ABSTRACT
As a consequence of expanded science and technical research, the market perception of consumers has shifted from standard traditional to valuable foods, which are furthermore nutritional as well as healthier in today’s world. This food concept, precisely referred to as functional, focuses on including probiotics, which enhance immune system activity, cognitive response, and overall health. This review primarily focuses on functional foods as functional additives in beverages and other food items that can regulate the human immune system and avert any possibility of contracting the infection. Many safety concerns must be resolved during their administration. Functional foods must have an adequate amount of specific probiotic strain(s) during their use and storage, as good viability is needed for optimum functionality of the probiotic. Thus, when developing novel functional food-based formulations, choosing a strain with strong technological properties is crucial. The present review focused on probiotics as an active ingredient in different beverage formulations and the exerting mechanism of action and fate of probiotics in the human body. Moreover, a comprehensive overview of the regulative and safety issues of probiotics-based foods and beverages formulations.
Graphical abstract
Highlights
Nutritional status is one of the leading risk factors for health ailments.
Probiotics-based functional food is one way of nutritional enrichment of beverages.
Probiotics reduce intestinal inflammation by NF-κB activation inhibition.
The process-based parameters pH and temperature, affects the probiotic formulation.
Present impinged ”Immunity” awareness boosts the technological sector.
1. Introduction
Due to modernization and a growing movement toward a healthy society, customers are more annoyed with exercise and diet, including food protection, to improve their living standards. The sustainable development goals of UNO (Goal 3) also impinges the well-being of individuals of all ages is a prerequisite to combat the pandemic outbreaks such as COVID-19 (https://www.un.org/sustainabledevelopment/health/). Nutritional status has been described as one of the leading risk factors for severe illness, including corpulence and malnutrition, when the economy is struggling with a global pandemic of sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [Citation1]. Hence in the current scenario, the only rational options for survival are safe, nutritious, and usable foods, which play an essential part in improving the immune system’s ability to fight the disease, thereby improving health [Citation2].
Although conventional preserved foods or fermented foods and drinks have been considered synonymous with certain indigenous practices as per the culture and are recognized as playing an essential part in ancient civilization and diversity that is commonly recognized across countries and continents [Citation3], initially these traditional food items were produced without taking into account the scientific application behind along with the role played by microorganisms. However, presently as the consumer consensus has grown with time, multiple shareholders in food networks are deviating from the traditional outlook of food processing by adding or increasing the nutritious and health advantages that make food items more effective and practical. These nutraceutical food items exceed essential nutrition and provide nutritional benefits beyond the generally balanced diet. Hence they’re also referred to as the ‘future foods.’ Further ahead, for selling these as functional foods, they must fulfill many requirements, including validation and meeting the local food safety regulations or supplying these outsides and adhering to international food quality standards, as well as providing free accessibility and evidence of nutritional benefits when eaten as a part of a regular meal. Presently, probiotics, prebiotics, antioxidants, herbal supplements, dietary fiber, minerals, and other phytochemicals dominate the functional foods sector [Citation4–6].
Probiotics-based food items and drinks are accounted for as one of the potential functional foods that are pretty popular and have a broader acceptance by customers among new functional foods on the market [Citation7]. When consumed in sufficient quantities, probiotics have different effects on the body, improving the host’s health by providing nutritional benefits [Citation8]. This outcome is anticipated to include the balancing of the intestinal homeostasis by suppressing or inhibiting microbes [Citation9,Citation10], strengthening the immunity [Citation11] to minimizing the chance of developing various cancers [Citation12], improving the assimilation of lactose [Citation13] as well as other advantages such as preventing the risk of heart diseases, diabetes, and allergic reactions [Citation14]. Furthermore, after the ingestion of probiotics, there are modifications to the intestinal tract microbiota [Citation15,Citation16]. Probiotic strains have been included in fermented foods and medications in the past as well. Probiotics are the integral parts of different fermented foods (Kimchi -Korea, Natto-Japan, Miso-Japan/Korea, Tempeh -Indonesia, Kombucha -China, Mabundu -Tanzania. Sourdough bread-Europe/USA, Sauerkraut – Europe). Different fermented dairy ingredients, such as cheese, tofu, soured/cultured milk, and drinking yogurt, have been the market’s most well-known probiotic distribution vehicles [Citation17,Citation18]. This can be attributed due to their distinctive physicochemical and nutritive capacities, which enable these to buffer the stomach’s harsh acidic environment (where pH is 2–3), allowing a possible amount of probiotics to survive in the lower gut and potentially exert their curative effects [Citation19,Citation20]. Yakult, composed of probiotic Lactobacillus casei Shirota, was the first fermented dairy beverage [Citation21].
While several probiotic strains have been shown to have beneficial effects on human immunity and overall health, any negative consequences should be seen in distinction from the standpoint of public welfare. To determine chiefly the protection of food items and drinks which are probiotics-based, factors such as their source and composition, infectivity, delivering mechanism, capacity to bear antibiotic-resistant genes or not, the extent of subjection to exposure, host health condition, and planned usage must all be taken into account [Citation22]. In several nations, regulatory frameworks have been established to shield consumers against any misleading claims made about probiotics’ intended use, in addition to protection. Depending on the planned use of probiotic ingredients, population trends, and consumer patterns, numerous regulatory assertions govern the commercial market of several nations [Citation23].
2. Role of probiotics in human health and necessity toward recombinat strains
It is essential to have a thorough understanding of the probiotic strain utilized in a product. This is a crucial initial step that is taken into account in vitro studies. In light of this, some researchers have been done on probiotic resistance to human microbiota and different circumstances like the pH of the human gut is acidic ranging from 1.5–3.5 with other gastric juices and bile juices secreting inside when the probiotic is in transit and in conclusion from all the studies an ideal probiotic strain is the one that can oppose the gastric acidity, is rigid against the bile acid, is capable of binding to mucus and epithelial cells or the cell lines, can act against microorganisms that can harm the body, can reduce pathogen adherence to body surfaces, viability enhancement and bile salt hydrolase activity [Citation24–27].
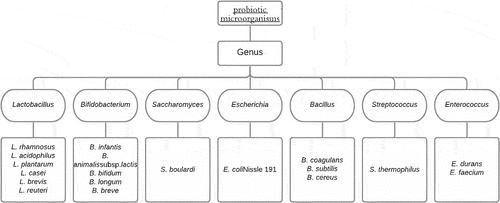
As a result, numerous bacterial species with the potential to become probiotics are studied. As per research, some Lactobacilli strains have been proven to reduce antibiotic-associated diarrhea [Citation28]. As a probiotic, Lactobacillus species are often chosen because they exhibit several essential characteristics, including a good level of resistance to acid and bile, the efficiency of adhering to intestinal surfaces, enduring low pH level and gastric juice, showing antimicrobial activity, and resistance to antibiotics, and the capacity to produce exopolysaccharides and eliminating cholesterol [Citation27,Citation29]. Lactobacillus rhamnosus CRL1505 has been shown to reduce viral pulmonary harm by modulating immune-coagulative responses and eliminating respiratory viruses [Citation30]. Lactobacilli strains commonly used as probiotics comprise L.casei, L. acidophilus, L. rhamnosus, L. paracasei, L. johnsonii, L. delbrueckii subsp. bulgaricus, L. plantarum L. fermentum and L. brevis [Citation31].
Apart from different Lactobacilli strains, Bifidobacterium strains are frequently employed as probiotic bacteria because they have a wide range of bile salt tolerance mechanisms, which is significant as the probiotic bacteria’s positive effects must be synthesized even in the vicinity of various biological fluidic surroundings. While bile resistance varies by strain, wild-type Bifidobacterium and Lactobacilli strains are susceptible to bile salts. They may be rendered tolerant by sub-culturing them in modest bile concentrations and steadily rising the concentrations, gradually making them resistant to bile fluids [Citation29]. Bifidobacterium strains commonly used as probiotics are B. adolescentis, B. adolescentis, B. infantis, B. animalis, B. bifidum, B. breve, B. longum. Different companies frequently develop scientifically sounding marketing labels as their trademark names for a few of these bifidobacteria.
