, Aladdin, USA) every day until they were euthanized.
2.2 Preparation of BMDMs
Bone marrow-derived monocytes were isolated from C57BL/6 J male mice (10–12 weeks of age) and were differentiated into BMDMs as previously described [Citation23]. Briefly, mice were sacrificed by cervical dislocation, and bone marrow-derived cells were harvested from the femur or tibia. Bone marrow-derived cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin for 12 hours before non-adherent cells were collected. Bone marrow-derived monocytes were differentiated into BMDMs using 40 ng/mL of GM-CSF (SRP3201, MERCK, USA) for 8 days. Lastly, BMDMs were polarized into M1 macrophages using 100 ng/mL of LPS and 20 ng/mL of IFN-γ for 24 hours. BMDMs were polarized into M2 macrophages using 20 ng/mL of IL-4 for 24 hours.
2.3 RNA extraction and qPCR
Total RNA was extracted from BMDMs and heart tissues using RNAiso plus (15,596,026, ThermoFisher Scientific, USA). Next, cDNA was performed using the First Strand cDNA Synthesis Kit (11,801–025, OriGene, USA) according to instructions provided by the manufacturer [Citation24]. Quantitative reverse transcription polymerase chain reactions (qPCRs) were performed as described by the manufacturer (A6001, Promega, USA) using the applied biosystems PCR system. PCR primers are listed in .
Table 1. Sequence of qPCR primers
2.4 Flow cytometry
Red blood cells were removed from a cardiac tissue single-cell suspension, counted and then incubated for 30 minutes on ice with one of the following antibodies: CD45-Pacific Blue (50–113-811, Invitrogen, USA), CD11b-APC-Cy7 (MABF512MI, MilliporeSigma, USA), Gr-1-APC (RM3005, Invitrogen, USA), CD86-PE-cy7 (11,527, Cell Signaling Technology, USA) or CD206-PE (12–2069-41, eBioscience, USA). Lastly, flow cytometry was used to detect the fluorescence and Flowjo 7.6.2 was used to analyze data.
2.5 Immunoblotting
As previous study, RIPA lysis buffer was used to extract total protein from mouse BMDMs and heart tissues [Citation25]. Protein concentration was detected using a BCA kit. Cell or tissue lysates were separated using 10% SDS-PAGE. After the protein transfer step, PVDF membranes (LC2002, ThermoFisher) were blocked with 5% skimmed milk and then membranes were probed with primary antibodies against IDO (86,630, CST, USA), Bax (ab32503, Abcam, UK) or Bcl2 (ab196495, Abcam, UK). β-Actin served as a loading as control. After three washes in PBS buffer, secondary antibodies were incubated for 1 hour at room temperature. Lastly, proteins were visualized using ECL solution (WBKLS0100, Beijing Xinjingke Biotechnologies Co., Ltd., China). Blots were analyzed using Image J 3.0 (IBM, USA).
2.6 Repression of IDO
Small interfering RNAs (siRNAs) were used to repress IDO expression. A total of 50 nmol/l of siRNAs (5ʹ-UAGUCAGAAGCAUAGUGAGCA-3ʹ) were transfected into 2.5 × 106 BMDMs using Lipofectamine 2000 based on manufacturer protocols. The experiments were performed 72 hours after transfection.
2.7 Immunochemistry
Heart tissues were harvested 24 hours after reperfusion and 5 μM histological sections were prepared. Next, a IDO (86,630, CST, USA) primary antibody was added to tissues before washing three times with PBS containing tween 20. Then, a secondary antibody (Goat Anti-Rabbit IgG H&L (HRP) (ab6721, abcam, UK)) was incubated with tissue samples for 2 hours at room temperature. Lastly, the number of positive cells staining with IDO were counted using a Leica TCS SP5 microscope (Leica microsystem) containing LAS AF Lite 4.0 image browser software.
2.8 Inflammatory cytokines and caspase 3 activity assays
Heart tissues were harvested 24 hours after reperfusion and homogenate was prepared on ice using a glass homogenizer. Caspase 3 activity was detected using a Caspase 3 Activity Assay Kit (C1116, Beyotime, China) based on manufacturer instructions. To detect TNF-α, IFN-γ, IL-4 and IL-10 levels, the Mouse TNF alpha ELISA kit (ab208348, abcam, UK), IFN-γ ELISA Kit (ab100689, abcam, UK), IL-4 ELISA Kit (ab100710, abcam, UK) and IL-10 ELISA Kit (ab255729, abcam, UK), were used, respectively.
