ABSTRACT
Accumulating studies have suggested that microRNAs (miRNAs) play vital roles in the pathogenesis of osteoarthritis (OA). Nevertheless, the specific function of miR-128-3p in OA remains unknown. In this study, we demonstrated that miR-128-3p was decreased and ZEB1 was increased in OA. Additionally, miR-128-3p expression was negatively correlated with ZEB1. miR-128-3p overexpression or ZEB1 silencing attenuated extracellular matrix degradation and cell apoptosis, and increased the proliferation of IL-1β-activated CHON-001 cells. Furthermore, ZEB1 was directly targeted by miR-128-3p. In addition, ZEB1 upregulation restored the effects of miR-128-3p overexpression on OA progression. Overall, our findings suggested that miR-128-3p might regulate the development of OA via targeting ZEB1.
Introduction
Osteoarthritis (OA) is considered as the most common age-related chronic joint disease, which is associated with severe inflammation, cartilage destruction and joint degeneration [Citation1–3]. OA may cause pain or even disability in some severe cases. Despite the efforts made to improve the therapeutic approaches to OA, the treatment outcomes remain unsatisfactory due to the complex pathogenesis of the disease [Citation4–6]. Therefore, it is important to investigate the molecular mechanisms underlying OA.
MicroRNAs (miRNAs) are a kind of non-coding RNAs with about 22 nt in length, that modulate gene expression at the post-transcriptional level [Citation7]. Extensive researches have indicated that miRNAs are associated with the pathogenesis of multiple diseases, including OA [Citation8]. For example, miR-448 accelerated interleukin-1β (IL-1β)-treated cartilage degradation in OA via targeting matrilin-3 [Citation9]. Additionally, silencing of miRNA-449a may inhibit IL-1β-treated cartilage destruction in OA via modulating sirtuin 1 (SIRT1) [Citation10]. miR-145 contributed to impaired extracellular matrix (ECM) degradation in OA cartilage via targeting Smad3 [Citation11]. Interestingly, miR-128-3p was identified to be downregulated and play a suppressive role in OA [Citation12]. However, the specific effects of miR-128-3p in the pathogenesis of OA remain unknown.
It has been reported that ZEB1 is implicated in inflammatory response. For example, miR-708 inhibited liver inflammation and lipid accumulation via targeting ZEB1 [Citation13]. In addition, ZEB1 promoted inflammation via suppressing the DNA repair glycosylase MPG in epithelial cells [Citation14]. Lai et al demonstrated that the expression levels of ZEB1 in synovial fluid and chondrocytes were higher in knee OA compared to the control group, and ZEB1 was inversely correlated with miR-100-5p [Citation15], suggesting that ZEB1 might act as a potential diagnostic biomarker in OA. Nevertheless, the effect of ZEB1 in OA remains elusive.
The present study was undertaken to determine the levels of miR-128-3p and ZEB1 in OA and explore the role of miR-128-3p in modulating cartilage ECM degradation, cell viability and apoptosis of IL-1β-stimulated chondrocytes. The aim of this study was to provide new insights into the treatment of OA.
Materials and methods
Samples
OA samples were collected from 32 patients (18 males and 14 females; age, 64.3 ± 4.1 years) who underwent total knee arthroplasty at the First People′s Hospital of Changzhou between January 2017 and August 2019. Normal cartilage samples were isolated from 32 traumatic amputees (20 males and 12 females; age, 65.1 ± 4.8 years). The cartilage tissues were frozen in liquid nitrogen immediately and stored at −80°C. Patients′ inclusion criteria: (1) patients were diagnosed with OA. (2) patients provided informed consent. Patients′ exclusion criteria: patients complicated with other diseases, such as chronic inflammatory diseases other than OA. Written informed consent was obtained from all participants. This research was approved by the Ethics Committee of the First People′s Hospital of Changzhou.
Cell culture and treatment
The chondrogenic cell line (CHON-001) was purchased from ATCC and grown in DMEM with 10% FBS at 37°C with 5% CO2. Then, CHON-001 cells were stimulated with 10 ng/ml IL-1β (Sigma-Aldrich) for 24 h to generate an in vitro OA model as previously described [Citation16,Citation17].