Other than these two prominent strains, a different bacterial genus that can be used as potential probiotics includes (i) Saccharomyces genus, where S. boulardii is frequently sold as a lyophilized probiotic for the treatment of diarrhea, and it has a good safety record as well [Citation32]. Other Saccharomyces strains include S. cerevisiae utilized in the production of wine, bread, and beer, S. bayanus used in the production of wine, and S. boulardii used as a probiotic in medicine. Saccharomyces yeasts also frequently make kefir [Citation33] by forming symbiotic networks with bacteria, and they’re occasionally found in kombucha as well [Citation34]; (ii) Bacillus genus where B. subtilis are being explored for animal feeding [Citation35,Citation36] and is recommended for treating diarrhea and eradicating H. pylori in people, however according to specific reports there is a risk of consumption of B. subtilis spores in o immunocompromised patients, and only under normal host circumstances [Citation37], consumption of these spores is exclusively safe for humans; (iii) Escherichia genus where E. coli is prominently known for being a lower intestinal resident and has its probiotic strain referred to as E. coli Nissle 1917 (EcN) which in combination with other probiotics, is known to be effective in the treatment of constipation [Citation38] and inflammatory bowel infections [Citation39]. However, further study is needed and may treat gastrointestinal disorders such as Crohn’s disease or ulcerative colitis [Citation40] and colorectal cancer [Citation39]. The commonly used probiotic strains have been summarized in .
The Department of Food and agriculture has defined probiotics as live microorganisms, confer a health benefit on the host when consumed in suitable amounts. Concerning health benefits, in adults, probiotics are used as therapeutic agents against gastroenteric microorganisms, lactose intolerance, metabolic disorders, protection against infections (vaginal/urinary tract), repair mechanisms stimulation of cells, ulcerative colitis, production of antimicrobial factors, cholesterol reduction attributes, irritable bowel syndrome, diarrhea associated with antibiotic, colorectal cancer, and control of obesity. In infants, consumption of probiotic aid perinatal health [Citation41,Citation42]. Despite all the scientific advancement, the main question arises: Can a probiotic protect against a specific disease knowing that the mode of action of probiotic action is nonspecific and nondiscriminatory against particular pathogens. In such cases, bioengineered probiotics are used for targeted/specific action pertain to specific disease prevention [Citation43].
Bioengineered probiotics have been in use for over a decade; these are designed for the delivery of therapeutic molecules such as peptides, enzymes, cytokines, DNA, allergens and, single-chain variable fragments [Citation44–46] and application including drug administration, immunomodulation and vaccine delivery [Citation47–54,Citation50]. There are particular attributes required for designing recombinant microorganisms. (1) During product manufacturing and storage should be able to tolerate stress, (2) Under the gastrointestinal environment able to express target antigen, (3) puissant antipathogen, and (4) robust mucosal colonization.
Most of the commonly used probiotics like Lactobacilli and Bifidobacteria are sensitive to different forms of stress. To confer the problem, stress tolerance can be attained through the persistent sublethal exposure of definite stresses or the genes alteration approaches. The methods are being successfully applied to Lactobacillus and Bifidobacterium strains, with various studies showing the adaptive response to temperature [Citation51], oxygen content [Citation52], and acid stress [Citation53]. The enhanced pathogen-specific activity of probiotics has been reported through the cloning and expression of toxin-specific host cell receptors, i.e., cloning of glycosyltransferase genes (N. meningitides/C. jejuni) and expression of chimeric lipopolysaccharide on the engineered probiotic E. coli for imitation of host cell receptor of cholera toxin which aids in the treatment of enterotoxin-associated with diarrhea [Citation54].
3. Probiotics as a functional ingredient in different beverages
The beverage is a liquid drink intended for human consumption to satisfy human thirst and constitutes human culture. Functional beverages are often referred to as nutraceuticals or as designer beverages [Citation55]. Value-added drinks presently have become quite prevalent in the Westward culture. Today, protein-rich beverages, including sports drinks, dominate the dairy-centered beverage sector [Citation56]. Previously, cheese whey, a by-product of cheese manufacture, mainly was used to make low-value-added items, but after its functional characteristics and high nutritional content were discovered, a wide variety of these have become accessible throughout the global markets, being used to manufacture relatively high-value products. These in particular often tend to comprise various types of drinks, including fermented beverages or non-fermented beverages or beverages prepared with probiotic strains or prebiotics or whey- fruit juices and so on. The dairy-based industry is dominated by nutritional drinks, which are liquids fortified or enhanced with functional additives such as bioactive peptides or minerals and vitamins, prebiotics, probiotics, etc. These utilitarian dairy drinks focused on probiotics, or prebiotics, were the first to be commercialized, and they continue to dominate the sector [Citation57]. Probiotic-based beverages with dairy-based foundations, whey-based foundations, and buttermilk-whey-based foundations, as well as probiotic strains used in the fermentation of some juices, are undoubtedly the most common on the market today.
3.1 Dairy-based formulation
Dairy-based probiotic drinks have been accessible in worldwide beverage retails for quite some time now. Fermented milk (drinkable and spoon-able), yogurt, and to a smaller degree, cheese have been used as probiotic carriers in dairy foods for an extended period [Citation58–60]. Probiotic yogurt has become one of the most popular value-added fermented foods available in the last two decades or so. The processing of yogurt necessitates the acidification of milk, which results in the formation of curd. Furthermore, yogurt production is an acidification method that is heavily reliant upon the development of native probiotic LAB (e.g., Lactobacillus paracasei, Lactobacillus acidophilus, L. delbrueckii, L. casei, and Bifidobacterium lactis). Probiotic yogurt containing potential strains of that of probiotic has been shown in several trials to have several health benefits, including decreasing cholesterol levels in the blood, reducing blood pressure and heart rate, and having an antihypertensive impact [Citation61–63,Citation14].
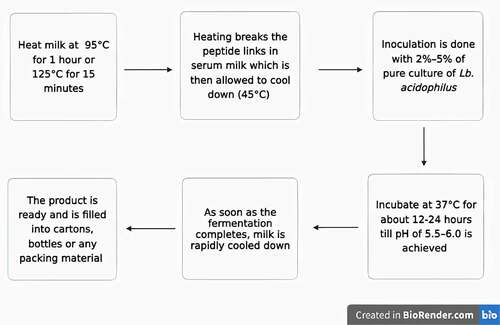
Acidophilus milk is made from milk that has been heated to a high temperature for an extended period (95°C for 1 h or 125°C for 15 minutes). Extreme thermal processing destroys the proteins and peptide linkages in serum milk, which are critical for the development of Lb. acidophilus. Following the thermal processing, all spores’ temperature is lowered to 37°C and left there for 3 to 4 hours to enable all spores to germinate. After that, the milk is reheated again; it further suppresses the actively growing and reproducing microorganisms. Fermentation occurs at 37°C for 18–20 hours, or before the pH reaches 5.5 or 1% lactic acid [Citation64] (). The average Lb. Acidophilus inoculation rate is 2–5% but replacing 25% of the Lb. Acidophilus with a yogurt culture mixture is recommended in certain situations. It has been proposed that probiotic fermented milk reduces plasma cholesterol levels, lowers low-density lipoprotein (LDL) levels in hypercholesterolemic people, blood pressure, plus hypertension prevention [Citation65]. Probiotic milk has also been shown to help hypertensive and prehypertensive people lower their blood pressure [Citation66].
3.2 Whey-based formulation
There has been a surge in using waste materials to create functional drinks in the last few years, with whey being the most notable example. Currently, whey is considered a cheese production by-product but rather a simple ingredient for making enhanced products with much greater value, like probiotic drinks. It comprises a variety of nutritious compounds, including dissolvable milk proteins, minerals, and lactose, and provides an ideal nutrient matrix for probiotic strain’s growth and viability [Citation67–69]. Whey beverages with probiotics being their basis and formulations for the enriched whey-based beverages have governed whey-based beverage studies over the last decade, as it has been exhibited that supplementing these with probiotics or, in fact with the prebiotics has certain health advantages, like reducing hypertension [Citation70]. There are multiple ways of using whey to make healthy probiotic drinks. It can be utilized directly, combined in addition to powders with a dairy foundation, or applied to beverage formulations in various ratios. Several experiments have looked at using whey during LAB fermentation to make lactic probiotic drinks, and probiotic bacteria have also illustrated that they can survive in whey [Citation34]. Numerous prebiotic additives can be operated in the food formulations to facilitate the advancement of the probiotic bacteria in whey-based drinks. The variety and the amount of prebiotics must be deliberately chosen not to harm the sensory properties of the finished product.
During the research, kefir grains were used to make a fermented beverage that resembled kefir by replacing milk with whey [Citation71]. Since multiple Lactobacillus yeasts and bacteria were found, the new beverage could be classified as a probiotic. This research opens up new possibilities for using whey with kefir grains. Apart from this, the growing success of fruit-flavored drinkable yogurt worldwide also presents an excellent opportunity to incorporate whey in producing refined fruit-flavored products [Citation72].
