ABSTRACT
DNA methylation is a process in which methyl (CH3) groups are added to the DNA molecule. The DNA segment does not change in the sequence, but DNA methylation could alter the action of DNA. Different enzymes like DNA methyltransferases (DNMTs) take part in methylation of cytosine/adenine nucleosides in DNA. In prokaryotes, DNA methylation is performed to prevent the attack of phage and also plays a role in the chromosome replication and repair. In fungi, DNA methylation is studied to see the transcriptional changes, as in insects, the DNA methylation is not that well-known, it plays a different role like other organisms. In mammals, the DNA methylation is related to different types of cancers and plays the most important role in the placental development and abnormal DNA methylation connected with diseases like cancer, autoimmune diseases, and rheumatoid arthritis.
Introduction
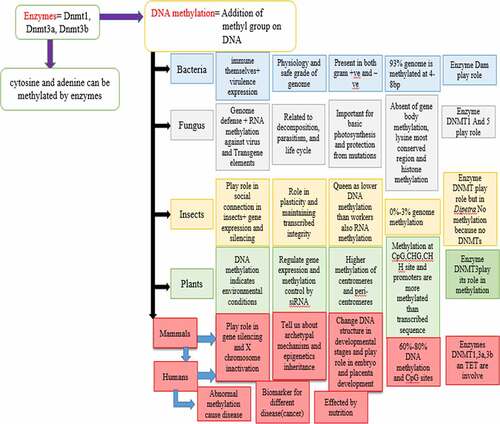
In the process of DNA methylation, methyl groups are added to the base pairs cytosine and adenine at the fifth position of the nucleoside in double-stranded DNA, at different sites like CpG, CHG, and CHH, DNA methylation takes place, and by using this process, the DNA molecule activity is changed easily without interrupting its sequence [Citation1]. The main methylation process widely used in prokaryotes and eukaryotes is the methylation of 5-methylcytosine. Two of the DNA’s four bases cytosine and adenine can be methylated. Its main importance is in the study of gene regulation, gene silencing, gene activation, and many other functions like cell differentiation [Citation2]. The catalyzation of this process is done by the enzymes methyltransferases (Dnmt1, Dnmt3a, and the third one is Dnmt3b), which have different functions.
Two ways of DNA demethylation are as follows:
An enzymatic process, which leads to the removal of the methyl group from 5mC, is called DNA demethylation.
In opposite passive DNA demethylation is the one which lack maintain methylation throughout successive DNA replication either in the absence of Dnmt1 or because of its inhibition and patterns in methylation are overall methylation patterns Methylation of specific genes, tissue-specific methylation pattern. DNA markers are introduced by these methylase enzymes, which give hints for a diversity of processes that include physiological processes of cell cycle and gene expression in epigenetics control in bacteria and another organism [Citation3]. DNA methylation plays a significant role in the physiology and is examined in two methyltransferase models, from Alphaproteo bacteria CcrM methylase and from Gamma proteo bacteria Dam methylase [Citation4–7].
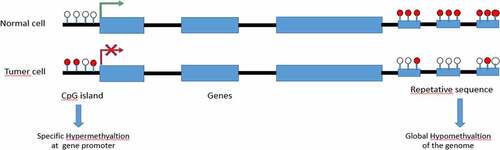
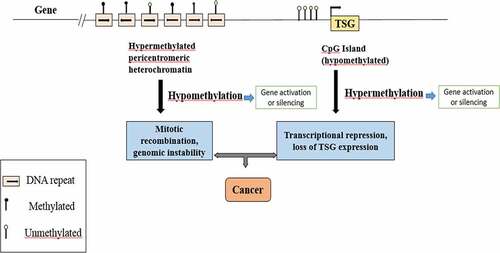
Graph.1; The diagram shows a representative region of genomic DNA in a normal cell. The region shown contains repeat-rich, hypermethylated pericentromeric heterochromatin and an actively transcribed tumor suppressor gene (TSG) associated with a hypomethylated CpG Island (indicated in red). In tumor cells, repeat-rich heterochromatin becomes hypomethylated and this contributes to genomic instability, a hallmark of tumor cells, through increased mitotic recombination events. De novo methylation of CpG Islands also occurs in cancer cells and can result in the transcriptional silencing of growth-regulatory genes. These changes in methylation are early events in tumorigenesis.
Due to DNA methylation, the chromatin structure can undergo changes directly and prevent transcription factor binding and methylation plays an important role in numerous cellular processes and components, including genomic imprinting, embryonic development, preservation of chromosome stability, and X-chromosome inactivation. DNA methylation also regulates transcription, chromosome replication, repair, and most possibly additional important processes. However, some motifs and DNA molecules are 100% methylated, while some motifs are nonmethylated [Citation8–11]. The researcher went through 230 species of bacterial and archaeal origin having varying genomes and got the evidence that 93% of genomes are methylated, while in fungi, the genome is methylated by DNA methyltransferase 1 (DNMT1) [Citation12] and DNA methyltransferase 5 (DNMT5) [Citation13] with unknown biological characteristics and methylation of cytosine (5-methylcytosine) 5mC has been concerned in defense of genome. By investigating insects’ DNA, methylation is mostly directed to broadly expressed genes [Citation14,Citation15]. But certain sets of insects have totally missing useful DNA methylation schemes and counting model dipterans [Citation14]. In insects, it is related to plasticity and regulates the phenotype of insects [Citation16–21]. Keeping in mind the global sustainability development goals to protect, restore, and use the sustainable use of ecosystem in which fungi and plants are major stock holder, we focus on both fungi and plants [Citation22]. In plants, animals, and fungi, the DNA methylation of histone at the nucleotide lysine is the most conserved marker that is correlated with the function of gene activation and gene silencing. In animals, 5mC is formed due to insertion or deletion of the methyl group in CG sites (CpG) due to which gene regulation is initiated [Citation23,Citation24]. All the sites methylated in the humans are done on the somatic cells [Citation25,Citation26].
DNA methylation in Bacteria:
DNA methylation is utilized as a basic tool for different studies in bacteria to know the functions of genes and their restriction/modification system [Citation27–29] by selective degradation of exogenous DNA of bacteriophage. Bacterial species immune themselves through the use of type II restriction-modification (R-M) systems, and different groups of prokaryotes immune their DNA from phage or extracellular DNA through methylation by sequence-specific restriction-modification (R-M) systems. In this system, DNA methyltransferases add the methyl group in bacterial DNA exactly at 4–8 base pairs (b.p.) and unmethylated DNA is degraded [Citation30]. Several other functions such as transcription, repair, chromosome replication, and most possible other essential procedures are regulated through that system. The regulation of transcription of few genes [Citation31–33] and chromosome replication [Citation5,Citation34] are regulated by Dam (DNA adenine methyltransferase) methylation in bacteria at GATC sequences (sequence at which methylation occurs). All GATC sequences of DNA are methylated at the 6th position of the adenine base in Escherichia coli, and this process is called dam methylation; for the role of dam methylation, one hypothesis is that mismatch repair enzymes are used to distinguish among new and old strands of DNA; at the replication fork, this process is followed by DNA methylation. To support this hypothesis, at the unmethylated strand of the heteroduplex DNA, mismatched bases are preferentially removed [Citation35,Citation36]. DNA adenine methyltransferase (Dam) methylates DNA in Escherichia coli at the adenine residue. Cellular functions such as DNA mismatch repair, gene transcription, nucleoid structure, and initiation of chromosome replication are influenced by the level of Dam and these methylated residues. Caulobacter crescentus CcrM enzymes and Escherichia coli Dam are the two best studied DNA methyltransferases. Caulobacter crescentus CcrM (cell cycle-regulated methyltrasferase) and Escherichia coli Dam and enzymes do not apparently relate to a restriction enzyme, which are the two best studied DNA methyltransferases.