Cell transfection
The miR-128-3p mimics (10 nM; 5′-GAGCUUAUUCAUAAAAUGCAG-3′), negative control (NC) mimics (10 nM; 5′-CGCCAAUAUCAUUAUACCUC-3′), miR-128-3p inhibitor (10 nM; 5′-AAAUAUGCUGUAUGUCAUGUGUU-3′), NC inhibitor (10 nM; 5′-GUCCAGUGAAUUCCCAG-3′), as well as the short hairpin RNAs (shRNAs) targeting ZEB1 (10 nM; shZEB1; 5′-GGAGGUGGAUGUGAAAGAU-3′) and its negative control (10 nM; shNC; 5′-GGCCUUGCGAUAGCGUAGC-3′), pcDNA3.1/ZEB1 vectors and pcDNA3.1 empty vectors were obtained from Shanghai GenePhama Co., Ltd. The plasmids were then transfected into cells (1x105) using Lipofectamine 2000® (Invitrogen) at room temperature for about 30 min.
RT-qPCR
Total RNA was extracted from tissues and cells using a TRIzol reagent (Invitrogen). Subsequently, 1 μg of total RNA was reverse-transcribed to cDNA using the PrimeScript RT reagent kit (Takara). RT-qPCR was carried out using the SYBR Premix Ex Taq II (Takara) on the LightCycler 480 II Real-Time PCR System (Roche). The relative expression of miRNA or mRNA was normalized to U6 or GAPDH. The primer sequences used were as follows: For miR-128-3p, forward, 5′-GACTGCCGAGCGAGCG-3′ and reverse, 5′-GACGCCGAGGCACTCTCTCCT-3′; for ZEB1, forward, 5′-AAGTGGCGGTAGATGGTA-3′ and reverse, 5′-TTGTAGCGACTGGATTTT-3′; for U6, forward, 5′-CCATCGGAAGCTCGTATACGAAATT-3′ and reverse, 5′-GGCCTCTCGAACTTGCGTGTCAG-3′; and for GAPDH, forward, 5′-CCCCTTCATTGACCTCAACT-3′ and reverse, 5′-ATGAGTCCTTCCACGATACC-3′.
Western blot analysis
CHON-001 cells were lysed with RIPA buffer (Thermo Fisher Scientific) to isolate proteins. Subsequently, total proteins were separated by 10% SDS-PAGE (Solarbio), and then transferred onto PVDF membranes. Then, membranes were incubated with the primary antibodies against aggrecan (ab36861; Abcam), collagen II (ab12268; Abcam), matrix metalloproteinase 13 (MMP-13) (ab39012; Abcam) or GAPDH (ab181602; Abcam). The protein bands were visualized using the ECL system (Bio-Rad). For MMP-13 in cell culture supernatants, cultured media were collected from the supernatant by centrifugation, followed by concentrated with trichloroacetic acid (TCA) and acetone as described previously [Citation18].
CCK-8 assay
CHON-001 cells (5x103) were seeded into 96-well plates and incubated for 24, 48 and 72 h. Then, 10 μl of CCK-8 solution was added to each well, and the cells were incubated for an another 2 h. The absorbance was measured using a microplate reader (Bio-Rad Laboratories, Inc.) at 450 nm [Citation19].
TUNEL assay
The One Step TUNEL Apoptosis Assay Kit (Beyotime) was utilized to determine CHON-001 cell apoptosis [Citation20]. Following dehydration in ethanol, cells were dyed and cultured with a TUNEL reaction mixture (Roche Diagnostics). Nuclear staining was performed with DAPI. TUNEL-positive cells were observed under a fluorescence microscope (Nikon Corporation).
Luciferase reporter assay
The plasmids containing the wild-type (WT) and mutant (MUT) ZEB1 3′UTR were synthesized by GenePharma Co., Ltd (Shanghai). Subsequently, these plasmids were co-transfected with miR-128-3p or NC mimics into cells. After 48 h, the luciferase activity was detected using the Dual-Luciferase Reporter Assay system (Promega Corporation) [Citation21].
Immunoprecipitation (RIP) assay
RIP assay was conducted by Magna RNA-binding protein RIP kit (EMD Millipore) [Citation22]. CHON-001 cells were lysed with RIP lysis buffer and incubated with magnetic beads conjugated with Ago2 or IgG. The RNAs in the immunoprecipitates were extracted with Trizol reagent and detected by RT-qPCR.