3.3 Buttermilk whey-based formulation
Probiotic drinks based on buttermilk-whey aren’t much popular compared to the cheese whey drinks or the ones found on milk. It is most likely attributed to the idea that the dairy industries do not discard large quantities of buttermilk or the buttermilk whey relative to the cheese whey. After 28 days of storage [Citation73], developed a probiotic drink made of buttermilk that has been supplemented with different flavors and sources of carbohydrate (like sucrose or sucralose) that had adequate sensorial content along with a large probiotic tally. Due to probiotic strains’ metabolic action on diacetyl, the buttermilk-based probiotic drink had a decreasing amount of diacetyl than the control beverage, which is a distinguishing feature of the drink. It was also stated by [Citation73], that the development and sustainability of B. animalis subsp. lactis. was encouraged by flavored probiotic buttermilk beverages.
3.4 Probiotics in juices
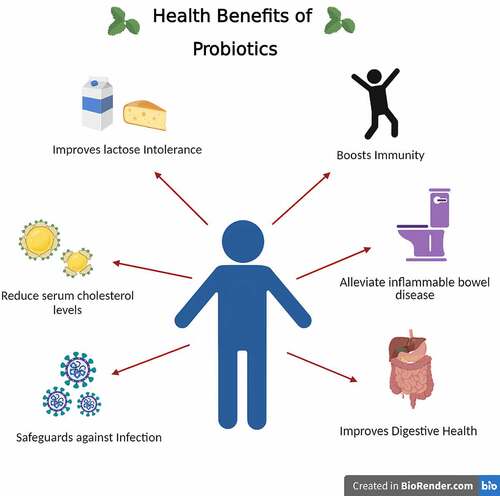
Over the years, probiotics have been used in fermented dairy products, but recent studies of alternate raw materials show suitable substrates for nondairy probiotics. Fruit juices are suitable substrates for probiotics because they already comprise valuable nutrients like vitamins, minerals, antioxidants, and fibers. Their natural sugar promotes the growth of probiotics. The consumption of dairy products is also limited as most individuals are lactose intolerant, hypercholesterolemia, or vegan, which eventually leads to more studies on probiotic fruit juices [Citation74]. Another advantage is that the digestion of juices is better than dairy products; they do not have allergens and are naturally cholesterol-free. The most common fruit juices used as fruit matrices for probiotic delivery are cashew apple, pineapple, apple, banana, orange, and blueberry [Citation75–77]. Overall probiotic juices increase the number of probiotic products in the population. The health benefits of probiotics have been depicted in .
4. Mechanisms of action of probiotics leading to health benefits
4.1 Epithelial barrier strengthening
Epithelial cells lining the intestine are constantly in touch with luminal contents and the continually changing enteric flora. Acting as a critical defensive mechanism, the intestinal barrier protects organisms from their surroundings while maintaining epithelial integrity. Increased gene expression involved in tight junction signaling has been a potential strategy for improving the integrity of the intestinal barrier [Citation78–80]. According to recent research, probiotics may restore the barrier role in the body after injury. For example, E. coli Nissle 1917 (EcN1917), apart from inhibiting enteropathogenic E. coli from disrupting the mucosal barrier, also restores mucosal integrity in T84 and Caco-2 cells. Therefore, increased production and relocation of the zonula occludens (ZO-2) and PKC tight junction proteins lead to rebuilding the close junction complexes [Citation81,Citation82]. Multiple intestinal disorders have been linked to increased pro-inflammatory cytokines and intestinal absorption [Citation83]. Avoiding cytokine-induced epithelium degradation, which is common in irritant bowel illness, may help strengthen the mucosal barrier when probiotics are used [Citation84]. Mucin glycoproteins (mucins) are significant macromolecular components of epithelial mucus, and they’ve been linked to both wellness and sickness for a long time. Thus, probiotics can increase mucus production to improve the barrier role and prevent infections from entering the system. Human intestine cell lines secrete more mucin when they are in contact with various Lactobacillus species. Although Lactobacillus adherence to the cell monolayer is required for this beneficial role, probably, this would not happen in vivo [Citation85,Citation86]. A minimal number of in vivo studies have been conducted. Therefore there is a lack of consistency at the moment. Probiotics may enhance mucus production in vivo, but further research is required in this domain.
4.2 Enhanced cell adhesion
To use a microbial strain as probiotic, several requirements ought to be satisfied. Adherence to the intestinal mucosa enabling colonization and subsequent interaction among the given probiotic strains and the host is a prerequisite [Citation87–89]. Antagonism toward infections and immune system activities are modulated by this unique relationship [Citation90,Citation91]. Thus, adherence has been identified as a critical selection criterion for novel probiotic strains and linked to specific probiotic benefits. Lactic acid bacteria have several surface characteristics that let them associate with intestinal epithelial cells (IECs) and mucus. IECs produce mucin, a complex glycoprotein combination that is the primary aspect of mucous, thereby preventing harmful agents from adhering to it [Citation92–94]. In addition, the mucous gel contains lipids, free proteins, immunoglobulins, and salts. This unique interconnection suggests a probable link amongst probiotic bacteria surface proteins with pathogen inhibition from mucus. Probiotics modify intestinal mucins in a way that prevents pathogens from adhering to them [Citation88,Citation95]. Additionally, the strains of probiotics may also trigger the emission of defensins from epithelial cells, preventing pathogenic infection. These tiny peptides/proteins have antibacterial, antifungal, and antiviral activity.
Furthermore, such tiny peptides/proteins help maintain the intestinal barrier’s overall functioning [Citation96]. Invertebrates, defensins are a large group of membrane-breaking peptides. The contact is nonspecific and occurs mainly via electrostatic interactions with anionic phospholipid molecules on the surface of the membrane. Throughout the bacterial membrane, this contact causes defensin apertures, which undermine membrane strength and lead to the death of microorganisms. Electrostatic interactions allow cathelicidins to attach to membranes of bacteria and, like defensin, cause membrane rupture [Citation97].
4.3 Pathogen competitive exclusion mechanism
Competitive exclusion occurs when one bacterium type contends fiercely for receptor sites within the digestive system than most other bacterial species [Citation87,Citation98]. The precise routes and critical support frameworks that underpin probiotics’ actions are largely unclear. Reduced luminal pH, conflict for nutritional resources, and the generation of bacteriocin or bacteriocin-like compounds are some of the primary processes hypothesized for pathogen inhibition [Citation99]. Human pathogens like Salmonella typhi and E.coli [Citation100] have been the subject of the majority of the research done to date. The regulation of various signaling and metabolic pathways in cells appears to be mediated by certain probiotic compounds. Probiotic metabolome constituents were known to relate with numerous targets in biochemical activities that control the proliferation of the cells, differentiation, programmed cell death, blood vessel formation, and inflammation [Citation101]. Bacteriocins are antimicrobial protein compounds produced by lactobacilli and bifidobacteria that hinder the spread of some diseases.
Further ahead, using probiotics as a preventative or therapeutic measure against enteric infections is referred to as colonization resistance. Each of the 30–60 amino acids in Bacteriocins makes it a little cationic molecule. These chemicals impair the proton motive force by acting on the bacterial plasma membranes and activated membrane vesicles [Citation102].
4.4 Modifications of immune system
The gut’s microbiota influences the immune response by producing chemicals with immunomodulatory and anti-inflammatory properties and stimulating immune cells. These effects on the immune system are attributed to probiotic bacteria interacting with epithelial cells, dendritic cells, monocytes/macrophages, and lymphocytes [Citation103]. Probiotics regulate the host immune system as one of their primary modes of action. As a result, the body’s defense system is split into two types: innate and adaptive. B and T cells, which attach to particular antigens, are essential for the adaptive immune response.
On the other hand, the innate system reacts to pathogen-associated molecular patterns (PAMPs), supported by most pathogenic microbes. Pattern recognition receptors (PRRs) that attach to PAMPs are responsible for the primary immune response to infections. Emerging from this, the PRR family also includes the Toll-like receptors (TLR) family, a group of transmembrane proteins found on variousimmune and nonimmune cells, including B- cells, natural killer dendritic cells (NK) as well as macrophages and fibroblasts. In addition, PRRs contain nucleotide-associated oligomerization domains, adhesion molecules, and lectins, among other things [Citation104]. Besides TLRs, PRRs have NOD-like intracellular receptors (NLRs), which defend the cytoplasmic region [Citation105].