In kingdoms bacteria and archaea, N6-methyl adenine (6 mA) has been found as a dynamic DNA epigenetic modification [Citation37]. N6-methyldeoxyadenosine (6 mA) is a type of DNA methylation, which has functional roles in living organisms, and DAMT1 is involved in it. Adenine methylation is catalyzed by DNA-adenine methyltransferase that adds the methyl group in adenine at the sixth position of the purine ring [Citation38,Citation39]. In bacteria, virulence gene expression is directly controlled by DNA adenine methylation [Citation31,Citation40]. Among the bacteria, some Gram-negative and Gram-positive organisms perform the phenomenon of DNA methylation. The best characterized orphan methyltransferase apart from Dam is CcrM. It is a necessary DNA methyltransferase of an aquatic bacterium with dimorphic nature. Both Dam and CcrM have the ability to catalyze the transmission of the methyl group from S-adenosylmethionin to the N-6 position of the adenine at a definite target sequence. However, they fit into distinct methyltransferase groups. There is also a difference in their target sequence such as GANTC for CcrM and GATC for DAM. In Escherichia coli, about 50 years ago, restriction modification (R-M) systems were first identified and are now known to be abundant among other bacterial species. Generally, two distinct enzyme activities are included in R-M systems: first, specific restriction endonuclease enzyme that cuts the DNA at a particular recoganization sequence and second, a transferase enzyme named DNA methyltransferase has the ability to methylate the DNA at the same particular sites. So DNA methyltransferase enzyme prevents the cleavage of DNA by the associated restriction enzyme. The type II R-M system includes two distinct types of proteins. These proteins possess independent enzymatic actions, endonucleases activity by restriction endonuclease enzyme, and DNA transferase activity by DNA methyl transferase [Citation41]. Not only the DNA methylation occurs, but also demethylase enzymes are catalyzed by the reversible modification (demethylation) [Citation42]. In bacteria, the methylation process works in bacterial physiology, making the bacteria safe from all other foreign antibacterial organisms like phages and R-M system.
(a) Role of DNA cytosine methylation in bacterial physiology
In bacterial genomics, T:G mismatches appear spontaneously due to deamination of C5-methylcytosine that creates thymine. The repair system in enteric bacteria precisely restores such mismatches, and it clearly appears for the establishment of the tricky side of C5-methylcytosine [Citation43]. An assumption was made that establishment of T:G mismatches could be a settlement for unidentified physiological paybacks of m5C. On the other hand, at least under laboratory conditions, phenotypic consequences in E. coli did not seem due to loss of the lonely methyltransferase Dcm [Citation44].
But recent studies propose that regulation of gene expression is a physiological role that may be due to DNA cytosine methylation. In H. pylori, gene expression, which was involved in adhesion, motility, and virulence, changes in the absence of an orphan C5-methylcytosine methyltransferase also called Hpy A – VIBM [Citation45], and in E.coli, expression of the stress response sigma factor Rpo S has increased in the absence of DNA cytosine methylation [Citation46]. Overexpression of a membrane transporter involves the ethidium bromide transporter found in E. coli dcm mutants [Citation47].
DNA methylation in Fungus
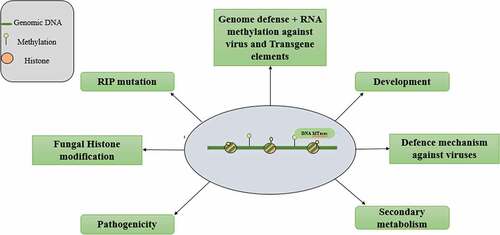
As camper to other eukaryotes, DNA methylation is less studied and less information is available about it in fungi. In the Uncinocarpus reesii, the DNA methylation is present in the noncoding region and in the coding region. It gives rise to the consensus that DNA methylation is initially dedicated to transposable elements (TEs), which are supposed to maintain the integrity of DNA in fungi [Citation12,Citation48], and there is negative correlation between the TE methylation level and TE expression as the more the TE methylation, the less the TE expression and vice versa. The DNA methylation of CpG positions plays a vital role in the conservation and strength of methylation designs, in usual and pathological circumstances, displaying that the DNA methylation position is extremely dependent on the native order CpG. This connection examines the DNA sequence related to methylation shapes in both pathological and physiological environments of fungus.
Currently, many research papers have concluded that different amounts of DNA methylation in different fungal species are present, according to which 0.22% DNA is methylated in Magnaporthe oryzae [Citation49], 0.38% to 0.42% genome is methylated in Metarhizium robertsii [Citation50], 1.8% in Ganoderma sinense [Citation51], 6.4%-7.7% genome is methylated in Cryphonectria parasitica [Citation52], and 36.9%-39.6% methylation of genome in Tuber melanosporum [Citation53]. More recent research showed that in fungi, the DNA methylation is around the genes [Citation49–52], which have more complex function than we have observed in the past; they are also present in more complicated and divergent patterns, for example, they show their role in defense of genome like in bacteria [Citation49,Citation51,Citation53], and also, research showed that they have dynamic epigenetic entity, play their role in the development of M.oryzea [Citation49,Citation50], and play role in the secondary metabolism and regulation of G. sinense [Citation51] and cell morphology delay in Candida Albicans [Citation54] by changing the transcriptional activities of related genes. DNA methylation is related to different lifestyles for example Decomposition, parasitism and life cycles of fungi, in H, parvipoerum 96,026 it is related to asexual (mycelia and conidia) lifestyle which was confirmed by Zhen Zeng et al in H, parvipoerum that DNA methylation have a role in the transcriptional regulation of asexual patterns, and N.carsaa before going to sexual cycle the spore fuses and before nuclear fusion take place a defense system is activated which works to prevent the repetitive sequencing such as TEs [Citation55].
(a) Fungal histone methylation distribution
In fungus, many novel enzymes are discovered, which can be easily studied because of their wide distribution, and also due to their high throughput sequencing, we came to know that most of the KMT genes in the kingdom mycota have the H3K4, H3K27, and H3K36 methylation; according to early capability, H3 potential lysine methylation cannot be absorbed in extant fungi [Citation56].
Methylation of histone is widespread among the hemiasco mycetes, best model organism for the understanding is S. cerevisiae don’t have H3K9, H3K27 and cytosine methylation, another model organism S. pombe lack H3K27 and methylation of histone but the specie Candida albicans don’t have H3K9 and H3K27 but they might have cytosine DNA methylation [Citation54].
In 2014, the DNA methyltransferase (DNMT5) was described, which methylates DNA in the basidiomycete a human pathogen C. neoforman [Citation13]. The genes that code for DNMT5 enzymes are distributed widely in the genome of fungi, which work as cytosine methylation, and new development and high-efficiency sequencing reveal the adenine methylation in the fungi; it is believed that adenine methylation is not present in fungi [Citation56]. In fungi, the loss of H3K27 methylation in a group with the loss of cytosine DNA is common and by comparison of structural SET domains, we came to know that no associations among lifestyle and ecology have occurred [Citation57].
In the future, research has to be carried out on the poorly understood taxa of fungi like chyrids and zygomycetes to know the histone modification and DNA methylation and process of these as they are very important and connected to plant and animals as well [Citation56]. Li et al. reveal that in C. parasitica, the influence of DNA methylation alters expression gene, as the RNA sequence shows that where genes are expressed lower and have higher methylation in the promoter region, DNA methylation takes place in the promoter and has a negative effect on gene expression [Citation58].
(b) Established gene body methylation is absent in fungi
In fungal genome, 5mC is not uniformly dispersed and 5mC occurs inside coding areas of the fungal genome, which is in divergence with CG DNA methylation enhancement in coding sequences of few species of insects and angiosperms as well and extremely preserved and expressed constitutively in some genes (gene body methylation) [Citation12,Citation59–62]. In species like Uncinocarpus reesii, a similar improvement has been observed at CH frameworks [Citation12]. This was confirmed in species U. reesii and will be continued in further species (A. bisporus, P. destructans, and C. cinerea) with confirmation of upgrading with the CG situation spending method of an enrichment used by these [Citation59,Citation63].
It was revealed that 5mC is need not be narrowed to coding areas. However, they showed that genes with 5mC DNA methylation that crossed genes and intergenic sequence enrichment stood localized, also that genes that are CG-enriched in fungi do not display the similar normal-like spreading of CG methylation as in plants' gene body and few insect species and in fungal species genome through investigation 'by Bewick et al., we came to know that usually 5mC is narrow (<1.0%) and a minor amount of genes contributes to the bulk of 5mC stages, which is why these are indications that in fungi species, canonical gene body methylation is absent [Citation61]. The genome methylation of fungi is very important for its basic photosynthetic ability and protection from different types of mutation and genome stability.
(c) RNA methylation in fungi and DNA methylation in S. pombe, Aspergillus, and S. cerevisiae
RNA-methylated gene silencing is newly discovered that suppresses the gene expression at the posttranscriptional level also known as RNA silencing or as an intrinsic defense mechanism against viruses, transgenes, and transposable elements [Citation64]. Double-stranded RNA regulates the degradation of homologous RNA by diminishing the gene expression [Citation65]. DNA methylation is necessary for normal development and growth of either mammals or plants and other organisms, but DNA methylation totally absent in some organisms like Saccharomyces cerevisiae and there is little DNA methylation as in Aspergillus [Citation66].