2.9 TUNEL staining to detect apoptosis of cardiomyocytes
Heart tissues were harvested 24 hours after reperfusion and 5 μM histological sections were prepared using the One Step TUNEL Apoptosis Assay Kit (C1088, Beyotime, China) based on manufacturer instructions. Briefly, sections were washed twice in PBS and 0.5% Triton X-100 was used to permeate cells after the section was fixed for 40 minutes using 4% paraformaldehyde. Lastly, 50 μl of TUNEL detection solution was added to the sections and incubated at 37°C for 60 minutes in the dark. The number of TUNEL positive cells was counted using a Leica TCS SP5 microscope (Leica microsystem) containing LAS AF Lite 4.0 image browser software.
2.10 Statistical analyses
SPSS 20.0 (IBM, USA) software was used to analyze data. Data were depicted as mean ± SD, and one-way ANOVA with a Turkey test as a post hoc test was used to compare the differences between multiple groups. P < 0.05 indicated statistical significance
3 Results
Herein, we study the effect of p-CA on the polarization of macrophages, and we hypothesized that p-CA reduces MI/R damage by promoting the activation of M2 macrophages. In the present study, we first study the effect of p-CA on the polarization of BMDM in vitro and found that p-CA promotes M2 macrophages polarization by increasing IDO expression. Next, we checked the effect of p-CA on the expression of IDO protein and macrophages polarization in MI/R mouse model and found that p-CA attenuated macrophage-mediated inflammation following by MI/R through promoting M2 macrophage polarization via increasing IDO expression.
3.1 p-CA regulates macrophage polarization in mouse BMDMs
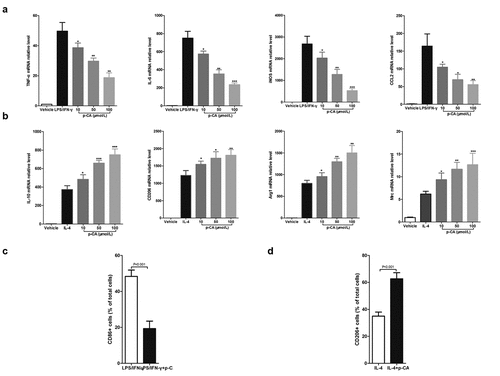
Mouse BMDMs have been used as the model for studying macrophage polarization. When treated with LPS (100 ng/mL) and IFN-γ (20 ng/mL) for 24 hours, BMDMs will polarize into M1 macrophages. BMDMs will polarize into M1 macrophages after being treated with IL-4 (20 ng/mL) for 24 hours. To investigate the effects of p-CA on macrophage polarization, we first treated BMDMs with LPS (100 ng/mL), IFN-γ (20 ng/mL) and different concentrations of p-CA (10, 50 and 100 μmol/L) for 24 hours. qPCR analysis indicated that p-CA significantly decreased the expression of LPS/IFN-γ-induced M1 macrophage markers (TNF-α, IL-6, iNOS and CCL2) in BMDMs (). Similarly, BMDMs were treated with IL-4 (20 ng/mL) and different concentrations of p-CA (10, 50 and 100 μmol/L) for 24 hours to find that p-CA significantly increased IL-4-induced M2 macrophage markers (IL-10, CD206, Arg1 and Mrc) in BMDM (). In addition, BMDMs were harvested to analyze M1 (CD86) and M2 (CD206) macrophage markers using flow cytometer, 24 hours after treatment. We found that p-CA significantly decreased CD86 positive cells () and increased CD206 positive cells ().