Probiotics have been shown to reduce intestinal inflammation by downregulating TLR expression, secreting compounds that prevent TNF from reaching blood mononuclear cells, thereby inhibiting NF-κBactivation in enterocytes. In this way, lactobacilli cell wall constituents may stimulate cytokine production by signaling via TLR2 and TLR6.TLR9 is yet another class of TLRs triggered by probiotics, and it has anti-inflammatory properties at the epithelial lining in vivo. Due to the intracellular signaling pathways caused by TLR9 activation, TNF – stimulated NF-κB is expressed. As a result, various probiotic species are expected to have varied capacities to activate TLR9. NLRs are another type of membrane-bound receptor. While there hasn’t been much research into how probiotics affect these receptors [Citation106], found that L. Salivarius’s protective ability is linked to local IL-10 synthesis, which has an anti-inflammatory effect that NOD2 mediates. Another significant aspect of NLRs is that they initiate a pathway in which NLRs create inflammasomes, activating the adaptor protein caspase 1 required for breaking pro-IL-1 β and pro-IL-18 into physiologically active forms.
TLR and NOD-like receptors, as well as NF-κB signaling pathways, have recently been characterized in terms of their capacity to promote or inhibit activation and affect downstream pathways. These studies will further throw insight into the intricate interplay of host-microbe interactions. By stimulating these receptors, communal bacteria can induce calibrated antimicrobial reactions that cause slight inflammation and tissue damage in the body. The fundamental mechanisms of probiotics toward different health benefits have been shown in .
Figure 4. Key mechanisms of action of probiotics leading to various health benefits [Modified and adapted from [Citation87]]
![Figure 4. Key mechanisms of action of probiotics leading to various health benefits [Modified and adapted from [Citation87]]](/cms/asset/3fb4b3a8-f1da-4155-bae9-5ffbc80a2df5/kbie_a_2005992_f0004_oc.jpg)
5. ADME (Absorption, distribution, metabolism, and excretion) of probiotics in the human body
The misuse of antibiotics resulting in multi-drug resistance microbes has again focused clinical attention on probiotics. Probiotics are living microbes that are regulated as food and drugs. However, the study and testing of probiotics as drugs are still developing, regulated by the Food and Drug Administration. Heedless of the way a probiotic is currently being marketed, it becomes a ‘drug’ for the prevention or treatment of an illness; therefore, probiotics are classified as ‘live biotherapeutics’ under the FDA work definition. For biological probiotics and new pharmaceutical entities, development pathways and requirements are similar, including preclinical tolerability studies, pharmacokinetics, and large, well-controlled clinical trials. However, pharmacokinetic studies such as absorption, distribution, metabolism, and excretion (ADME) are not convenient for Probiotics [Citation90]. When probiotics are taken into the body by oral or intravenous injection, the nutrients carried by probiotics are absorbed from the small intestine (oral administration) or absorbed into the bloodstream (other administration methods). The primary metabolism occurs in the liver and is excreted as urine from the kidneys or feces with bile. Then they pervade blood vessels from the bloodstream and exercise their medicinal and few toxic effects in several organs [Citation107]. For the particular pharmaceutical probiotic major challenge arises to explain factors that may affect absorption, distribution, metabolism, and excretion (ADME) [Citation108]. The ADME process of probiotics has been depicted in .
Figure 5. Probiotic ADME (Absorption, distribution, metabolism and excretion) process in the body. [Modified and adapted from [Citation108]]
![Figure 5. Probiotic ADME (Absorption, distribution, metabolism and excretion) process in the body. [Modified and adapted from [Citation108]]](/cms/asset/c64b2231-751c-4f99-b65b-2e1294a099dc/kbie_a_2005992_f0005_oc.jpg)
6. Health benefit and safety claims of Probiotic beverages
It is said that when a human takes beneficial bacteria via food, it regulates the gut flora resulting in improved health conditions of a consumer. However, a considerable study is required to conclude the same health benefits with an increase in the number of beneficial bacteria [Citation109]. More than 20,000 articles related to the health benefit of probiotics have been published in PUBMED, out of which 2200 were of a clinical trial. The most studied bacteria with over 9000 articles published are Lactobacillus sp., followed by Bifidobacterium sp., Streptococcus sp., Bacillus sp. and Saccharomyces sp. The health impacts of dairy-based probiotic beverages are extensively studied through in-vitro cell culture and in-vivo animal models, yet clinical trials on humans are still limited.
The maximum number of studies focuses on the effect on the immune system, microbial resistance. The hypocholesterolaemic effect of probiotic strains is studied by in-vitro methods, resulting in probiotics that can remove cholesterol by attaching the cholesterol to their cell surface [Citation110]. Miremadi, Sherkat, and Stojanowska had well-reviewed the Anti-hypertensive properties and hypocholesterolaemic effect of prebiotics and probiotics in 2016 in one of their studies found that the daily consumption of probiotics through dairy products will decrease the chances of cardiovascular disease. The viability of Propionibacterium freudenreichii 138 and Lactobacillus casei BL23 is stimulated under acid, bile salts, and cold storage stress conditions by the augmentation of probiotic skim milk with whey protein isolate at a level of 30% [Citation111]. However, it is found that whey protein isolate increases the anti-inflammatory effect of Lactobacillus casei BL23.
In-vitro and In-vivo (animal trials) studies do not impart adequate data to conclude the effect of probiotics on the human body as they are only helpful for pilot assessment. The health claims related to humans are only applicable if the proper approach executes them. The joint WHO/FAO union in Cordoba, Argentina, from 1st – 4th of October, 2001, identified the rising demand for probiotic-based products. For the safety of a consumer, it is necessary that before reaching them, the product has gone through the proper assessment and approach. The working groups have made proper guidelines and identified the minimum requirements required to be characterized as ‘probiotic food’ [Citation112]. The commercially used different probiotics have been tabulated under .
Table 1. Some probiotics products used commercially [Citation143]
6.1 Microorganisms screening
Isolation and identification are considered to be the initial step for any probiotic used in food implementation. 16s RNA method is used to identify the species and verify with the help of genotypic and phenotypic tests. The presence of Plasmid (extra chromosomal material) supports the strain properties [Citation112,Citation113].
6.2 Probiotic potential screening: in vitro
The efficacy of the identified microbe needs to be supported by the in vitro and in vivo tests followed by human clinical trials. There are different categories such as: gastric juice resistance, ability to reduce pathogens in the gut, antimicrobial activity, adherence to human epithelial cells and safety assessment to check whether the identified microbe has a probiotic [Citation113]. To certify the probiotics in beverages, guidelines of FAO/WHO working groups are followed. For the safety assessment following points need to be considered:
Production of toxins, undesirable metabolites.
Potential side effects in human clinical studies.
Chances of antimicrobial activity.
Epidemiological studies of the effect on consumers and its assessment.
In-vitro studies are more likely to provide more appropriate data on genomic analysis, strain characteristics, viability computation, and DNA- based identification [Citation114].
For the safety assessment, all the approaches should be included in an integrated manner.
6.3 Studying human and animal: in- vivo
After successful in-vitro studies, a sustainable in vivo study is required for the safety interest. Before human trials, approved animal models are used to support in vitro studies. The process for Human clinical studies regarding applying probiotic food is done in four stages: safety evaluation, efficacy, effectiveness, and Surveillance [Citation115].
Safety assessment
Detailed screening of isolates is done, and factors like antibiotic resistance pattern, possible undesirable side effects, and measure of toxin production are recognized. All the procedures are followed, which are recommended by the working officials.
Efficacy
To evaluate the effectiveness, animal studies are necessary [Citation114]. The animal study results are required to be assessed conscientiously and expounded like morphology, physiology, anatomy, pathology, phytology, test groups, and other factors.
Effectiveness
The effectiveness of the probiotic in food needs to be evaluated, and human trials do it. The number of viable cells of the tested probiotic strain in terms of ml/CFU in the carrier drink/ food should be indicated, providing health claims.
Surveillance
As safety is a priority, before human trial, the authorizing body should approve it. The study depends on the category of probiotic strain utilized in the beverages and studied population size.
The in-vivo studies of probiotics-based beverages have been summarized under .
Table 2. In-vivo studies of probiotic based beverages
Labeling requirements
Following standard international nomenclature, Genus, species and strain should be labeled.
Minimum viable number of probiotics and the level at which efficacy is claimed
Clearly stated health claims
Serving size for efficacy
Storage conditions should be mentioned
Probiotics beverages are inspected in open label- studies so a risk factor is associated with it as it can influence microbe viability and effect on the host can be altered [Citation113].
7. Fate of probiotic activity during major formulations
Probiotic formulations have been utilized as dietary supplements for several years. For a successful probiotic product, specific criteria must be met; optimization of the number of viable cells in Formulation is one of them. Liquid or semisolid formulations are the most common forms of probiotic products available in the market. The probiotic products carrying definite probiotic strain are developed in various formulations like fermented milk [Citation116], sachets [Citation117], chewing gum [Citation118], and capsules [Citation119]. The fate of probiotic activity depends on several factors such as pH, storage temperature, evaporation, oxygen, humidity, hydrogen peroxide, etc.