DNA methylation in insects
In insects, the occurrence of 5-methylcytosine has been testified in many species. The DNA methylation is not always present in insects, and some species also show the absence of methylation and those that have DNA methylation need to be understood with great care since the methylation can be confined to the specific development stage to particular sequences and the DNA methylation is preserved in most species if not in all. In insects, DNA methylation is still poorly recognized as we do not have such a good model organism to understand insects’ DNA methylation function'; according to data, insects have a variable number of DNA methylations, which might have variable factions [Citation67]. In the insects, the methylation of the gene body was very much considered [Citation14].
Investigational facts have revealed that the stages of methylation of gene body of the honey bee and silk moth are considerably inferior associated with further invertebrates (sea anemone, Ciona intestinally, Nematostella vectensis, and sea squirt) [Citation68]. All these results have supported the assumption that in insects, ancestor DNA methylation was decreased in Drosophila melanogaster [Citation69], Fruit fly, and Tribolium castaneum, flour beetles; like model organisms, insects donot have notable DNA methylation level in their genome [Citation12,Citation70]. On the other hand, in honey bee, Apis mellifera nutritionally changing the DNA methylation levels contributes to the oncogenic establishment of substitute castes [Citation21,Citation71].
All this supports the hypothesis that caste development and sociality evolution are connected with the DNA methylation, and recent actual evidence from the social insects like Hymenoptera (ants, wasps, and bees) reveals that this relationship is not general [Citation72–76]. tRNA aspartic acid methyltransferase 1 (TRDMT1), most usually recognized as DNMT2, is the noncanonical member of the DNMT family (an enzyme that works in DNA methylation) known to methylate the tRNA, not DNA [Citation77,Citation78], and it is usually considered that these functions are preserved in insects [Citation79]. This hypothesis is underpinned by the opinion that in D. melanogaster, the nonappearance of DNMT1 and DNMT3 is related to the damage or else dangerous drop of DNA methylation [Citation80].
DNA methylation is absent in Diptera due to lost DNMT1 and DNMT3
DNA methylation is based on the two types of DNMT1 and DNMT3 methylates in mammals, while in insects, these methyltransferases are absent due to the great limitation toward DNA methylation as in D. melanogaster [Citation81]. But in contrast, Hemiptera, Coleoptera, and Lepidoptera lack DNMT3, but DNA methylation is observed in the absence of DNMT3 as DNA methylation is not conserved in insect than mammals as in Hemiptera, Coleoptera, Lepidoptera, silk moth, and many others.
In distinction to mammals, theDNMT toolset of insects displays considerable points that in their genomes, DNA methylation is not preserved (as mention above), i.e. Bombyx mori and silk moth have verifiable firm DNA methylation in its genome but copies of DNMT3 are absent [Citation70,Citation82]. In insects, functional methylation systems can be realized in the absence of DNMT3. Loss in altered lineages but the frequency of DNMT3 is unidentified due to the absence of widespread relative data. Ten-eleven Translocation (TET) (enzymes that provided a mechanistic foundation for a frequently imaginary pathway for DNA demethylation) in the honey bee newly shows that the sole Tet enzyme is the competent of changing 5mC to 5hmC [Citation83]. In insects, Tet enzymes could exhibit handy promiscuity, as 5mC demethylation and N6-methyladenine demethylation were observed due to the Tet enzyme homolog in DNA and mRNA of D. melanogaster [Citation84,Citation85]. In insects, the Tet enzyme dispersal and its association with the occurrence of 5mC are presently unknown.
DNA methylation in contrast to mammals and CpG methylation differ in insects from mammals
DNA methylation differs significantly in insects from mammals due to different regulatory functions. The percentage of methyl cytosine varies in eukaryotes as it is 0–3% in insects and 5% in mammals. In mammals, 60%-90% CpGs are methylated in the whole genome and the DNA methylation is well characterized in them as compared to the insects [Citation86]. CpG dinucleotides are heavily methylated in mammals except for the CpG Islands in which promoter regions that overlap are unmethylated. In invertebrates including insects, DNA methylation at the CpG sites is almost negligible and linked to the gene bodies (transcribed part of the gene) [Citation87]. In Drosophila melanogaster, there are two proteins that encode for DNA methylation and resemble cytosine DNA methyltransferases and a mammalian methyl-CpG-binding domain (MBD) protein [Citation88].
By doing the profiling of methylation in insects, we come to know that methylation typically representing Hymenoptera and to a minor degree Coleoptera and Lepidoptera exposed mostly alike designs of DNA methylation, which mainly aims to exons of protein-coding genes [Citation72,Citation74–76,Citation82,Citation89–92]. Through different experiments on different insects, we came to know that DNA methylation-targeted genes were universally disclosed among several kinds of tissues, between diverse transforms in ants [Citation72,Citation75], and between progressive phases in the Nasonia vitripennis, parasitoid wasp [Citation90].
A majority of gene ontology explanations disclosed that most genes assist in simple cellular functions and reveal a significantly methylated state between species, and at the sequence level, they are extremely preserved [Citation89–94]. Through different research studies, different findings are obtained, which strongly suggest that in insect genomes, DNA methylation is not random, but a concrete clarification for this opinion remains indefinable [Citation87].
DNA methylation role in social connection
DNA methylation has a great influence on the social behavior of insects. Honey bees are the best example for their social behavior, memory formation, and age-related behavior for working due to DNA methylation. Queens have a lower level of DNA methylation as compared to workers. So, inhibition or silencing of DNA methylation in newly hatched embryos produces queens rather than workers [Citation71].
Role in gene expression or gene silencing
DNA methylation has both repressive and expressive effects, as in the case of honey bees, lower production of DNA methyltransferases (DNMTs) affects the social behavior by showing the repressive effect, while in other insects, a higher level of DNMTs causes higher epigenetic modifications due to the higher level of DNA methylation [Citation87].
Importance of DNA methyltransferase 1 and posttranslational modification, as predictive
The level of DNA methylation can be estimated by the presence or absence of DNA methyltransferases and normalized CpG dinucleotide content. DNMT1 is much wider than DNMT3. So, DNMT1 helps in the prediction of DNA methylation [Citation87].
(a) In insects, the role of DNA methylation awareness
Evidence in insects recommends gene bodies DNA methylation preform part in the maintenance of transcript integrity, and also presented as the evidence that mRNA starts or splices outline are influenced by it and might show a particular part in it [Citation88,Citation92,Citation95–99]. In mammalian studies, we came to know the idea that DNA methylation in gene might control substitute intragenic promoters and disturbing alternative transcription [Citation88,Citation100].
A hypothesis is given that de novo DNA methylation is a significant part in developing responsiveness to natural features and the arrangement of growing plasticity in honeybee Ap. mellifera possible case [Citation21,Citation101,Citation102]. Through epigenetic evidence, we know that during the sequence of organismal growth, the freshly presented difference in DNA methylation might point to changes in the direction of gene transcription, which might improve progressive plasticity and deliver a significant mechanism by stimuli of environment and responsiveness [Citation14].
DNA methylation in plants
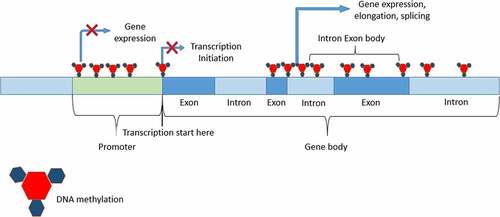
Plants are the most influential system to study DNA methylation. DNA methylation’s outcomes and associations are context-dependent. DNA methylation has been judged by various techniques. The production of DNA methylation resolution maps through the entire genome are possible by Whole Genome bisulfite sequencing (WGBS). It has been used to sequence an increasing number of plant methylomes ranging from a model plant A. thaliana to economical crops like Z. mays. DNA methylation in plants methylates DNA in three different context: dinucleotide CpG or CG and trinucleotide CHG and CHH sites (C, T and H = A) [Citation103,Citation104]. CpG and CHG sites are symmetrical, and CHH sites are asymmetrical. In plants, epigenetic mechanisms indicate that environmental conditions alter the DNA methylation position [Citation105]. It is to be noted that in coding regions and promoter region are more methylated than actively transcribed sequences in plants [Citation106], showing that DNA methylation regulates gene expression [Citation107]. DNA methylation reassures the wrapping of DNA into the so-called heterochromatin-imposed silent state, for example, by constructing the DNA reserved to transcription activators in plants [Citation108].
DNA methyltransferases (DNMTs) are working as a generator of DNA methylation in plants, and methyl groups are enzymatically transferred from S-adenosyl methionine (SAM) to cytosine [Citation109]. Small interfering RNAs (siRNAs) are controlling the DNA methylation in plants, This RNA-directed DNA methylation (RdDM) is referred by two corresponding pathways, controlling initiation and establishment of DNA methylation in every sequence [Citation110,Citation111].