Statistical analysis
The data are expressed as the mean ± SD. Statistical analysis was performed using the SPSS software (IBM.). Comparisons between two groups were carried out by Student′s t-test, while those among multiple groups with one-way ANOVA. P < 0.05 was considered to indicate a statistically significant difference.
Results
The aim of this study was to investigate the role of miR-128-3p in modulating cartilage ECM degradation, cell viability and apoptosis of IL-1β-stimulated chondrocytes. Through a series of functional experiments, we demonstrated that miR-128-3p suppresses IL-1β-stimulated cartilage degradation and chondrocyte apoptosis via targeting ZEB1 in osteoarthritis.
miR-128-3p is downregulated and ZEB1 is upregulated in OA
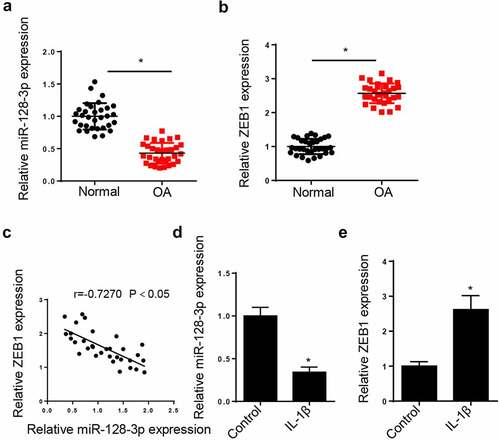
To determine whether miR-128-3p and ZEB1 were dysregulated in OA patients, the expressions of miR-128-3p and ZEB1 were measured in OA cartilage tissues. The results demonstrated that the expression of miR-128-3p was reduced and that of ZEB1 was enhanced in OA cartilage tissues (). In addition, a negative correlation was observed between miR-128-3p and ZEB1 expression in OA tissues (). Furthermore, CHON-001 cells were treated with IL-1β to establish in vitro OA model. As shown in , RT-qPCR revealed that miR-128-3p expression was notably reduced in IL-1β-treated cells. In addition, ZEB1 expression was strongly upregulated following cell treatment with IL-1β (). Overall, the aforementioned findings suggested that the abnormal levels of miR-128-3p and ZEB1 might be involved in the progression of OA.
Figure 1. MiR-128-3p expression was reduced and ZEB1 was enhanced in OA tissues and IL-1β-activated chondrocytes. (a and b) RT-qPCR assay was applied to detect the expression of miR-128-3p and ZEB1 in OA cartilage tissues (n = 32) and normal cartilage tissues (n = 32). (c) Pearson′s correlation analysis showed the correlation between miR-128-3p and ZEB1 in OA cartilage tissues (n = 32). (d) RT-qPCR assay showed the expression of miR-128-3p in IL-1β-induced CHON-001 cells. (e) RT-qPCR assay showed ZEB1 expression in IL-1β-induced CHON-001 cells. Data are expressed as the mean± SD (n = 3) *P < 0.05.
miR-128-3p inhibits the apoptosis and facilitates the viability of IL-1β-treated CHON-001 cells
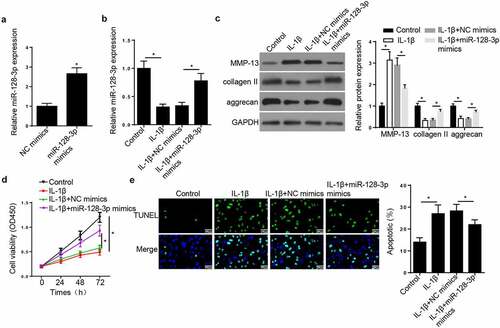
To evaluate the function of miR-128-3p in OA, CHON-001 cells were treated with IL-1β, IL-1β + NC mimics or IL-1β + miR-128-3p. Firstly, transfection with miR-128-3p mimics increased the expression of miR-128-3p (). Moreover, RT-qPCR analysis showed that the expression of miR-128-3p was decreased in CHON-001 cells treated with IL-1β, while miR-128-3p overexpression reversed this effect (), suggesting that miR-128-3p overexpression partially abrogated the inhibitory effect of IL-1β treatment on miR-128-3p expression in CHON-001 cells. Additionally, Western blot analysis revealed that cell treatment with IL-1β increased the protein levels of MMP-13 and reduced those of collagen II and aggrecan (). CHON-001 cell transfected with miR-128-3p mimics downregulated MMP-13 and upregulated collagen II and aggrecan expression (). Furthermore, CCK-8 showed that miR-128-3p overexpression rescued the repressive effect of IL-1β on CHON-001 cell viability (). On the contrary, TUNEL assay demonstrated that the treatment of IL-1β promoted CHON-001 cell apoptosis, but miR-128-3p upregulation abrogated this effect (). These results suggested that miR-128-3p modulated ECM degradation and the IL-1β-induced CHON-001 cell proliferation and apoptosis.