The temperature has a significant impact on bacterial strain growth, as is well documented. Bacterial strains are inactivated by low temperatures and killed by high temperatures. Thus, the temperature has a significant role in the probiotic microbial activity, as has been described by many researchers. In conjunction with the increase in temperature, there is also an increase in activity levels of various strains. Too much heat, on the other hand, renders the bacteria’s enzyme activity inactive. As described by [Citation120], several factors in probiotics are affected by temperature. They showed that the fermentation time needed to reach the required H+ ion concentration is proportional to the temperature. The higher the temperature, the lower the fermentation time required to achieve the necessary H+ ion concentration. Similarly, the titratable acidity of the probiotic is also inversely proportional to the temperature at which the fermentation is carried out [Citation121].stated that the biomass concentration produced by the probiotic bacteria during fermentation is also temperature-dependent. They showed that the Lactobacillus casei species showed higher biomass concentration than other lactobacillus species, notably at 35°C and 37°C [Citation122], showed the effect of temperature on the growth of bacteria and the production of the acid in the goat yogurt probiotic. Overall, the cumulative bacterial count grew significantly within 3 hours and slowed down at each temperature over the next 24 hours. The overall bacterial counts were 1.26, 1.36, 1.8, 1.85, and 1.65 (109 CFU/mL) at 37, 39, 41, 43, and 45°C, respectively. The acidity was seen to be moderate at 43°C. It was also seen that the probiotic (yogurt) had a strong smell if it was fermented at a lower temperature (which is 37°C) and had a good taste and state if fermented at a higher temperature. The development of beverage formulation as a probiotic carrier is a complicated process. Designing a product with probiotics is crucial for a strain to persist low and high pH. The most critical factor is that the probiotic must first survive the unfavorable effect of the gastric pH and then bile salts in the small intestine to ensure maximum health benefits. To survive, various techniques are used; microencapsulation is used to survive the stomach’s acidic pH [Citation123]. Probiotic strains have different survival rates at different pH; for example, Lactobacillus reuteri DSM 17938 when cultured at pH 6.5, the survival rate of cells was highest while at pH 4.5, the survival rate was lowest [Citation124] while in the case of Lactobacillus rhamnosus cells when grown at pH 5.0 found to have better survival rate than grown at pH 5.8 [Citation125].
8. Conventional fermented fruits, vegetables and beverages of India as a source of probiotic/functional food ingredients
Conventional fermented foods and beverages are widely consumed and have been a source of dietary nutrition since the dawn of time. It may be made using reasonably rudimentary procedures and tools in the home or a cottage enterprise [Citation126,Citation127]. Fermentation is a cost-effective and ancient food preparation and storage [Citation128]. Thus fermented dishes and beverages have long been popular in India. Fermented foods made from regional food crops and other biological resources are prevalent throughout the Indian subcontinent. However, the product’s composition and the underlying ingredient vary by area [Citation129]. A vast spectrum of fermented vegetable products is produced in India’s eastern Himalayan areas for bioprocessing biodegradable vegetables for preservation and later ingestion. Sinki, a native fermented radish taproot pit fermented product of Sikkim Himalayas, is a distinctive variety of lactic acid fermentation found in the food biopreservation. Sinki fermentation is accomplished by a variety of LAB, including Leuconostoc plantarum, Leuconostoc brevis, Leuconostoc casei, and Leuconostoc fallax [Citation130]. Another typical fermented food found in India is Khalpi, a fermented cucumber product popular among Sikkim’s Brahmin Nepalis [Citation131]. The microorganisms isolated from Khalpi fermentation comprise L. Plantarum, Leuconostoc fallax and L. Brevis [Citation132]. In 2018, Singh et al. demonstrated the molecular characterization of isolated bacterial strains from Manipur’s Utonga-kupsu, Hentak, and Ngari fish species. The researchers found bacillus and Staphylococcus species with significant antibacterial and probiotic capabilities in all three fermented fish products. Antimicrobial, antioxidant, and probiotic properties are all present in the isolated product HNS60. Another example of a conventional fermented food product is Soidon; a fermented bamboo shoot tip is a staple food of Manipur made by the Meitei women. The process for preparing it begins with collecting Bamboo shoots from the shoot apex of Teinostachyum wightii, a bamboo species. The bottom sections of the casing, as well as the exterior housings, are removed. The entire tips are buried in a clay pot of water. The prior batch’s soidon, or sour liquid, is reintroduced as a starter (1:1 dilution) and kept for fermentation for 3–7 days at room temperature. The leaves of Garcinia pedunculata Roxb. (heibung, a local name) are added during the fermentation stage to improve the flavor of soidon [Citation133]. The fermented soidon consists of the LAB’s of L. Brevis, Lactococcus lactis and Leuconostoc fallax [Citation132]. Besides these, India’s prominent fermented foods comprise Mesu, Ngari, Ziang-sang, Ipoh, Gnocchi, Kinema, Bekang, etc. These are rich in microbial strains like Bacillus sp., Saccharomyces cerevisiae, Candida sp. and Lactobacillus sp [Citation134].
The use of nondairy probiotic fermentation is possible for sweet lime and sugarcane juice [Citation135]. Different products have been used to make juice enriched with phytochemical herbs such as Ashwagandha (Camellia sinensis), Oat, Green Tea (Camellia sinensis), and whey. Sweet lime and cane juice serve as a functional probiotic drink for lactose intolerant individuals. Kanji, a functional non-milk-based probiotic drink of Indian origin made of carrots and beetroot, is one of the rich sources of β-carotene, tocopherol, and ascorbic acid. The fermentation is mediated by the lactic acid bacteria, i.e., L. plantarum, L. casei and L. acidophilus. Haria is a cultural beverage produced in eastern-central India by the fermentation of milk [Citation64]. Handia, fermented cooked rice, is a wine; undistilled rice beverage consumed in India predominantly by tribals [Citation136]. Other rice-based beverages like Chhang [Citation137] and Chicha [Citation138] are also produced in India. Palm wine is a traditional drink made with the sap of various palm trees consumed in India. Cauliflower, mustard, and radish using probiotic bacteria Pediococcus sp. and Lactobacillus sp. produce Gundruk (Probiotic drink) in India [Citation132,Citation139].
9. Future perspectives
Today, probiotics can be found in a wide variety of food items and drinks, and it is a rapidly expanding industry that has broadened its horizons attributed to the reason that health issues have become an ongoing concern, drawing the attention of the consumers to these functional foods [Citation140]. The present world is besieged by the lethal COVID-19 outbreak triggered by a novel coronavirus that puts forth the importance and awareness of ‘Immunity-based supplements,’ which can boost the proposed functional food-based beverage technology. Due to such prevalent reasons, there has been more discovery and development in conventional and modern generation probiotic foods and beverages. In the not-too-distant future, there is a high probability of more probiotic-based food supplements and drinks. With this imminent emerging prospect of probiotics as potential food, the primary consideration to be taken into account is health and safety. For decades, the basic principle of using probiotics has been that they do more benefit than harm to human health. Still, the advent of specific welfare issues, especially with the growing number of probiotic strains, has triggered a safety examination. For the concern expressed about the strain type, it would be safe to say that the strain used in making probiotic beverages must meet specific requirements imposed by the probiotic beverage’s composition. The development of probiotics is usually undesirable in the case of non-fermented drinks. However, this can be quite the opposite in the case of fermented beverages, where these probiotics prove to be very beneficial. As a result, selecting the appropriate probiotic strain for a particular food application makes a significant contribution. Therefore, choosing a strain with strong technical properties would be critical [Citation141]. However, when it comes to strain selection, the essential protection criteria include that it must be of human origin, which has been sequestered from the human gastrointestinal tract. Also, they must not be infective or have any virulent factor, must not facilitate any disease-related behaviors, and at last, they must not contain any antibiotic resistance gene which can be transferred. Aside from these health and safety concerns, a regulatory mechanism that aims to protect consumers from false, inaccurate, and deceptive statements and harmonize the trading market is also an essential requirement [Citation142].