There are a significant group of enzymes that assist plant-specific DNA methylation, known as CHROMOMETHYLASES (CMTs), which are characterized by the presence of a chromatin organization modifier (CHROMO) domain between the cytosine methyltransferase catalytic motifs I and IV [Citation112]. The highest levels of DNA methylation are typically found in centromeres and peri-centromeric regions in plants [Citation113,Citation114]. There are five different classes of methylated genes in flowering plants:
(a) UM (Unmethylated)
(b) gbM (gene body methylated)
(c) TSS (transcriptional start and termination site)
(d) Mcg/mcgh (methylated CG or methylated CGH)
(e) CHH/RdDM. In plants, generally, high DNA methylation occurs in these transposable elements [Citation104,Citation113,Citation115,Citation116]. Using recent studies in the model plant Arabidopsis thaliana, we examine the biological importance of DNA methylation, as well as demethylation, in plant immunity against nonviral pathogens.
(a) DNA Methylation in Arabidopsis
DNA methylation targets tandem, and dispersed repeats generally result in transposable elements [Citation117,Citation118]. At these loci, DNA methylation occurs in three diverse sequence contexts: symmetrical CG dinucleotides (usually highly methylated at 80–100%); symmetrical CHG, where it resembles A, T, or C (methylated at 20–100%); and asymmetrical CHH (usually slowly methylated at 10% or less) [Citation114,Citation119]. DNA methylation can also be present at gene bodies, exclusively at CG residues. In this particular case, DNA methylation function is at present unclear and hence the focus on repeat-associated DNA methylation in promoters and introns () [Citation120].
Table 1. Summary of arabidopsis genome DNA methyltransferase [Citation187]
DNA methylation in mammals
As in other organisms, the cytosine methylation occurs in mammals too and enzymes DNA methyltransferase (DNMT) and ten eleven translocation (TET) are involved in it. In mammals, DNA methylation plays its role in silencing of transposable elements, X-chromosome inactivation, regulation of gene expression, and genomic simprinting. In mammalian DNA, 70%–80% somatic tissue DNA is methylated at all CpG positions, sequences that have high methylation include exons of genes, nonrepetitive intergenic DNA, repetitive elements (including transposons and their inert relics), and satellite DNAs, and researchers show that the CpG dinucleotides have more frequency of methylation [Citation121]. DNA methylation is important for the proper placenta and embryo development [Citation122]. For normal placenta development, DNMT is important (DNMT10) [Citation123]. Loss of dnmt10 results in placenta dysmorphology in mice [Citation124,Citation125]. In comparison with other tissues, placentas of mouse and humans are hypomethylated [Citation126–128]. On a symbolic set of mammalian placentas by performing Methy l C-seq, high methylation is found in single genes rather than an complete group of genes, for example, in placentas with PMD (partially methylated domain)/ HMD (highly methylated domain).
De novo DNA methylation mechanism and enzyme
In mammals, the establishment of DNA methylation patterns occurs through embryonic growth by the de novo DNA methylating enzymes known as Dnmt3a and Dnmt3b. These enzymes are conserved by the Dnmt-1-mediated process during cell division, and epigenetic markers are based on DNA methylation patterns of the genome of an organism during cell division. It is the reason that DNA methylation tells us about archetypal mechanism of epigenetic inheritance [Citation71].
Developmental period
DNA methylation is necessary for mammalian’s development as addition of methyl groups to the 5-position of cytosine changes the structure of the DNA groove to which DNA binding protein binds. Methylation of CpG promoters is used to prevent transcriptional initiation and makes sure the gene silencing and parasitic DNAs. CpG poor promoters are less efficient for a tissue-specific gene expression maintenance [Citation129]. Mammalian genome is greatly CpG-depleted, and 60%-80% human genome is methylated out of 28 million CpGs [Citation130].
DNA methylation and diseases
Aberrant DNA methylation is a possible cause of various health problems in cloned organisms [Citation130]. Aberrant hypermethylation CpG Islands are observed in human’s colorectal tumors that are due to upregulation of DNA tranferases, changes in DNA methylation occur in a developmental stage, and tissue-specific gene’s DNA methylation often leads to tumor development in the form of CpG Island hypermethylation [Citation131]. Loss of de novo DNA methylation can be beneficial to tumor progression as it is linked to cancer due to hypermethylation [Citation132].
Pathological diseases
Concernments in epigenetic factors (DNA methylation, histone modification, and genomic imprinting) are the cause of developing many diseases such as diabetes, cardiovascular diseases, and cancer. Oxidative stress is related to pathophysiology of many diseases referred to high synthesis of reactive oxygen species (ROS). Oxidative stress causes the limitation of antioxidant defenses, which is responsible for their metabolism and causes an equilibrium state between production and removal [Citation133].
(a) DNA Methylation in humans
Keeping in mind the global Sustainable Development goals, ensuring healthy lives and promoting well-being for all ages and every station are focused [Citation22]. In human, DNA CpG might be alternating but is extremely methylated, and segments containing GC bases more than 50% are known as the CpG Island (CGI) and roughly 200–300 bp long [Citation134,Citation135]. DNA methyltransferases (DNMTs) catalyze the methylation reactions in humans, and enzymes DNMT1, DNMT3a, and DNMT3b catalyze the addition of methyl groups mostly at the CpG dinucleotide [Citation136]. In humans during early embryogenesis, DNA methyltransferases (DNMTs) 1, 3a, and 3b are the enzymes that establish methylation patterns.
Abnormal DNA methylation is also related to several diseases [Citation137], which include cancer [Citation138–140], autoimmune diseases, rheumatoid arthritis [Citation139,Citation141], diabetes [Citation142,Citation143], and neurodegenerative diseases such as Parkinson's and Alzheimer’s[Citation144–146]. Several new results show that psychological factors such as aggression, anxiety, happiness, sadness, and the life satisfaction also initiate abnormal patterns of DNA methylation and results lead to numerous mental diseases [Citation147–150]. Epigenome-wide association (EWA) studies have ever found a durable link among methylation and psychological exposures in humans [Citation151].
(b) DNA methylation in cancer in humans
The earliest proof about DNA methylation involved in carcinogenesis directly came in 1994 when Herman et al. disclosed that tumor suppressor gene VHL may be silenced by hypermethylation of the VHL gene promoter in some renal carcinoma cases [Citation152]. Abnormal DNA methylation of fixed loci has been observed in many types of cancers such as colon, breast, ovarian, esophagus, bladder, and bone cancer [Citation153].
(c) DNA methylation in early cancer diagnosis
When DNA methylation is deregulated, it frequently interrupts signal pathways, which contribute to the growth of numerous sicknesses, and all these are initial epigenetic variations for tumor identification [Citation154].
In several tumor cells, irregular DNA methylation has been examined, in cells like cells of the colon, breast, cervical, and ovarian cancer, which are connected with acknowledged tumor or oncogene suppressor genes' appearance. This is why in malignancy diagnosis, DNA methylation was used as a biomarker [Citation155].
(d) DNA methylation biomarker in cancer
DNA methylation is not only related to cancer but also to levels of mRNA. Barrett’s esophagus (BE) was checked in 195 patients who had passed methylation, 125 times higher danger of changing into esophageal adenocarcinoma than in healthy and controlled genes like CDH13, TAC1,NELL1, AKAP12, p16, RUNX3, HPP1, and SST, which individually were initiated to anchorage methylated sites [Citation156].
In ESR1, promoters and 14-3-3-sigma methylation status were diverse among healthy people and patients with breast cancer [Citation157]. Also, research showed that tissue factor pathway inhibitor 2 (TFPI2) methylation was noticeable in colorectal cancers of human [Citation158]. Cancer is helped by not only abnormal promoter methylation, which deregulated tumor-related gene expression [Citation155], but also biomarkers for different types of cancers like pan cancer [Citation159], prostate cancer [Citation160], ovarian cancer [Citation161], and bladder cancer [Citation162].
(e) Barriers in applying DNA methylation biomarkers
In spite of the potential of biomarkers, quite a little biomarkers attained the probability for acceptance in clinic; in malignant tumor, the most applicant biomarkers are elaborated; as with disease phenotypes, approaches used in gaging DNA methylation need to be corrected and be reproducible; and DNA methylation must be delicate and fixed and also have high analytics in cancer [Citation155].
(f) Nutrition and its role in DNA methylation in human
Nutrition has a significant part in vigor, sickness circumstances, and impacts in utero growth, DNA has the areas that are delicate to methylation built on little earlier reviews on nutrition, and these areas are recognized as methylation variable positions (MVPs); MVP observation and explanation showed that for part of nutrition in the fetal origin of mature diseases, several theories are put forward to clarify the fetal origin of adult diseases [Citation163]. Different theories define that nutrition has a significant part in adult disease danger and transmission to the progeny [Citation164]. Fetal growth is related to insulin resistance, and diabetes and adult cardiovascular disease are also expected that they will affect impairment in them [Citation165,Citation166].