Figure 2. MiR-128-3p inhibited the apoptosis and facilitated the proliferation of CHON-001 cells treated with IL-1β. (a) RT-qPCR analysis showed miR-128-3p expression in CHON-001 cells transfected with NC mimics or miR-128-3p mimics. (b) RT-qPCR analysis showed miR-128-3p expression in CHON-001 cells treated with IL-1β, IL-1β+NC mimics or IL-1β+miR-128-3p mimics. (c) Western blot assay measured the levels of MMP-13, collagen II and aggrecan in CHON-001 cells treated with IL-1β, IL-1β+NC mimics or IL-1β+miR-128-3p mimics. (d) CCK-8 was used to test the proliferation in CHON-001 cells transfected with IL-1β, IL-1β+NC mimics or IL-1β+miR-128-3p mimics. (e) TUNEL assay showed the apoptosis in CHON-001 cells transfected with IL-1β, IL-1β+NC mimics or IL-1β+miR-128-3p mimics. Data are expressed as the mean± SD (n = 3). *P < 0.05.
ZEB1 silencing ameliorates the effect of IL-1β on chondrocytes
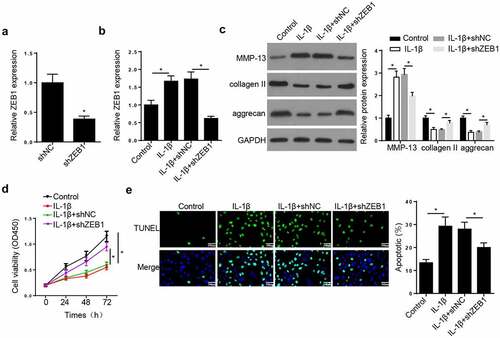
Next, the effects of ZEB1 on OA development were evaluated. RT-qPCR assay showed that ZEB1 expression was reduced by ZEB1 silence (). Meanwhile, ZEB1 knockdown restored the IL-1β-mediated upregulation of ZEB1 in CHON-001 cells (). Western blot indicated that IL-1β upregulated MMP-13 and downregulated collagen II and aggrecan in CHON-001 cells. However, these effects were reversed by ZEB1 silencing (). CCK-8 uncovered that ZEB1 knockdown rescued the suppressive impact of IL-1β on CHON-001 cell proliferation (). Additionally, TUNEL assay demonstrated that treatment of CHON-001 cells with IL-1β accelerated cell apoptosis, which was abolished by ZEB1 knockdown (). These findings revealed that silencing of ZEB1 accelerated cell viability and inhibited ECM degradation and apoptosis of IL-1β-stimulated chondrocytes.
Figure 3. ZEB1 depletion ameliorated the effects of IL-1β treatment on OA progression in chondrocytes. (a) RT-qPCR assay showed ZEB1 expression in CHON-001 cells transfected with shNC or shZEB1. (b) RT-qPCR assay showed ZEB1 expression in CHON-001 cells treated with IL-1β, IL-1β+shNC or IL-1β+shZEB1. (c) Western blot assay measured the levels of MMP-13, collagen II and aggrecan in CHON-001 cells treated with IL-1β, IL-1β+shNC or IL-1β+shZEB1. (d) CCK-8 was used to test the proliferation in CHON-001 cells treated with IL-1β, IL-1β+shNC or IL-1β+shZEB1. (e) TUNEL assay showed the apoptosis in CHON-001 cells treated with IL-1β, IL-1β+shNC or IL-1β+shZEB1. Data are expressed as the mean± SD (n = 3). *P < 0.05.