Figure 1. Effect of p-CA on the polarization of mouse BMDM in vitro. A, 24 hours after being stimulated with p-CA (10, 50 and 100 μmol/L), LPS (100 ng/mL) and IFN-γ (20 ng/mL), qPCR analysis was used to detected the expression of M1 macrophages markers (TNF-α, IL-6, iNOS and CCL2) in BMDM; B, 24 hours after being stimulated with p-CA (10, 50 and 100 μmol/L) or IL-4 (20 ng/mL), qPCR analysis was used to detected the expression of M2 macrophages markers (IL-10, CD206, Arg1 and Mrc) in BMDM; C-D, 24 hours after being treated with different methods, BMDM were harvested to analyze M1 (CD86) (c) and M2 (CD206) (d) macrophages markers using flow cytometer. Data was expressed as (mean ± SD) from 3 independent tests, and P value was calculated by post-hoc comparisons in (a) and (b), and by student’s t test in (c) and (d). * was P < 0.05, ** was P < 0.01 and *** was P < 0.001 vs LPS+IFN-γ group
3.2 p-CA promotes M2 macrophage polarization by increasing IDO expression
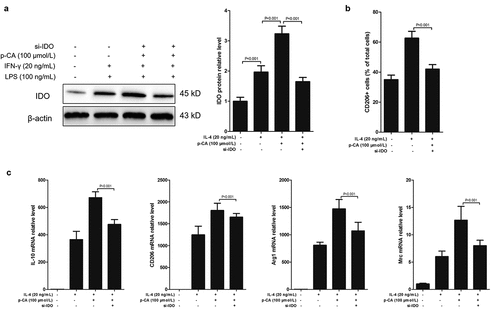
p-CA inhibits the expression of IDO in mouse immune cells [Citation19], and IDO plays an important role in the polarization of THP-1 (a cell line widely used as a model for monocytes/macrophages differentiation) [Citation20]. Therefore, we assumed that p-CA induces M2 macrophage polarization by promoting IDO expression. To test this hypothesis, we first tested the effects of p-CA on the expression of IDO induced by IL-4 to find that p-CA treatment significantly increased IDO protein expression levels through IL-4 in BMDMs. Knockdown of IDO using siRNAs significantly reversed the effects of p-CA promoting IDO expression (). Importantly, knockdown of IDO significantly reversed the M2 macrophage ratio induced by p-CA (). At the same time, we also detected M2 macrophage markers, such as IL-10, CD206, Arg1 and Mrc, and qPCR analysis show that knockdown of IDO also significantly decreased the expression of IL-10, CD206, Arg1 and Mrc mRNAs promoted by p-CA (). Altogether, these results suggested that p-CA promotes IL-4-induced M2 macrophage polarization by increasing IDO expression.
Figure 2. Inhibition of p-CA on LPS-induced M1 macrophage polarization by inhibiting IDO expression. A-C, after 24 hours of stimulation in different ways, BMDM were harvested to analyze IDO protein expression using Western blot, to analyze CD206 positive macrophage using flow cytometer, and to detect the expression of M2 macrophages markers (IL-10, CD206, Arg1 and Mrc) using qPCR. Data was expressed as (mean ± SD) from 3 independent tests, and P value was calculated by post-hoc comparisons in (a) and (b), and by student’s t test in (c)
3.3 p-CA increases IDO expression caused by MI/R injury
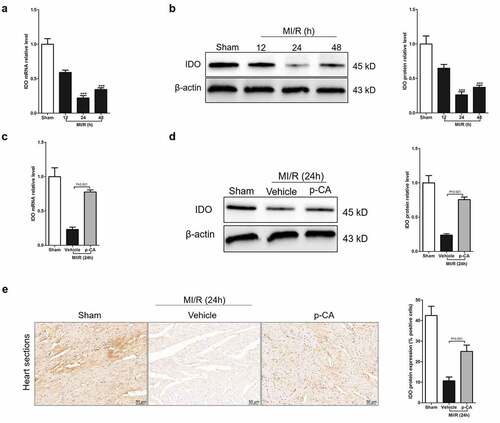
Macrophage-mediated inflammation is a key cause of myocardial damage resulting from MI/R [Citation5]. The increased polarization of M1 macrophages causes macrophage-mediated inflammation [Citation26]. p-CA regulates macrophage polarization by increasing IDO expression. Theoretically, if IDO expression decreases in MI/R mice, p-CA suppresses macrophage-mediated inflammation in MI/R mice by promoting IDO. Fortunately, we first detected dynamic changes of IDO mRNAs using RT-qPCR in mouse heart tissues at 12, 24 and 48 hours after reperfusion to find that IDO mRNA levels were rapidly down regulated and reached a plateau 24 hours after reperfusion (). Next, IDO mRNAs rapidly increased 24 hours after reperfusion and remained at a low level 48 hours after reperfusion. Similarly, dynamic changes in IDO protein levels in the heart tissues after reperfusion showed a rapid decrease in IDO a 24 hours after reperfusion, and then an increase in IDO protein (). To confirm that p-CA increased IDO expression induced by external factors in vivo, we compared the expression levels of IDO protein in MI/R heart tissues in mice with or without p-CA pre-treatment using qPCR, immunoblotting and immunohistochemistry. We found that p-CA pre-treatment significantly increased IDO protein expression in MI/R mouse hearts ().