Through an earlier study, I was exposed that prenatal and initial postnatal experience to the shortage of food might upsurge the danger of fatness [Citation167], lung diseases, schizophrenia, and also women breast cancer [Citation168]. Methionine and folic acid in humans have a vital part in DNA modifications and in pyrimidine and purine synthesis, and folic acid is known for roles in providing one-carbon breakdown that delivers carbon to them. In utero and in adults, it is vital to have a decent comprehension of its part in DNA modifications [Citation163].
DNA methylation is increased by periconceptional additions of folic acid up to 4.5% of IGF2 [Citation169]. Homocysteine and folic acid status are inversely related to each other, these were shown by different studies of cord blood sample analysis, and they are vice versa of each other [Citation170].
Nutritional values for fetal development
Development of a zygote to an individual or whole organism is maintained by the series of cellular processes. DNA methylation causes certain epigenetic modifications during DNA methylation, which is necessary for mammalian development [Citation130]. Nutrition at different stages of life can affect the epigenetic gene function. High consumption of methionine causes the DNA methylation and deregulation of the gene expression [Citation171].
Limits or short comings of DNA methylation
DNA methylation is a time-consuming and relatively expensive process. Detection, which is highly dependent on assay conditions and DNA quantitation, assesses methylation status and CpG residues, and we cannot determine differences in methylation between different CpGs contained in primer recognition sequences.
It requires large amounts of DNA and lengthy procedure and only assesses methylation status of CpG residues at specific DNA restriction sites for detection.
Complexity of PCR-based measurement of methylation and problems in primers with respect to different sites of methylation, and also, we cannot be sure that either the require region is amplified or not and the primer and other condition are working optimally, if so, after that, the Qualification and Quantification of Methylation is a lengthy procedure.
Future prospective of DNA methylation
Epigenetics is a unique and successful research. Today, many queries related to epigenetics are unanswered, but there is hope that they will be attempted tomorrow. The unique features of epigenetics m,ade it useful and necessary for targeted therapeutics, pathogenic insight, transplantation, and biomarker discovery.
As a biomarker DNA methylation is not that delicate technique to tissue handling in comparison with proteins and RNA because it can be achieved even from little amount of DNA isolated from fixed tissues [Citation172]. Future education must involve a balanced method by examining the DNA methylome in a genome-wide way to offer an extensive and more detailed study.
Nutrition has significant part in health, utero development and also has an impact on disease conditions. There are some areas on DNA, known as methylation variable positions (MVPs), and these regions are delicate to methylation based on a few earlier studies on diet. Some theories revealed that nutrition plays a role in adult disease risk and also its transfer to offsprings [Citation164]. It is known that folic acid and methionine play an important role in DNA modification, so it is important to understand the role of folic acid in both utero and adult. The DNA methylation is increased by 4.5% of IGF2 due to periconceptional supplementation of folic acid [Citation169]. DNA methylation of 12 genes (AMN,BDH2, C9 or f64, FBN3, EIF2C3, MGC33486, PDE2A, PVRL2, RUNX1T1, ZBTB11, ZPBP2, and ZNF187) is directly connected to methylation of five genes (ATP5F1, BMX, MDS032, FSTL3. and CYP26C1), and homocysteine levels displayed an opposite connection with the Homocysteine level [Citation173]; why it happened?
The layering and conversation of different kinds of epigenetic material is another stimulating and unfamiliar direction for future education in insects. DNA methylation contains the relations between the large set of proteins in vertebrates and fungi [Citation174], such as those related to histone modification systems, and has to be understood more [Citation175–177]. DNMTs and MBPs play a role in the recruitment of histone modification proteins [Citation178–183] and the proteins responsible for the transformation of chromatin in the mammals [Citation181,Citation184]; and understanding genes and their working is required with more knowledge.
The global genomic methylation studies in mammalian genomes, especially in genomes of human, are renewing the idea that DNA methylation play its role in development and differentiation that require more research and that has to be solved and understood. The role of anomalous DNA methylation in cancer has been credibly discussed [Citation179], but still more information and research areneeded on it; in the future, we might be able to prevent cancer and will also cure it. Various other human diseases are also linked with abnormalities in DNA methylation [Citation185].
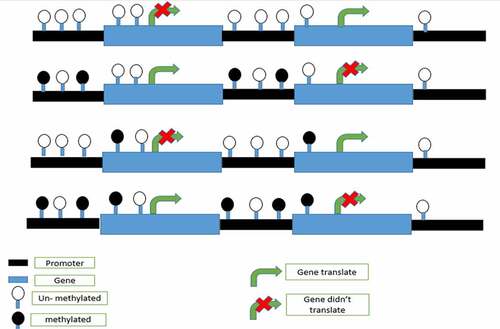
Graph.2; In the graph, it is shown that DNA methylation on any part of gene may stop translation or may not depend on the DNA methylation role.
Graph.4; The diagram explains how the DNA methylation is distributed and the presence of DNA methylatransferase across the eukaryotes. The black and white circles indicate the presence and absence of DNA methyltransferase/methylation, respectively [Citation186].
![Graph.4; The diagram explains how the DNA methylation is distributed and the presence of DNA methylatransferase across the eukaryotes. The black and white circles indicate the presence and absence of DNA methyltransferase/methylation, respectively [Citation186].](/cms/asset/deecf8c7-1174-4765-b01c-963ac4f8f548/kbie_a_2014387_uf0005_oc.jpg)
Conclusion
As goals be global Sustainable Development we try to focus on those goals like health in which bacteria as disease causing agent, fungi rule in disease and also in environment and plants are also focused [Citation22]. By going through all of these, we came to the conclusion that DNA methylation is a very important thing in the tree of life. Starting from simple organism like bacteria, it plays its role in their genome and also perform functions like saving the bacteria attached to its own restriction enzymes (RE), which act against the viral phage genome and other agents. Also as camper to other eukaryotes, DNA methylation is present in the noncoding region and in the coding region and it is absent and gives rise to consensus that DNA methylation is initially dedicated to transposable elements (TEs), which are supposed to maintain the integrity of DNA, and also that different levels of methylation are present in it and lack the gene body methylation. In the insects, methylation of the gene body was considered and does not have such a good modal organism for study due to which lack of information regarding the DNA methylation in insects is present; in plants, it is also present in different areas of its genome and has different functions and also is the case with humans in which they play a role in cancer and other diseases related to genome. As the area of genomics will gain more information and more advanced techniques will come onward, the function and role of DNA methylation will be clearer and will be used for the betterment of life and betterment of nature.
Ethics approval and consent to participate
Authors and supervisors
Consent for publication
By all authors
Availability of data and material
Books, research articles, and internet
Authors’ contributions
All the authors contributed equally
Acknowledgements
No acknowledgments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xiao F-H, Wang H-T, Kong Q-P. Dynamic DNA methylation during aging: a “Prophet” of age-related outcomes. Frontiers in Genetics. 2019;10:107.
- Du J, Johnson LM, Jacobsen SE, et al. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015;16:519–532.
- Casadesús J. Bacterial DNA methylation and methylomes. Adv Exp Med Biol. 2016;945:35–61.
- Mohapatra SS, Fioravanti A, Biondi EG. DNA methylation in Caulobacter and other Alphaproteobacteria during cell cycle progression. Trends Microbiol. 2014;22:528–535.
- Wion D, Casadesús J. N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. Nat Rev Microbiol. 2006;4:183–192.
- Collier J. Epigenetic regulation of the bacterial cell cycle. Curr Opin Microbiol. 2009;12:722–729.
- Sánchez-Romero MA, Cota I, Casadesús J. DNA methylation in bacteria: from the methyl group to the methylome. Curr Opin Microbiol. 2015;25:9–16.
- Casadesus J, Low D. Epigenetic gene regulation in the bacterial world. Microbiol Mol Biol Rev. 2006;70:830–856.
- Flusberg BA, Webster DR, Lee JH, et al. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods. 2010;7:461–465.
- Eid J, Fehr A, Gray J, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138.
- Blow MJ, Clark TA, Daum CG, et al. The epigenomic landscape of prokaryotes. PLOS Genet. 2016;12:e1005854.
- Zemach A, McDaniel IE, Silva P, et al. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328:916–919.
- Huff JT, Zilberman D. Dnmt1-independent CG methylation contributes to nucleosome positioning in diverse eukaryotes. Cell. 2014;156:1286–1297.
- Glastad KM, Hunt BG, Yi SV, et al. DNA methylation in insects: on the brink of the epigenomic era. Insect Mol Biol. 2011;20:553–565.