10. Conclusion
The industrial probiotic products indubitably depict the most appropriate and the simplest way for improving the daily intake of dairy and nondairy-based beverages. The global market has shown increased sales of probiotic beverages in the last few years, especially dairy-based products. For the production of probiotic beverages, there is a potential to use cereals, vegetables, and fruits as substrates. Even so, a lot of study and research is required in the field regarding juices. Each country has a different set of rules and guidelines for the approval of human and clinical trials. To be used for effective industrial production, it is necessary to conduct relevant tests and understand a specific probiotic strain’s property and metabolite production. Although the specification and policies are continuously improving with time, the rapidly growing market of probiotic-based beverages demands more rigorous attempts to assure the safety of consumers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- James PT, Ali Z, Armitage AE, et al. Could nutrition modulate COVID-19 susceptibility and severity of disease? A systematic review. medRxiv. 2020. DOI:10.1101/2020.10.19.20214395
- Aman F, Masood S. How nutrition can help to fight against COVID-19 pandemic. Pak J Med Sci. 2020;36(COVID19–S4):S121–S123.
- Flach J, van der Waal MB, van Den Nieuwboer M, et al. The underexposed role of food matrices in probiotic products: reviewing the relationship between carrier matrices and product parameters. Crit Rev Food Sci Nutr. 2018;58:2570–2584.
- Annunziata A, Vecchio R. Consumer perception of functional foods: a conjoint analysis with probiotics. Food Qual Prefer. 2013;28(1):348–355.
- Corbo MR, Bevilacqua A, Petruzzi L, et al. Functional beverages: the emerging side of functional foods: commercial trends, research, and health implications. Compr Rev Food Sci Food Saf. 2014;13(6):1192–1206.
- Granato D, Barba FJ, Bursać Kovačević D, et al. Functional foods: product development, technological trends, efficacy testing, and safety. Annu Rev Food Sci Technol. 2020;11:93–118.
- Shi LH, Balakrishnan K, Thiagarajah K, et al. Beneficial properties of probiotics. Trop Life Sci Res. 2016;27(2):73.
- Noomhorm A, Ahmad I, Anal AK. Functional foods and dietary supplements: processing effects and health benefits. First ed. United States: John Wiley & Sons; 2014.
- Cordeiro M, Souza E, Arantes R, et al. Fermented whey dairy beverage offers protection against Salmonella enterica sp. enterica serovar Typhimurium infection in mice. J Dairy Sci. 2019;102(8):6756–6765.
- Khaneghah AM, Abhari K, Eş I, et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: a review. Trends Food Sci Technol. 2020;95:205–218.
- Galdeano CM, Cazorla SI, Dumit JML, et al. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019;74(2):115–124.
- Pino A, de Angelis M, Chieppa M, et al. Gut microbiota, probiotics and colorectal cancer: a tight relation. World J Cancer Res. 2020;7:e1456.
- Oak SJ, Jha R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr. 2019;59(11):1675–1683.
- Shafi A, Naeem RH, Farooq U, et al. Antimicrobial and antidiabetic potential of synbiotic fermented milk: a functional dairy product. Int J Dairy Technol. 2019;72:15–22.
- Guimarães JT, Balthazar CF, Silva R, et al. Impact of probiotics and prebiotics on food texture. Curr Opin Food Sci. 2020;33:38–44.
- Roobab U, Batool Z, Manzoor MF, et al. Sources, formulations, advanced delivery and health benefits of probiotics. Curr Opin Food Sci. 2020;32:17–28.
- Anal AK. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: a review. Fermentation. 2019;5(1):8.
- Zucko J, Starcevic A, Diminic J, et al. Probiotic–friend or foe? Curr Opin Food Sci. 2020;32:45–49.
- Kalicka D, Znamirowska A, Pawlos M, et al. Physical and sensory characteristics and probiotic survival in ice cream sweetened with various polyols. Int J Dairy Technol. 2019;72(3):456–465.
- Linares DM, Gomez C, Renes E, et al. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front Microbiol. 2017;8:846.
- Katan M. Why the European food safety authority was right to reject health claims for probiotics. Benef Microbes. 2012;3(2):85–89.
- de Simone C. The unregulated probiotic market. Clin Gastroenterol Hepatol. 2019;17(5):809–817.
- Melchor SR, Skoblikov L, Urazbaeva SBA, et al. The regulatory framework for the use of probiotics claims in food and food supplements around the world: a comparative analysis. Funct Field Food Law: Reconcil Market Human Rights. 2019;11:155–169.
- Anwar AA, Thikra AA, Saeed AM. Adhesion, autoaggregation and hydrophobicity of six Lactobacillus strains. Br Microbiol Res J. 2014;4:381–391.
- Food and Agriculture Organization of the United Nations/World Health Organization FAO/WHO. Guidelines for the evaluation of probiotics in food; Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food: London, Ontario, Canada; 2002.
- Seale JV, Millar M. Probiotics: a new frontier for infection control. J Hosp Inf. 2013;84:1–4.
- Tulumoglu S, Yuksekdag ZN, Beyatli Y, et al. Probiotic properties of Lactobacilli species isolated from children’s feces. Anaerobe. 2013;24:36–42.
- Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic- associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959–1969.
- Ruiz L, Margolles A, Sánchez B. Bile resistance mechanisms in lactobacillus and bifidobacterium. Front Microbiol. 2013;4:396.
- Zelaya H, Tsukida K, Chiba E, et al. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation-coagulation interactions. Int Immunopharmacol. 2014;19:161–173.
- Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11(5):4745–4767.
- McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97–105.
- Witthuhn RC, Schoeman T, Britz TJ. Characterisation of the microbial population at different stages of Kefir production and Kefir grain mass cultivation. Int Dairy J. 2005;15:383–389.
- Marsh AJ, Hill C, Ross RP, et al. Fermented beverages with health promoting potential: past and future perspectives. Trends Food Sci Technol. 2014;38(2):113–124.
- Larsen N, Thorsen L, Kpikpi EN, et al. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl Microbiol Biotechnol. 2013;98(3):1105–1118.
- Zokaeifar H, Babaei N, Saad CR, et al. Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2014;36:68–74.
- Hosoi T, Hirose R, Saegusa S, et al. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int J Food Microbiol. 2003;82:255–264.
- Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010;16:69–75.
- Behnsen J, Deriu E, Sassone-Corsi M, et al. Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med. 2013;3(3):a010074.
- Xia P, Zhu J, Zhu G. Escherichia coli Nissle 1917 as safe vehicles for intestinal immune targeted therapy—A review. Acta Microbiol Sin. 2013;53:538–544.
- Azcarate-Peril MA, Sikes M, Bruno-Barcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401–G424.
- Ly NP, Litonjua A, Gold DR, et al. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127:1087–1094.
- Sleator RD, Hill C. New frontiers in probiotic research. Lett Appl Microbiol. 2008d;46:143–147.
- Amalaradjou MAR, Bhunia AK. Bioengineered probiotics, a strategic approach to control enteric infections. Bioengineered. 2013;4(6):379–387.
- Sleator RD, Hill C. Battle of the bugs. Science. 2008;321:1294–1295.
- Wells J. Mucosal vaccination and therapy with genetically modified lactic acid bacteria. Annu Rev Food Sci Technol. 2011;2:423–445.
- Culligan EP, Hill C, Sleator RD. Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut Pathog. 2009;1:19.
- Sleator RD. Probiotics: a viable therapeutic alternative for enteric infections especially in the developing world. Discov Med. 2010d;10:119–124.
- Sleator RD. Designer probiotics: development and applications in gastrointestinal health. World J Gastrointest Pathophysiol. 2015a;6:73–78.
- Sleator RD, Hill C. Designer probiotics: a potential therapeutic for Clostridium difficile? J Med Microbiol. 2008b;57:793–794.
- Aakko J, Sanchez B, Gueimonde M, et al. Assessment of stress tolerance acquisition in the heat- ´ tolerant derivative strains of Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus rhamnosus GG. J Appl Microbiol. 2014;117:239–248.
- Li Chen Q, Ruan H, Zhu D, et al. Isolation and characterisation of an oxygen, acid and bile resistant Bifidobacterium animalis subsp. lactis Qq08. J Sci Food Agric. 2010;90:1340–1346.
- Broadbent JR, Larsen RL, Deibel V, et al. Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J Bacteriol. 2010;192:2445–2458.
- Paton AW, Morona R, Paton JC. Designer probiotics for prevention of enteric infections. Nat Rev Microbiol. 2006;4:193–200.
- Shori AB. Influence of food matrix on the viability of probiotic bacteria: an overview based on dairy and non-dairy beverages. Food Biosci. 2016;13:1–8.
- Parker MN, Lopetcharat K, Drake MA. Consumer acceptance of natural sweeteners in protien beverages. J Dairy Sci. 2018;101(10):8875–8889.
- Özer B, Kirmaci HA. Functional milks and dairy beverages. Int J Dairy Technol. 2010;63(1):1–15.
- Granato D, Branco GF, Cruz AG, et al. Probiotic dairy foods as functional foods. Compre Rev Food Sci Food Saf. 2010;9:455–470.