Figure 3. Effect of p-CA on elevated IDO expression in the heart tissues caused by MI/R. A-B, we harvested the mouse heart tissues following 12 h, 24 h and 48 h of MI/R injury, and then analyze IDO gene expression using qPCR (a) and immunoblotting (b); C-E, mice were treated with100 mg/kg of p-CA prior to MI/R injury, and mouse heart tissues were harvest following 24 h of MI/R injury to be analyzed IDO gene expression using qPCR (c), immunoblotting (d) and immunochemistry (e) for studying the effect of p-CA on IDO expression in MI/R mice. Data was expressed as (mean ± SD) from 7 mice per group, and P value was calculated by post-hoc comparisons in (A) and (B), and by student’s t test in (C), (D) and (E). *** was P < 0.001 vs Sham group
3.4 p-CA regulates macrophage polarization in heart tissues of MI/R mice
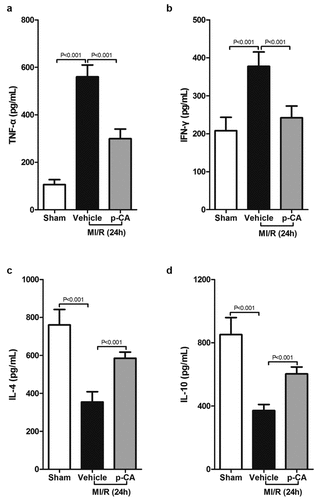
We next investigated the effects of p-CA on macrophage polarization in the heart tissues of MI/R mice since p-CA significantly decreased the expression of IDO in MI/R mouse hearts. First, we determined the expression of M1 macrophage markers (iNOS and CCL2) and M2 macrophage markers (Arg1 and CD206), and found that both M1 and M2 markers were increased in MI/R mouse compared to the sham group. We also found that p-CA pre-treatment significantly decreased the expression of M1 macrophage markers () and significantly increased the expression of M2 macrophage markers (). To further analyze the subtypes of macrophages infiltrating the heart tissues of MI/R mice, we prepared a single-cell suspension of heart tissues and detected the ratio of M1 macrophages (CD16+ CD45+ Gr-1-CD11b+) and M2 macrophages (CD206+ CD45+ Gr-1-CD11b+) using a flow cytometer (). Flow-cytometry analysis revealed that p-CA pre-treatment significantly decreased the ratio between M1 macrophages caused by MI/R injury (), while p-CA significantly increased the ratio of M1 macrophages caused by MI/R injury (). Moreover, we also found that p-CA pre-treatment significantly decreased TNF-α () and IFN-γ () mRNA expression levels and significantly increased IL-4 () and IL-10 () mRNA expression levels caused by MI/R injuries.