- Hunt BG, Glastad KM, Yi SV, et al. The function of intragenic DNA methylation: insights from insect epigenomes. Integr Comp Biol. 2013;53:319–328.
- Weiner SA, Toth AL. Epigenetics in social insects: a new direction for understanding the evolution of castes. Genet Res Int. 2012;2012 :1–11.
- Yan H, Bonasio R, Simola DF, et al. DNA methylation in social insects: how epigenetics can control behavior and longevity. Annu Rev Entomol. 2015;60:435–452.
- Alvarado S, Rajakumar R, Abouheif E, et al. Epigenetic variation in the Egfr gene generates quantitative variation in a complex trait in ants. Nat Commun. 2015;6:6513.
- Li-Byarlay H, Li Y, Stroud H, et al. RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. Proc Natl Acad Sci U S A. 2013;110:12750–12755.
- Herb BR, Wolschin F, Hansen KD, et al. Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat Neurosci. 2012;15:1371–1373.
- Kucharski R, Maleszka J, Foret S, et al. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319:1827–1830.
- THE 17 GOALS | Sustainable development, (n.d.). https://sdgs.un.org/goals (cited 2021 Oct 6).
- Vilcinskas A. The role of epigenetics in host-parasite coevolution: lessons from the model host insects Galleria mellonella and Tribolium castaneum. Zoology (Jena). 2016;119:273–280.
- Jeltsch A, Jurkowska RZ eds. DNA methyltransferases - role and function. Cham: Springer International Publishing; 2016. DOI:10.1007/978-3-319-43624-1
- Xu X, Li Y, Liang Y, et al. MiR-18a and miR-17 are positively correlated with circulating PD-1+ICOS+ follicular helper T cells after hepatitis B vaccination in a Chinese population. BMC Immunol. 2018 25;19. doi:10.1186/s12865-018-0263-y
- Dubey A, Jeon J. Epigenetic regulation of development and pathogenesis in fungal plant pathogens. Mol Plant Pathol. 2017;18:887–898.
- Kobayashi I. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res. 2001;29:3742–3756.
- Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol. 2010;8:317–327.
- Makarova KS, Wolf YI, Snir S, et al. Defense Islands in bacterial and archaeal genomes and prediction of novel defense systems. J Bacteriol. 2011;193:6039–6056.
- Wilson GG. Type II restriction–modification systems. Trends Genet. accessed 2019 May 19 1988;4:314–318. http://www.ncbi.nlm.nih.gov/pubmed/3070854
- Braaten BA, Nou X, Kaltenbach LS, et al. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. accessed 2019 May 19 1994;76:577–588. http://www.ncbi.nlm.nih.gov/pubmed/7906204
- Nou X, Skinner B, Braaten B, et al. Regulation of pyelonephritis-associated pili phase-variation in Escherichia coli: binding of the Papl and the Lrp regulatory proteins is controlled by DNA methylation, Mol. Microbiol. 1993;7:545–553.
- Barras F, Marinus MG. The great GATC: DNA methylation in E. coli. Trends Genet. accessed 2019 May 19 1989;5:139–143. http://www.ncbi.nlm.nih.gov/pubmed/2667217
- Vasu K, Nagaraja V. Diverse functions of restriction-modification systems in addition to cellular defense. Microbiol Mol Biol Rev. 2013;77:53–72.
- Lu AL, Clark S, Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci. 1983;80:4639–4643.
- Radman M, Wagner R. Mismatch repair in escherichia coli. Annu Rev Genet. 1986;20:523–538.
- O’Brown ZK, Greer EL. N6-methyladenine: a conserved and dynamic DNA mark. Adv Exp Med Biol. 2016;945:213–246.
- Iyer LM, Abhiman S, Aravind L. Natural history of eukaryotic DNA methylation systems. Prog Mol Biol Transl Sci. 2011;25–104. DOI:10.1016/B978-0-12-387685-0.00002-0
- Luo G-Z, He C. DNA N6-methyladenine in metazoans: functional epigenetic mark or bystander? Nat Struct Mol Biol. 2017;24:503–506.
- Wang J, Yang C, Zhang C, et al. Complete genome sequence of the Clostridium difficile LCL126. 2021;12:745–754.
- Kahng LS, Shapiro L. The CcrM DNA methyltransferase of Agrobacterium tumefaciens is essential, and its activity is cell cycle regulated. J Bacteriol. 2001;183:3065–3075.
- Wu TP, Wang T, Seetin MG, et al. DNA methylation on N(6)-adenine in mammalian embryonic stem cells. Nature. 2016;532:329–333.
- Poole A, Penny D, Sjöberg BM. Confounded cytosine! Tinkering and the evolution of DNA. Nat Rev Mol Cell Biol. 2001;2:147–151.
- Marinus MG, Casadesus J. Roles of DNA adenine methylation in host-pathogen interactions: mismatch repair, transcriptional regulation, and more. FEMS Microbiol Rev. 2009;33:488–503.
- Kumar R, Mukhopadhyay AK, Ghosh P, et al. Comparative transcriptomics of H. pylori strains AM5, SS1 and their hpyAVIBM deletion mutants: possible roles of cytosine methylation. PLoS One. 2012;7:e42303.
- Kahramanoglou C, Prieto AI, Khedkar S, et al. Genomics of DNA cytosine methylation in Escherichia coli reveals its role in stationary phase transcription. Nat Commun. 2012;3:886.
- Militello KT, Mandarano AH, Varechtchouk O, et al. Cytosine DNA methylation influences drug resistance in Escherichia coli through increased sugE expression. FEMS Microbiol Lett. 2014;350:100–106.
- Zemach A, Zilberman D. Evolution of eukaryotic DNA methylation and the pursuit of safer sex. Curr Biol. 2010;20:R780–5.
- Jeon J, Choi J, Lee G-W, et al. Genome-wide profiling of DNA methylation provides insights into epigenetic regulation of fungal development in a plant pathogenic fungus, Magnaporthe oryzae. Sci Rep. 2015;5:8567.
- Li W, Wang Y, Zhu J, et al. Differential DNA methylation may contribute to temporal and spatial regulation of gene expression and the development of mycelia and conidia in entomopathogenic fungus Metarhizium robertsii. Fungal Biol. 2017;121:293–303.
- Zhu Y, Xu J, Sun C, et al. Chromosome-level genome map provides insights into diverse defense mechanisms in the medicinal fungus Ganoderma sinense. Sci Rep. 2015;5:11087.
- So -K-K, Ko Y-H, Chun J, et al. Global DNA methylation in the chestnut blight fungus cryphonectria parasitica and genome-wide changes in DNA methylation accompanied with sectorization. Front Plant Sci. 2018;9:103.
- Montanini B, Chen P-Y, Morselli M, et al. Non-exhaustive DNA methylation-mediated transposon silencing in the black truffle genome, a complex fungal genome with massive repeat element content. Genome Biol. 2014;15:411.
- Mishra PK, Baum M, Carbon J. DNA methylation regulates phenotype-dependent transcriptional activity in Candida albicans. Proc Natl Acad Sci U S A. 2011;108:11965–11970.
- Zeng Z, Wu J, Kovalchuk A, et al. Genome-wide DNA methylation and transcriptomic profiles in the lifestyle strategies and asexual development of the forest fungal pathogen Heterobasidion parviporum. Epigenetics. 2019;14:16–40.
- Freitag M. Histone methylation by SET domain proteins in fungi. Annu Rev Microbiol. 2017;71:413–439.
- Volpe TA, Kidner C, Hall IM, et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837.
- Li R, Zhou S, Li Y, et al. Comparative methylome analysis reveals perturbation of host epigenome in chestnut blight fungus by a hypovirus. Front Microbiol. 2018;9:1026.
- Niederhuth CE, Bewick AJ, Ji L, et al. Widespread natural variation of DNA methylation within angiosperms. Genome Biol. 2016;17:194.
- Feng S, Cokus SJ, Zhang X, et al. Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A. 2010;107:8689–8694.
- Bewick AJ, Hofmeister BT, Powers RA, et al. Diversity of cytosine methylation across the fungal tree of life. Nat Ecol Evol. 2019;3:479–490.
- Stroud H, Greenberg MVC, Feng S, et al. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell. 2013;152:352–364.
- Takuno S, Gaut BS. Body-methylated genes in Arabidopsis thaliana are functionally important and evolve slowly. Mol Biol Evol. 2012;29:219–227.
- Kadotani N, Nakayashiki H, Tosa Y, et al. RNA silencing in the phytopathogenic fungus Magnaporthe oryzae. Mol Plant-Microbe Interact. 2003;16:769–776.
- Nakayashiki H, Nguyen QB. RNA interference: roles in fungal biology. Curr Opin Microbiol. 2008;11:494–502.