- Nadelman P, Monteiro A, Balthazar CF, et al. Probiotic fermented sheep’s milk containing Lactobacillus casei 01: effects on enamel mineral loss and Streptococcus counts in a dental biofilm model. J Funct Foods. 2019;54:241–248.
- Özer B, Kirmaci HA. Technological and health aspects of probiotic cheese. In: Foster RD, editor. Cheese: types, nutrition and consumption. NY, USA: Nova Science Publishers; 2011. p. 1–42.
- Panda SK, and Shetty PH. Innovations in technologies for fermented food and beverage industries. Cham, Switzerland: Springer; 2018.
- Ranadheera CS, Naumovski N, Ajlouni S. Non-bovine milk products as emerging probiotic carriers: recent developments and innovations. Curr Opin Food Sci. 2018;22:109–114.
- Sarfraz F, Farooq U, Shafi A, et al. Hypolipidaemic effects of synbiotic yoghurt in rabbits. Int J Dairy Technol. 2019;72:545–550.
- Kandylis P, Pissaridi K, Bekatorou A, et al. Dairy and non-dairy probiotic beverages.Curr. Opin Food Sci. 2016;7:58–63.
- Sahu L, and Panda SK. Innovative technologies and implications in fermented food and beverage industries: an overview. In: Panda SK, and Shetty PK, editors. Innovations in technologies for fermented food and beverage industries. Cham, Switzerland: Springer; 2018. p. 1–23.
- Dong JY, Szeto IM, Makinen K, et al. Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1188–1194.
- Buriti FCA, Freitas SC, Egito AS, et al. Effects of tropical fruit pulps and partially hydrolysed galactomannan from Caesalpinia pulcherrima seeds on the dietary fibre content, probiotic viability, texture and sensory features of goat dairy beverages. LWT-Food Sci Technol. 2014;59:196–203.
- Castro WF, Cruz AG, Bisinotto MS, et al. Development of probiotic dairy beverages: rheological properties and application of mathematical models in sensory evaluation. J Dairy Sci. 2013;96:16–25.
- Pescuma M, Hébert EM, Mozzi F, et al. Functional fermented whey-based beverage using lactic acid bacteria. Int J Food Microbiol. 2010;141:73–81.
- Fluegel SM, Shult TD, Powers JR, et al. Whey beverages decrease blood pressure in prehypertensive and hypertensive young man and women. Int Dairy J. 2010;20(11):753–760.
- Londero A, Hamet MF, De Antoni GL, et al. Kefir grains as a starter for whey fermentation at different temperatures: chemical and microbiological characterisation. J Dairy Res. 2012;79:262–271.
- Shiby VK, Mishra HN. Fermented milks and milk products as functional foods—a review. Crit Rev Food Sci Nutr. 2013;53(5):482–496.
- Antunes AEC, Silva ERA, Agf VD, et al. Probiotic buttermilk-like fermented milk product development in a semiindustrial scale: physicochemical, microbiological and sentsory acceptability. Int J Dairy Technol. 2009;62(4):556–563.
- Martins EMF, Ramos, AM, and Vanzela, ESL, et al. Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51(2):764–770.
- Fonteles TV, Garcia M, Costa M. Stability and quality parameters of probiotic cantaloupe melon juice produced with sonicated juice. Food Bioprocess Technol. 2013;6:2860–2869.
- Kyung YY, Woodams E, Hang E, et al. Fermentation of beet juice by beneficial lactic acid bacteria. LWT Food Sci Technol. 2005;38(1):73–75.
- Pereira ALF, Maciel TC, Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res Int. 2011;44(5):1276–1283.
- Anderson RC, Cookson AL, McNabb WC, et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316.
- Dunne C. From biocontrol to cancer, probiotics and beyond. Bioengineered. 2013;4(4):185–190.
- Wang M, Liu P, Kong L, et al. Promotive effects of sesamin on proliferation and adhesion of intestinal probiotics and its mechanism of action. Food Chem Toxicol. 2021;149:112049.
- Stetinova V, Smetanova L, Kvetina J, et al. Caco-2 cell monolayer integrity and effect of probiotic Escherichia coli Nissle 1917 components. Neuro Endocrinol Lett. 2010;31:51–56.
- Zyrek AA, Cichon C, Helms S, et al. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKC redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816.
- Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann NY Acad Sci. 2006;1072:242–252.
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407.
- Mack DR, Ahrne S, Hyde L, et al. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833.
- Mattar AF, Teitelbaum DH, Drongowski RA, et al. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18:586–590.
- Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, et al. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–174.
- Chang YH, Jeong CH, Cheng WN, et al. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J Dairy Sci. 2021;104(7):7415–7425.
- Linares DM, Ross P, Stanton C. Beneficial Microbes: the pharmacy in the gut. Bioengineered. 2016;7(1):11–20.
- Plaza-Díaz J, Ruiz-Ojeda FJ, Gil-Campos M, et al. Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients. 2018;10(1):42.
- Yadav AK, Tyagi A, Kumar A, et al. Adhesion of lactobacilli and their anti-infectivity potential. Crit Rev Food Sci Nutr. 2017;57:2042–2056.
- Collado MC, Gueimonde M, Hernández M, et al. Adhesion of selected Bi-fidobacterium strains to human intestinal mucus and the role of adhesion in entero-pathogen exclusion. J Food Prot. 2005;68:2672–2678.
- González-Rodríguez I, Sánchez B, Ruiz L, et al. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl Environ Microbiol. 2012;78:3992–3998.
- Zadravec P, Štrukelj B, Berlec A. Heterologous surface display on lactic acid bacteria: non-GMO alternative? Bioengineered. 2015;6(3):179–183.
- Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330.
- Furrie E, Macfarlane S, Kennedy A, et al. Synbiotic therapy (Bifidobacterium longum /Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249.
- Bals R, Wilson JM. Cathelicidins – a family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60:711–720.
- Kurian SJ, Unnikrishnan MK, Miraj SS, et al. Probiotics in prevention and treatment of COVID-19: current perspective and future prospects. Arch Medical Res. 2021;52(6):582–594.
- Collado MC, Gueimonde M, Salminem S. Probiotics in adhesion of pathogens: mechanisms of action. In: Watson RR, Preedy VR, editors. Bioactive foods in promoting health: probiotics and prebiotics. 1st ed. London: Academic Press, Elsevier; 2010. p. 353–370.
- Muñoz-Quezada S, Bermudez-Brito M, Chenoll E, et al. Competitive inhibition of three novel bacteria isolated from faeces of breast milk-fed infants against selected enteropathogens. Br J Nutr. 2013;109:S63–S69.
- Kumar M, Nagpal R, Verma V, et al. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev. 2013;71:23–34.
- Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbicidal alphadefensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118.
- D’Amelio P, Sassi F. Gut microbiota, immune system, and bone. Calcif Tissue Int. 2017;102(4):415–425.
- Gómez-Llorente C, Muñoz S, Gil A. Role of toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc. 2010;69:381–389.
- Claes AK, Zhou JY, Philpott DJ. NOD-like receptors: guardians of intestinal mucosal barriers. Physiology (Bethesda). 2015;30:241–250.
- Fernandez M, Valenti V, Rockel C, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–1059.
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, et al. Mechanisms of action of probiotics. Adv Nutr. 2020;10(1):S49–S66.
- Ishida S. Organs-on-a-chip: current applications and consideration points for in vitro ADME-Tox studies. Drug Metabol Pharmaco. 2018;33(1):49–54.
- Plaza-Diáz J, Fernándéz-Caballero JA, Chueca N, et al. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients. 2015;7:3999–4015.
- Lye HS, Rusul G, Liong MT. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J Dairy Sci. 2010;93:1383–1392.
- Cordeiro BF, Oliveira ER, Da Silva SH, et al. Whey protein isolate-supplemented beverage, fermented by Lactobacillus casei BL23 and Propionibacterium freudenreichii 138, in the prevention of mucositis in mice. Front Microbiol. 2018;9:2035.
- FAO/WHO. Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; 2002.
- Zielińska D, Sionek B, Kołożyn-Krajewska D. Safety of probiotics. In: Grumezescu A, Holban AM, editors. Diet, microbiome and health. New York: Elsevier; 2018. p. 131–161.
- Sanders ME, Tompkins T, Heimbach JT, et al. Weight of evidence needed to substantiate a health effect for probiotics and prebiotics. Eur J Nutr. 2005;44(5):303–310.
- Gupta RC. Nutraceuticals: efficacy, safety and toxicity. First ed. United States: Academic Press; 2016.
- Lavermicocca P. Highlights on new food research. Digest Liv Dis. 2006;38(2):S295–S299.