miR-128-3p directly targeted ZEB1
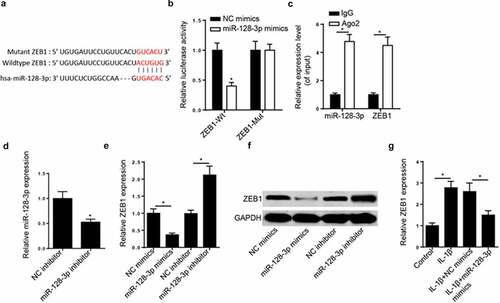
Subsequently, using the StarBase database, ZEB1 was predicted as a target of miR-128-3p. The potential binding sequences between miR-128-3p and ZEB1 are shown in . Then, a luciferase reporter assay was performed to verify that miR-128-3p mimics markedly suppressed the luciferase activity of ZEB1-WT. However, the luciferase activity was not affected in cells transfected with ZEB1-MUT (). The direct binding between miR-128-3p and ZEB1 was further confirmed by RIP assay. The results uncovered that the enrichment of ZEB1 and miR-128-3p was higher in the Ago2 group compared to IgG group (). miR-128-3p expression was decreased in CHON-001 cells transfected with miR-128-3p inhibitor (). Additionally, the mRNA and protein levels of ZEB1 was down- and up-regulated in cells by miR-128-3p upregulation and downregulation, respectively (). ZEB1 was also significantly downregulated following miR-128-3p overexpression in IL-1β-activated CHON-001 cells (). Collectively, the data confirmed miR-128-3p could directly target ZEB1 and negatively regulate its expression.
Figure 4. ZEB1 was directly targeted by miR-128-3p. (a) Binding sequences between miR-128-3p and ZEB1 were predicted by starBase website. (b) Luciferase reporter assay was adopted to verify the binding ability between miR-128-3p and ZEB1 in CHON-001 cells. (c) RIP assay was used to analyze the enrichment of miR-128-3p and ZEB1 in CHON-001 cells of anti-Ago2 group compared with anti-IgG group. (d) RT-qPCR analysis showed miR-128-3p expression in CHON-001 cells transfected with miR-128-3p inhibitor. (e and f) RT-qPCR and Western blot analysis was applied to detect ZEB1 expression in CHON-001 cells transfected with miR-128-3p mimics and miR-128-3p inhibitor. (g) RT-qPCR analysis showed ZEB1 expression in CHON-001 cells treated with IL-1β, IL-1β+NC mimics or IL-1β+miR-128-3p mimics. Data are expressed as the mean± SD (n = 3). *P < 0.05.
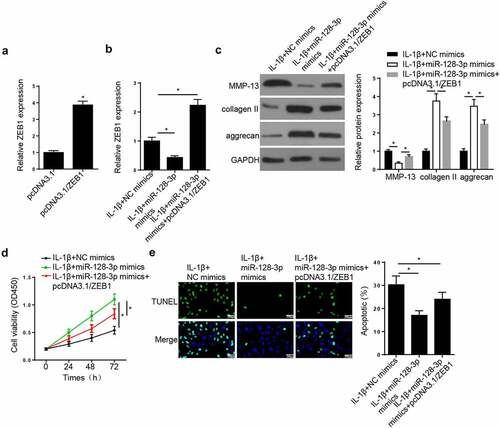
ZEB1 overexpression reverses the effect of miR-128-3p overexpression on OA progression
RT-qPCR showed that ZEB1 expression was increased in CHON-001 cells transfected with ZEB1 overexpression plasmid (). To verify whether miR-128-3p could regulate OA progression via ZEB1, rescue assays were conducted. RT-qPCR demonstrated that the expression of ZEB1 was reduced following transfection of IL-1β-treated CHON-001 cells with miR-128-3p mimics, while ZEB1 overexpression neutralized this effect (). Besides, miR-128-3p overexpression decreased MMP-13, and increased collagen II and aggrecan expression levels, while ZEB1 overexpression had the opposite effect (). Furthermore, CCK-8 and TUNEL experiments revealed that miR-128-3p mimics promoted cell viability and attenuated apoptosis, while ZEB1 overexpression had the opposite effect (). In sum, the aforementioned results demonstrated that miR-128-3p may be associated with OA development via targeting ZEB1.