- Lee DW, Freitag M, Selker EU, et al. A cytosine methyltransferase homologue is essential for sexual development in Aspergillus nidulans. PLoS One. 2008;3. DOI:10.1371/journal.pone.0002531
- Field LM, Lyko F, Mandrioli M, et al. DNA methylation in insects. Insect Mol Biol. 2004;13:109–115.
- Sarda S, Zeng J, Hunt BG, et al. The evolution of invertebrate gene body methylation. Mol Biol Evol. 2012;29:1907–1916.
- Glastad KM, Hunt BG, Goodisman MA. Evolutionary insights into DNA methylation in insects. Curr Opin Insect Sci. 2014;1:25–30.
- Bewick AJ, Vogel KJ, Moore AJ, et al. Evolution of DNA methylation across insects. Mol Biol Evol. 2017;34:654–665.
- Foret S, Kucharski R, Pellegrini M, et al. DNA methylation dynamics, metabolic fluxes, gene splicing, and alternative phenotypes in honey bees. Proc Natl Acad Sci U S A. 2012;109:4968–4973.
- Patalano S, Vlasova A, Wyatt C, et al. Molecular signatures of plastic phenotypes in two eusocial insect species with simple societies. Proc Natl Acad Sci U S A. 2015;112:13970–13975.
- Kapheim KM, Pan H, Li C, et al. No title. 2015;348. DOI:10.1126/science.aaa4788
- Bonasio R, Li Q, Lian J, et al. Genome-wide and caste-specific DNA methylomes of the ants Camponotus floridanus and Harpegnathos saltator. Curr Biol. 2012;22:1755–1764.
- Libbrecht R, Oxley PR, Keller L, et al. Robust DNA methylation in the clonal raider ant brain. Curr Biol. 2016;26:391–395.
- Standage DS, Berens AJ, Glastad KM, et al. Genome, transcriptome and methylome sequencing of a primitively eusocial wasp reveal a greatly reduced DNA methylation system in a social insect. Mol Ecol. 2016;25:1769–1784.
- Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19:81–92.
- Goll MG, Kirpekar F, Maggert KA, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398.
- Wang Y, Jorda M, Jones PL, et al. Functional CpG methylation system in a social insect. Science. 2006;314:645–647.
- Raddatz G, Guzzardo PM, Olova N, et al. Dnmt2-dependent methylomes lack defined DNA methylation patterns. Proc Natl Acad Sci U S A. 2013;110:8627–8631.
- Provataris P, Meusemann K, Niehuis O, et al. Signatures of DNA methylation across insects suggest reduced DNA methylation levels in holometabola. Genome Biol Evol. 2018;10:1185–1197.
- Xiang H, Zhu J, Chen Q, et al. Single base–resolution methylome of the silkworm reveals a sparse epigenomic map. Nat Biotechnol. 2010;28:516–520.
- Wojciechowski M, Rafalski D, Kucharski R, et al. Insights into DNA hydroxymethylation in the honeybee from in-depth analyses of TET dioxygenase. Open Biol. 2014;4: 140110–140110.
- Zhang G, Huang H, Liu D, et al. N6-methyladenine DNA modification in Drosophila. Cell. 2015;161:893–906.
- Delatte B, Wang F, Ngoc LV, et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285.
- Beeler SM, Wong GT, Zheng JM, et al. Whole-Genome DNA methylation profile of the jewel wasp (Nasonia vitripennis), G3 genes. Genomes, Genet. 2014;4:383–388.
- Provataris P, Meusemann K, Niehuis O, et al. Signatures of DNA methylation across insects suggest reduced DNA methylation levels in holometabola. Genome Biol Evol. 2018;10:1185–1197.
- Maunakea AK, Nagarajan RP, Bilenky M, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257.
- Cunningham CB, Ji L, Wiberg RAW, et al. The genome and methylome of a beetle with complex social behavior, nicrophorus vespilloides (coleoptera: silphidae). Genome Biol Evol. 2015;7:3383–3396.
- Wang X, Wheeler D, Avery A, et al. Function and evolution of DNA methylation in Nasonia vitripennis. PLoS Genet. 2013;9:e1003872.
- Rehan SM, Glastad KM, Lawson SP, et al. The genome and methylome of a subsocial small carpenter bee, ceratina calcarata. Genome Biol Evol. 2016;8:1401–1410.
- Lyko F, Foret S, Kucharski R, et al. The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLoS Biol. 2010;8:e1000506.
- Elango N, Hunt BG, Goodisman MAD, et al. DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci U S A. 2009;106:11206–11211.
- Hunt BG, Glastad KM, Yi SV, et al. Patterning and regulatory associations of DNA methylation are mirrored by histone modifications in insects. Genome Biol Evol. 2013;5:591–598.
- Young JI, Hong EP, Castle JC, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci. 2005;102:17551–17558.
- Mandrioli M. A new synthesis in epigenetics: towards a unified function of DNA methylation from invertebrates to vertebrates. Cell Mol Life Sci. 2007;64:2522–2524.
- Suzuki MM, Kerr ARW, De Sousa D, et al. CpG methylation is targeted to transcription units in an invertebrate genome. Genome Res. 2007;17:625–631.
- Foret S, Kucharski R, Pittelkow Y, et al. Epigenetic regulation of the honey bee transcriptome: unravelling the nature of methylated genes. BMC Genomics. 2009;10:472.
- Hunt BG, Brisson JA, Yi SV, et al. Functional conservation of DNA methylation in the pea aphid and the honeybee. Genome Biol Evol. 2010;2:719–728.
- Cheong J, Yamada Y, Yamashita R, et al. Diverse DNA methylation statuses at alternative promoters of human genes in various tissues. DNA Res. 2006;13:155–167.
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254.
- Maleszka R. Epigenetic integration of environmental and genomic signals in honey bees: the critical interplay of nutritional, brain and reproductive networks. Epigenetics. 2008;3:188–192.
- Ramsahoye BH, Biniszkiewicz D, Lyko F, et al. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc Natl Acad Sci U S A. 2000;97:5237–5242.
- Lister R, Pelizzola M, Dowen RH, et al. No title. Nature. 2009;462. DOI:10.1038/nature08514
- Grimanelli D, Roudier F. Epigenetics and development in plants: green light to convergent innovations. Curr Top Dev Biol. 2013;104:189–222.
- Finnegan EJ, Brettell RI, Dennis ES. The role of DNA methylation in the regulation of plant gene expression. EXS. accessed 2019 May 19 1993;64:218–261. http://www.ncbi.nlm.nih.gov/pubmed/8380350
- Martienssen RA, Colot V. DNA methylation and epigenetic inheritance in plants and filamentous fungi. Science. 2001;293:1070–1074.
- Chan SW-L, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet. 2005;6:351–360.
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220.
- Cao X, Jacobsen SE. Role of the arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol. accessed 2019 May 19 2002;12:1138–1144. http://www.ncbi.nlm.nih.gov/pubmed/12121623
- Niederhuth CE, Schmitz RJ. Covering your bases: Inheritance of DNA methylation in plant genomes. Mol Plant. 2014;7:472–480.
- Bartee L. Arabidopsis cmt3 chromomethylase mutations block non-CG methylation and silencing of an endogenous gene. Genes Dev. 2001;15:1753–1758.
- Matzke MA, Mosher RA. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat Rev Genet. 2014;15:394–408.
- Cokus SJ, Feng S, Zhang X, et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219.
- Zhang X, Yazaki J, Sundaresan A, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell. 2006;126:1189–1201.
- Lister R, O’Malley RC, Tonti-Filippini J, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536.
- Maumus F, Quesneville H. Deep investigation of Arabidopsis thaliana junk DNA reveals a continuum between repetitive elements and genomic dark matter. PLoS One. 2014;9:e94101.
- Orgel LE, Crick FH. Selfish DNA: the ultimate parasite. Nature. accessed 2019 May 19 1980;284:604–607. http://www.ncbi.nlm.nih.gov/pubmed/7366731
- Cavrak VV, Lettner N, Jamge S, et al. How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genet. 2014;10:e1004115.
- Chan SW-L, Zhang X, Bernatavichute YV, et al. Two-step recruitment of RNA-directed DNA methylation to tandem repeats. PLoS Biol. 2006;4:e363.
- Li E, Zhang Y. DNA methylation in mammals. Cold Spring Harb Perspect Biol. 2014;6:a019133.
- Kaneda M, Okano M, Hata K, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903.
- Ding F, Patel C, Ratnam S, et al. Conservation of Dnmt1o cytosine methyltransferase in the marsupial Monodelphis domestica. Genesis. 2003;36:209–213.