Figure 4. Effect of p-CA on macrophage polarization in the heart tissues caused by MI/R. A-B, 24 hours after MI/R injury, mice heart tissues were harvested to analysis M1 macrophages markers (iNOS and CCL2) (a) and M2 macrophages markers (CD206 and Arg1) (b) by qPCR; C-D, as described in (a and b), we prepared a single cell suspension of heart tissue, and used flow cytometer (c) to count the ratio of M1 macrophages (d) and M2 macrophages (e). Data was expressed as (mean ± SD) from 7 mice per group, and P value was calculated by student’s t test
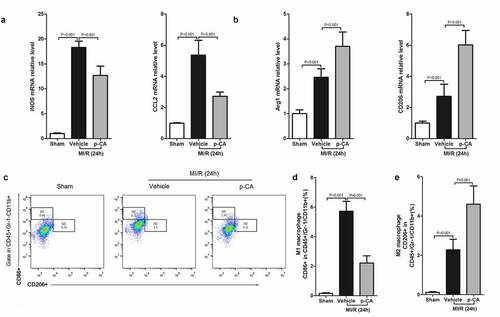
Figure 5. Effect of p-CA on inflammatory cytokines expression in the heart tissues caused by MI/R. A-D, 24 hours after MI/R injury, mice heart tissues were harvested to analysis TNF-α (a), IFN-γ (b), IL-4 (c) and IL-10 (d) by ELISA assay kit. Data was expressed as (mean ± SD) from 7 mice per group, and P value was calculated by student’s t test
3.5 p-CA protects cardiomyocytes against MI/R injury
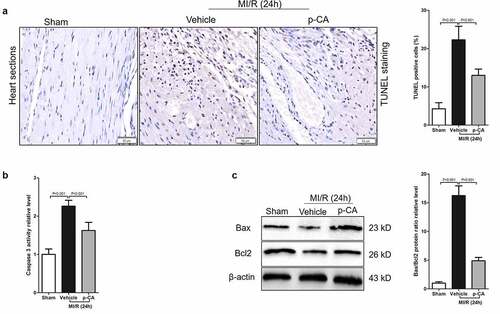
Cardiomyocyte apoptosis caused by MI/R injury results in cardiac dysfunction and myocardial damage [Citation27]. Here, we investigated the effects of p-CA on cardiomyocyte apoptosis caused by MI/R to find that the apoptosis of cardiomyocytes significantly increased and p-CA treatment significantly decreased cardiomyocyte apoptosis caused by MI/R, as shown by TUNEL staining ( and b). p-CA not only significantly decreased the activity of caspase 3 caused by MI/R in mouse heart tissues (), but it also significantly decreased the increased ratio of Bax/Bcl2 protein expression caused by MI/R (). Overall, p-CA treatment decreased MI/R-induced cardiomyocyte apoptosis mouse heart tissues.
Figure 6. Effect of p-CA on cardiomyocyte apoptosis in the heart tissues caused by MI/R. A-C, 24 hours after MI/R injury, mice heart tissues were harvested to analysis cardiomyocyte apoptosis by TUNEL staining (a), to detect the caspase 3 activity by a kit (b), and to measure the expression of apoptosis-related proteins (Bax and Bcl2) by immunoblotting (c). Data was expressed as (mean ± SD) from 7 mice per group, and P value was calculated by student’s t test
4. Discussion
In this study, we investigated the effects of p-CA on MI/R injury caused by macrophage-mediated inflammation, and we also touched on the mechanism behind this. Luckily, we found that p-CA suppressed M1 macrophage polarization by increasing the expression of IDO both in vitro and in vivo. This results in the attenuation of cardiomyocyte apoptosis caused by macrophage-mediated inflammation. Altogether, the results of this study indicated that p-CA is a potential drug that can prevent and treat MI/R injuries due to its ability to inhibit macrophage-mediated inflammation by repressing IDO expression.
p-CA is derived from natural plants, and its pharmacological activities have been reported as beneficial for MI/R. This compound contains both antioxidant and anti-inflammatory characteristics and has the ability to reduce atherosclerosis and inhibit apoptosis. p-CA contains a phenolic hydroxyl structure, providing hydrogen atoms to scavenge reactive oxygen species (ROS), terminating ROS free chain reactions and transferring electrons to form stable products through the conjugation of a benzene ring and double bond side chains [Citation28,Citation29]. Using different evaluation systems, coumaric acid has been shown to have free radical scavenging abilities, but its antioxidant activity is weaker than ortho-diphenol derivatives or ortho-position methoxy base derivatives, since there is only one phenolic hydroxyl group provided by p-CA [Citation30,Citation31]. For example, the antioxidant activity of p-CA is almost the same as butyl hydroxyanisole (BHA) and di-tert-butyl p-cresol (BHT), but better than vitamin C (VC) and vitamin E (VE) in the ABTS method. The scavenging ability of p-CA on O2 is lower than BHT, and the antioxidant activity of p-CA is second to VE based on an iron ion reduction ability experiment [Citation30,Citation31]. So far, we have not observed p-CA protected myocardial ischemia-reperfusion injury through anti-oxidative stress. There is no direct proof to show that the antioxidant activity of p-CA is responsible for the cardioprotective action observed in our study.