- Cruywagen CW, Jordann I, Venter L. Effect of Lactobacillus acidophilus supplement of milk replacer on preweaning performance of calves. J Dairy Sci. 1996;79:483–486.
- Caglar E, Kavaloglu SC, Kuscu OO, et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Invest. 2007;11:425–429.
- Bruno FA, Shah NP. Viability of two freeze-dried strains of Bifidobacterium and of commercial preparations at various temperatures during prolonged storage. J Food Sci. 2003;68:2336–2339.
- Nguyen HTH, Ong L, Kentish SE, et al. The effect of fermentation temperature on the microstructure, physicochemical and rheological properties of probiotic buffalo yoghurt. Food Bioprocess Technol. 2014;7:2538–2548.
- Mustafa SM, Chua LS, El-Enshasy HA, et al. Effect of temperature and pH on the probiotication of Punica granatum juice using Lactobacillus species. J Food Biochem. 2019;43:e12805.
- Shu G, Li C, Chen H, et al. Effect of inoculum and temperature on the fermentation of goat yogurt. Adv J Food Sci Technol. 2014;1:68–71.
- Alemzadeh E, Oryan A. Application of encapsulated probiotics in health care. J Exp Path. 2020;1(1):16–21.
- Hernández A, Larsson CU, Sawicki R, et al. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. AMB Exp. 2019;9(1):66.
- Saarela MH, Alakomi HL, Puhakka A, et al. Effect of the fermentation pH on the storage stability of Lactobacillus rhamnosus preparations and suitability of in vitro analyses of cell physiological functions to predict it. J Appl Microbiol. 2009;106(4):1204–1212.
- Aidoo KE, Nout NJR, Sarkar PK. Occurrence and function of yeasts in Asian indigenous fermented foods. FEMS Yeast Res. 2006;6(1):30–39.
- Ilango S, Antony U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food SciTechnol. 2021;118(A):617–638.
- Tamang JP, Watanabe K, Holzapfel WH. Review: diversity of microorganisms in global fermented foods and beverages. Front Microbiol. 2016;7:377.
- Sekar S, Mariappan S. Usage of traditional fermented products by Indian rural folks and IPR. Indian J Tradit Knowl. 2007;6(1):111–120.
- Tamang JP, Tamang B, Schillinger U, et al. Identification of predominant lactic acid bacteria isolated from traditionally fermented vegetable products of the Eastern Himalayas. Int J Food Microbiol. 2005;105(3):347–356.
- Yan PM, Xue WT, Tan SS, et al. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control. 2008;19(1):50–55.
- Tamang JP. Himalayan fermented foods: microbiology, nutrition and ethnic values. New Delhi, India: CRC Press; 2009.
- Behera P, Balaji S. Health benefits of fermented bamboo shoots: the twenty-first century green gold of Northeast India. Appl Biochem Biotechnol. 2021;193(6):1800–1812.
- Das AJ, Deka SC. Fermented foods and beverages of the North-East India. Int Food Res J. 2012;19(2):377–392.
- Khatoon N, Gupta RK. Probiotics beverages of sweet lime and sugarcane juices and its physiochemical, microbiological & shelf-life studies. J Pharmacogn Phytochem. 2015;4(3):25–34.
- Panghal A, Janghu S, Virkar K, et al. Potential non-dairy probiotic products – a healthy approach. Food Biosci. 2018;21:80–89.
- Thakur N, Pej SS, Bhalla TC. Microorganisms associated with amylolytic starters and traditional fermented alcoholic beverages of North Western Himalayas in India. Food Biosci. 2015;11:92–96.
- Puerari C, Magalha˜ es-Guedes K, Rf S. Physicochemical and microbiological characterization of chicha, a rice-based fermented beverage produced by Umutina Brazilian Amerindians. Food Microbiol. 2015;46:210–217.
- Dahal NR, Karki TB, Swamylingappa B, et al. Traditional foods and beverages of Nepal—a review. Food Rev Int. 2005;21(1):1–25.
- Aguilar CN, Ruiz HA, Rios AR, et al. Emerging strategies for the development of food industries. Bioengineered. 2019;10(1):522–537.
- Saarela M. Probiotics as ingredients. In: Paquin P, editor. Functional beverages, Functional and speciality beverage technology. Sawston, United Kingdom: Woodhead Publishing; 2009. p. 55–70.
- Koirala S, Anal AK. Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims. Fut Foods. 2021;3:100013.
- Turkmen N, Akal C, Özer B. Probiotic dairy-based beverages: a review. J Funct Food. 2019;53:62–75.
- Banerjee D, Hassarajani SA, Maity B, et al. Comparative healing property of kombucha tea and black tea against indomethacin-induced gastric ulceration in mice: possible mechanism of action. Food Funct. 2010;1:284–293.
- Yang J, Huang K, Qin S, et al. Antibacterial action of selenium-enriched probiotics against pathogenic Escherichia coli. Dig Dis Sci. 2009;54:246–254.
- Yang ZW, Ji BP, Zhou F, et al. Hypocholesterolaemic and antioxidant effects of kombucha tea in high-cholesterol fed mice. J Sci Food Agric. 2009;89:150–156.
- Adriani L, Mayasari N, Kartasudjana RA. The effect of feeding fermented kombucha tea on HLD, LDL and total cholesterol levels in the duck bloods. Biotechnol Anim Husb. 2011;27:1749–1755.
- Jayabalan R, Chen PN, Hsieh YS, et al. Effect of solvent fractions of kombucha tea on viability and invasiveness of cancer cells—characterization of dimethyl 2-(2-hydroxy-2- methoxypropylidine) malonate and vitexin. Indian J Biotechnol. 2011;10:75–82.
- Sato J, Kanazawa A, Azuma K, et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci Rep. 2017;7:12115.
- Tanida M, Takada M, Kato-Kataoka A, et al. Intragastric injection of Lactobacillus casei strain Shirota suppressed spleen sympathetic activation by central corticotrophin-releasing factor or peripheral 2-deoxy-d-glucose in anesthetized rats. Neurosci Lett. 2016;619:114–120.
- Toghyani M, Mosavi SK, Modaresi M, et al. Evaluation of kefir as a potential probiotic on growth performance, serum biochemistry and immune responses in broiler chicks. Animal Nutri. 2015;1(4):305–309.
- Punaro GR, Maciel FR, Rodrigues AM, et al. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide. 2014;37:53–60.
- Adiloǧlu AK, Gönülateş N, Işler M, et al. The effect of kefir consumption on human immune system: a cytokine study. Bir Sitokin Çalişmasi. 2013;47:273–281.
- Ostadrahimi A, Taghizadeh A, Mobasseri M, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health. 2015;44:228–237.
- Rosa D, Dias M, Ł G, et al. Milk kefir: nutritional, microbiological and health benefits. Nutri Res Rev. 2017;30(1):82–96.
- Hartajanie L, Fatimah-Muis S, and Heri-Nugroho K, et al. Probiotics fermented bitter melon juice as promising complementary agent for diabetes type 2: study on animal model. J Nutri Metabol. 2020; 2020 :1–7.
- Yasmin A, Butt MS, van Baak M, et al. Supplementation of prebiotics to a whey-based beverage reduces the risk of hypercholesterolaemia in rats. Int Dairy J. 2015;48:80–84.
- Hernandez-Mendoza A, Robles VJ, Angulo JO, et al. Preparation of a whey-based probiotic product with Lactobacillus reuteri and Bifidobacterium bifidum. Food Technol Biotechnol. 2007;45:27–31.
- Borowicki A, Michelmann A, Stein K, et al. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr Cancer. 2011;63:151–160.
- Rafter J, Bennett M, Caderni G, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–496.
- Skopinska E, Lachowicz D, Wultanska D, et al. Assessment of antagonistic activity in vitro Lactobacillus spp. strains againtsClostridum difficile strains isolated from gastrointestinal tract of patients hospitalized in three hospitals in region Mazovia. Med Dosw Mikrobiol. 2012;64:109–114.
- DeVries L, Horstman C, Fossell M, et al. Ingestion of Bifidobacterium longum changes miRNA levels in the brains of mice. PLoS ONE. 2021;16(4):e0249817.
- Baldwin C, Millette M, Oth D, et al. Probiotic lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer. 2010;62:371–378.
- Desrouillères K, Millette M, Vu KD, et al. Cancer preventive effects of a specific probiotic fermented milk containing Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 on male F344 rats treated with 1,2-dimethylhydrazine. J Funct Foods. 2015;17:816–827.
- Merenstein D, Murphy M, Fokar A, et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: the DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010;64(7):669–677.