- Himes KP, Koppes E, Chaillet JR. Generalized disruption of inherited genomic imprints leads to wide-ranging placental defects and dysregulated fetal growth. Dev Biol. 2013;373:72–82.
- McGraw S, Oakes CC, Martel J, et al. Loss of DNMT1o disrupts imprinted X chromosome inactivation and accentuates placental defects in females. PLoS Genet. 2013;9:e1003873.
- Ehrlich M, Gama-Sosa MA, Huang LH, et al. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982;10:2709–2721.
- Cotton AM, Avila L, Penaherrera MS, et al. Inactive X chromosome-specific reduction in placental DNA methylation. Hum Mol Genet. 2009;18:3544–3552.
- Fuke C, Shimabukuro M, Petronis A, et al. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet. 2004;68:196–204.
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070.
- Smith ZD, Meer A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220.
- Rauch T, Li H, Wu X, et al. MIRA-assisted microarray analysis, a new technology for the determination of DNA methylation patterns, identifies frequent methylation of homeodomain-containing genes in lung cancer cells. Cancer Res. 2006;66:7939–7947.
- Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88:899–910.
- Franco R, Schoneveld O, Georgakilas AG, et al. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11.
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38.
- Ghosh S, Yates AJ, Frühwald MC, et al. Tissue specific DNA methylation of CpG Islands in normal human adult somatic tissues distinguishes neural from non-neural tissues. Epigenetics. 2010;5:527–538.
- Bestor TH, Edwards JR, Boulard M. Notes on the role of dynamic DNA methylation in mammalian development. Proc Natl Acad Sci U S A. 2015;112:6796–6799.
- Shirodkar AV, Marsden PA. Epigenetics in cardiovascular disease. Curr Opin Cardiol. 2011;26:209–215.
- Aran D, Sabato S, Hellman A. DNA methylation of distal regulatory sites characterizes dysregulation of cancer genes. Genome Biol. 2013;14:R21.
- Yari K, Payandeh M, Rahimi Z. Association of the hypermethylation status of PTEN tumor suppressor gene with the risk of breast cancer among Kurdish population from Western Iran. Tumor Biol. 2016;37:8145–8152.
- Mokarram P, Kumar K, Brim H, et al. Distinct high-profile methylated genes in colorectal cancer. PLoS One. 2009;4:e7012.
- Cribbs A, Feldmann M, Oppermann U. Towards an understanding of the role of DNA methylation in rheumatoid arthritis: therapeutic and diagnostic implications. Ther Adv Musculoskelet Dis. 2015;7:206–219.
- Toperoff G, Aran D, Kark JD, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21:371–383.
- Toperoff G, Kark JD, Aran D, et al. Premature aging of leukocyte DNA methylation is associated with type 2 diabetes prevalence. Clin Epigenetics. 2015 35;7. doi:10.1186/s13148-015-0069-1
- Lu H, Liu X, Deng Y, et al. DNA methylation, a hand behind neurodegenerative diseases. Front Aging Neurosci. 2013 85;5. doi:10.3389/fnagi.2013.00085
- Boks MP, de Jong NM, Kas MJH, et al. Current status and future prospects for epigenetic psychopharmacology. Epigenetics. 2012;7:20–28.
- J. B-S, R. C, O. M, et al. Promoter DNA methylation regulates progranulin expression and is altered in FTLD. Acta Neuropathol Commun. 2013;1:16.
- Lam LL, Emberly E, Fraser HB, et al. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17253–17260.
- Kim D, Kubzansky LD, Baccarelli A, et al. Psychological factors and DNA methylation of genes related to immune/inflammatory system markers: the VA Normative Aging Study. BMJ Open. 2016;6:e009790.
- Ota VK, Noto C, Gadelha A, et al. Changes in gene expression and methylation in the blood of patients with first-episode psychosis. Schizophr Res. 2014;159:358–364.
- Puglia MH, Lillard TS, Morris JP, et al. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci U S A. 2015;112:3308–3313.
- Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. accessed 2019 May 19 1997;277:1659–1662. http://www.ncbi.nlm.nih.gov/pubmed/9287218
- Herman JG, Latif F, Weng Y, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci. 1994;91:9700–9704.
- Murphy SK, Huang Z, Wen Y, et al. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res. 2006;4:283–292.
- Riggs AD, Jones PA. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. accessed 2019 May 19 1983;40:1–30. http://www.ncbi.nlm.nih.gov/pubmed/6197868
- Pan Y, Liu G, Zhou F, et al. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med. 2018;18:1–14.
- Jin Z, Cheng Y, Gu W, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in barrett’s esophagus. Cancer Res. 2009;69:4112–4115.
- Martínez-Galán J, Torres B, Del Moral R, et al. Quantitative detection of methylated ESR1 and 14-3-3-sigma gene promoters in serum as candidate biomarkers for diagnosis of breast cancer and evaluation of treatment efficacy. Cancer Biol Ther. 2008;7:958–965.
- Li M, Gao F, Xia Y, et al. Filtrating colorectal cancer associated genes by integrated analyses of global DNA methylation and hydroxymethylation in cancer and normal tissue. Sci Rep. 2016;6:31826.
- Tang B. DMAK: a curated pan-cancer DNA methylation annotation knowledgebase. Bioengineered, English. 2016;8 4;8(2):182–190. doi:10.1080/21655979.2016.1218582.
- Cheng W, Cao J, Xia Y, et al. A DNA methylation profile of long non-coding RNAs can predict OS in prostate cancer. Bioengineered. 2021;12 12(1) :3252–3262. doi:10.1080/21655979.2021.1945991. Dec 2021.
- Zhang C, Liu J, Guo H, et al. m6A RNA methylation regulators were associated with the malignancy and prognosis of ovarian cancer. Bioengineered . 2021;12(1):3159–3176. doi:10.1080/21655979.2021.1946305. Dec 2021.
- Yi W, Yu Y, Li Y, et al. The tumor-suppressive effects of alpha-ketoglutarate-dependent dioxygenase FTO via N6-methyladenosine RNA methylation on bladder cancer patients. Bioengineered. 2021;12(1) :5323–5333. doi:10.1080/21655979.2021.1964893 Dec 2021.
- Kandi V, Vadakedath S. Effect of DNA methylation in various diseases and the probable protective role of nutrition: a mini-review. Cureus. 2015;7:e309.
- Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111.
- Barker DJP. The fetal origins of type 2 diabetes mellitus. Ann Int Med. 1999;130:322.
- Barker DJ. Fetal nutrition and cardiovascular disease in later life. Br Med Bull. 1997;53:96–108.
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353.
- Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–491.
- Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845.
- Fryer AA, Emes RD, Ismail KMK, et al. Quantitative, high-resolution epigenetic profiling of CpG loci identifies associations with cord blood plasma homocysteine and birth weight in humans. Epigenetics. 2011;6:86–94.
- Waterland RA. Assessing the effects of high methionine intake on DNA methylation, in: j. Nutr. Am Inst Nutr. 2006. DOI:10.1093/jn/136.6.1706s
- Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326.
- Deaton AM, Bird A. CpG Islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022.
- Viré E, Brenner C, Deplus R, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874.
- Ben-Porath I, Cedar H. Epigenetic crosstalk. Mol Cell. accessed 2019 May 23 2001;8:933–935. http://www.ncbi.nlm.nih.gov/pubmed/11741529
- Okitsu CY, Hsieh C-L. DNA methylation dictates histone H3K4 methylation. Mol Cell Biol. 2007;27:2746–2757.
- Tamaru H, Selker EU. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature. 2001;414:277–283.
- Fuks F, Hurd PJ, Deplus R, et al. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 2003;31:2305–2312.
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692.
- López-Rodas G, Brosch G, Georgieva EI, et al. Histone deacetylase. A key enzyme for the binding of regulatory proteins to chromatin. FEBS Lett. accessed 2019 May 19 1993;317:175–180. http://www.ncbi.nlm.nih.gov/pubmed/8425601
- Geiman TM, Sankpal UT, Robertson AK, et al. DNMT3B interacts with hSNF2H chromatin remodeling enzyme, HDACs 1 and 2, and components of the histone methylation system. Biochem Biophys Res Commun. 2004;318:544–555.
- Feng Q, Zhang Y. The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes Dev. 2001;15:827–832.
- Bai S, Ghoshal K, Datta J, et al. DNA methyltransferase 3b regulates nerve growth factor-induced differentiation of PC12 cells by recruiting histone deacetylase 2. Mol Cell Biol. 2005;25:751–766.
- Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11:285–296.
- Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610.
- Zhong X. Comparative epigenomics: a powerful tool to understand the evolution of DNA methylation. New Phytol. 2016;210:76–80.
- Tariq M, Paszkowski J. DNA and histone methylation in plants. Trends Genet. 2004;20:244–251.