In addition, the anti-inflammatory effects of p-CA are closely related to its antioxidant activity. During inflammation, macrophages and neutrophils release ROS and cause tissue damage, while p-CA eliminates O2 induced by chemotactic peptides in human neutrophils, restores lipid peroxidation levels and induces endogenous antioxidant gene expression levels. This restored the antioxidant status of the body to near normal levels [Citation32]. Importantly, p-CA inhibits the expression of inflammatory factors in a dose-dependent manner [Citation33,Citation34], including cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF- α), interleukin-1β (IL-1β) and interleukin-6 (IL-6). In this study, we found that p-CA suppressed the polarization of M1 macrophages and promoted the polarization of M2 macrophages by inhibiting IDO expression in vitro and in the heart tissues of MI/R mice, thereby reducing MI/R injury caused by MI/R.
M1 macrophages promote inflammation by secreting pro-inflammatory factors (IL-1β, IL-6, IL-12, TNF-α), chemokines (MCP-1) and iNOS. M2 macrophages exert anti-inflammatory effects by producing large amounts of anti-inflammatory factors such as IL-10 and inhibiting the secretion of pro-inflammatory factors IL-12, IL-1, TNF-α [Citation35,Citation36]. Macrophages, especially M1 macrophages, have increased infiltration in tissues, which is an important pathological manifestation of tissue inflammation and one main causes of tissue damage [Citation22,Citation37]. The differentiation and activation of macrophages depend on specific growth and differentiation factors, receptors, signaling pathways and transcription factors [Citation38,Citation39]. Local microenvironmental signals play an important impact in the polarization of macrophages. Different microenvironmental signals induce macrophage polarization into two different states, including the immune activation or immune suppression states [Citation40].
Indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme that catalyzes the degradation of tryptophan. An increase in IDO accelerates the metabolism of local tryptophan, exhausting tryptophan and resulting in the accumulation of its metabolite kynurenine, thereby changing the microenvironmental signals around macrophages [Citation41]. Wang et al. found that IFN-γ up-regulated the expression of IDO at both the mRNA and protein levels in dTHP-1 cells in a concentration-dependent manner. In addition, this study also found that overexpression of IDO promoted the polarization of M2 macrophages, while knockdown of IDO promoted polarization of M1 macrophages in dTHP-1 cells [Citation20]. A previous study also found that p-CA regulated the expression of IDO protein in mouse immune cells [Citation19].
Conclusion
Taken together, p-CA attenuates macrophage-mediated inflammation following MI/R by promoting M2 macrophage polarization through increasing IDO expression.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen H, Shi L, Xue M, et al. Geographic variations in in‐hospital mortality and use of percutaneous coronary intervention following acute myocardial infarction in China: a nationwide cross‐sectional analysis. J Am Heart Assoc. 2018;7(8):e008131.
- Marquis-Gravel G, Thiele H, Jones WS, et al. Size of anterior wall acute myocardial infarction treated by primary percutaneous coronary intervention in United States versus Europe/Australia versus India (from the CRISP-AMI randomized controlled trial). Am J Cardiol. 2019;123(7):1026–1034.
- Levine GN, Bates ER. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive Summary †. 2011; 124: 2574–2609.
- Formigli L, Manneschi LI, Nediani C, et al. Are macrophages involved in early myocardial reperfusion injury? Ann Thorac Surg. 2001;71(5):1596–1602.
- Vinten-Johansen J, Jiang R, Reeves JG, et al. Inflammation, proinflammatory mediators and myocardial ischemia–reperfusion injury. Hematol Oncol Clin North Am. 2007;21(1):123–145.
- Fujiwara N, Kobayashi K. Macrophages in Inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–286.
- Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–184.
- Liu Y, Yang H, Liu LX, et al. NOD2 contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and inflammation. Life Sci. 2016;149:10–17.
- Cheng Y, Rong J. Prostaglandin 15-keto PGF2α skews macrophage M1/M2 polarization against myocardial ischemia reperfusion injury via covalent modification of glycolytic enzyme PKM2. 2017.
- Cao D, Schiattarella GG, Villalobos E, et al. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation. 2018; 137: 2613-2634.
- Kiliç I, Yeşiloğlu Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. 2013;Spectrochim Acta A Mol Biomol Spectrosc. 115:719–724.
- Shairibha SR, Rajadurai M. Anti-diabetic effect of p-coumaric acid on lipid peroxidation, antioxidant status and histopathological examinations in streptozotocin-induced diabetic rats. Int j integr sci innov technol. 2014;3:1–11.
- Shen Y, Song X, Li L, et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed Pharmacother. 2019;111:579–587.
- Lee S, Kim H-B, Hwang E-S, et al. Antidepressant-like effects of p-coumaric acid on LPS-induced depressive and inflammatory changes in rats. Exp Neurobiol. 2018;27(3):189.
- Huang X, You Y, Xi Y, et al. p-Coumaric acid attenuates IL-1β-induced inflammatory responses and cellular senescence in rat chondrocytes. Inflammation. 2020;43: 619-628.
- Pragasam SJ, Venkatesan V, Rasool M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013;36(1):169–176.
- Sakamula R, Thong-asa W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab Brain Dis. 2018;33(3):765–773.
- Abdel-Wahab M. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol Res. 2003;48(5):461–465.
- Kim SI, Jeong Y-I, Jung ID, et al. p-Coumaric acid inhibits indoleamine 2, 3-dioxygenase expression in murine dendritic cells. Int Immunopharmacol. 2007;7(6):805–815.
- Wang X-F, Wang H-S, Wang H, et al. The role of indoleamine 2, 3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Cell Immunol. 2014;289(1–2):42–48.
- Gao E, Lei YH, Shang X, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107(12):1445–1453.
- Takawale A, Fan D, Basu R, et al. Myocardial recovery from ischemia–reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circulation. 2014;7:652–662.
- Zhao M, Li F, Jian Y, et al. Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced glycolysis. Eur J Pharmacol. 2020;871:172916.
- Tong L, Tang C, Cai C, et al. Upregulation of the microRNA rno-miR-146b-5p may be involved in the development of intestinal injury through inhibition of Kruppel-like factor 4 in intestinal sepsis. Bioengineered. 2020;11(1):1334–1349.
- Huang W, Chen J, Weng W, et al. Development of cancer prognostic signature based on pan-cancer proteomics. Bioengineered. 2020;11(1):1368–1381.
- Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2015;5:683.
- Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20(6):445.
- Cheng JC, Dai F, Zhou B, et al. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: mechanism and structure-activity relationship. Food Chem. 2007;104(1):132–139.
- Camarero S, Canas AI, Nousiainen P, et al. p-Hydroxycinnamic acids as natural mediators for laccase oxidation of recalcitrant compounds. Environ Technol. 2008;42(17):6703–6709.
- Kili I, YeIloLu YI. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochimica Acta Part A. Mol Biomol Spectrosc. 2013;115:719–724.
- Mathew S, Abraham TE, Zakaria ZA. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol. 2015;52(9):5790–5798
- Pragasam SJ, Rasool M. Dietary component p-coumaric acid suppresses monosodium urate crystal-induced inflammation in rats. Inflammation Res. 2013;62(5):489–498.
- Xiong L, Jin Z, Jianxing L, et al. Systematic analysis of absorbed anti-inflammatory constituents and metabolites of sarcandra glabra in rat plasma using ultra-high-pressure liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry. PLoS One. 2016;11(3):e0150063.
- Yen GC, Chen YC, Chang WT, et al. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J Agric Food Chemistry. 2011;59(2):546–551.
- Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010; 30: 245-57.
- Tang PM-K, Nikolic-Paterson DJ, Lan H-Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–158.
- Oh DJ, Dursun B, He Z, et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol. 2008; 294:F264-71.
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. 32:593–604.
- Murray PJ. Macrophage polarization. Annu Rev Physiol. 2016;79(1):541.
- Davis MJ, Tsang TM, Qiu Y, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4(3). 10.1128/mBio.00264-13.
- Liu P, Xie B-L, Cai S-H, et al. Expression of indoleamine 2, 3-dioxygenase in nasopharyngeal carcinoma impairs the cytolytic function of peripheral blood lymphocytes. BMC Cancer. 2009;9(1):416.