Figure 5. ZEB1 addition reversed the effects of miR-128-3p overexpression on OA progression in IL-1β-treated chondrocytes. (a) RT-qPCR assay showed ZEB1 expression was upregulated in CHON-001 cells transfected with pcDNA3.1/ZEB1. (b) RT-qPCR assay showed ZEB1 expression in CHON-001 cells treated with IL-1β+miR-128-3p mimics, IL-1β+miR-128-3p mimics+pcDNA3.1/ZEB1. (c) Western blot assay measured the levels of MMP-13, collagen II and aggrecan in CHON-001 cells treated with IL-1β+miR-128-3p mimics, IL-1β+miR-128-3p mimics+pcDNA3.1/ZEB1. (d and e) CCK-8 and TUNEL assays were used to test the proliferation and apoptosis in CHON-001 cells treated with IL-1β+miR-128-3p mimics, IL-1β+miR-128-3p mimics+pcDNA3.1/ZEB1. Data are expressed as the mean± SD (n = 3). *P < 0.05.
Discussion
OA is a highly prevalent chronic joint disease [Citation23]. This study confirmed that miR-128-3p level was reduced and that of ZEB1 was enhanced in OA. Furthermore, miR-128-3p modulated cartilage ECM degradation, cell viability and apoptosis via ZEB1 in IL-1β-treated chondrocytes.
Several miRNAs have been reported to be involved in the pathogenesis of OA [Citation24,Citation25]. miR-128-3p was found to be associated with inflammatory response and ECM degradation. For example, miR-128-3p could directly target SIRT1 to mediate the TNF-α-induced inflammatory response in bone marrow mesenchymal stem cells [Citation26]. miR-128-3p also attenuated chondrocyte apoptosis, ECM degradation and inflammation via targeting Wnt-induced secreted protein 1 in OA [Citation12]. Herein, we demonstrated that miR-128-3p was downregulated in chondrocytes and OA tissues. IL-1β is a cytokine that can induce a series of pathogenic responses in chondrocytes, thus playing a crucial role in the progression of OA [Citation27]. Consistently, miR-128-3p expression was also suppressed in IL-1β-treated CHON-001 cells in the present study. Furthermore, miR-128-3p overexpression reduced the expression levels of MMP-13 and improved those of collagen II and aggrecan levels in IL-1β-activated CHON-001 cells. It was verified that MMP-13 was implicated in ECM degradation in OA. Cartilage ECM mainly consist of collagen II and aggrecan [Citation28]. These findings indicated that upregulated miR-128-3p could attenuate cartilage ECM degradation in OA. Besides, miR-128-3p overexpression overturned the suppression on CHON-001 cell viability and the increased cell apoptosis caused by IL-1β.
ZEB1, a member of the ZEB family, is a known transcriptional regulator that plays critical roles in the induction of epithelial‑to-mesenchymal transition and other biological processes [Citation29,Citation30]. It has been reported that ZEB1 is involved in the excessive production of ECM proteins via the transforming growth factor β1 signaling pathway in corneal endothelial cells [Citation31]. Furthermore, NEAT1 promoted ECM progression via regulating the miR-27b-3p/ZEB1 axis in diabetic nephropathy [Citation32]. Another study demonstrated that miR-200b attenuated inflammatory responses via ZEB1 in gingival fibroblasts [Citation33]. In the present study, ZEB1 was predicted as a downstream gene of miR-128-3p in IL-1β-induced chondrocytes. Silencing of ZEB1 suppressed ECM degradation and cell apoptosis and increased the viability of IL-1β-treated CHON-001 cells. In addition, ZEB1 was downregulated following miR-128-3p overexpression. Functional assays revealed that miR-128-3p mimics attenuated ECM degradation and cell apoptosis and increased the proliferation of IL-1β-activated CHON-001 cells, while ZEB1 overexpression restored these effects.
Conclusion
Our data demonstrated that miR-128-3p is overexpressed in patients with OA and can regulate OA cartilage ECM degradation, cell apoptosis and proliferation via targeting ZEB1 in chondrocytes. These findings indicated that miR-128-3p might be of value as a potential target for OA treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441.
- Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12(Suppl A):S10–19.
- Castaneda S, Roman-Blas JA, Largo R, et al. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–323.
- Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531.
- Saltzman CL, Kadoko RG, Suh JS. Treatment of isolated ankle osteoarthritis with arthrodesis or the total ankle replacement: a comparison of early outcomes. Clin Orthop Surg. 2010;2:1–7.
- Man GS, Mologhianu G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. J Med Life. 2014;7:37–41.
- Romaine SP, Tomaszewski M, Condorelli G, et al. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101(12):921–928.
- Goldring MB, Marcu KB. Epigenomic and microRNA-mediated regulation in cartilage development, homeostasis, and osteoarthritis. Trends Mol Med. 2012;18(2):109–118.
- Yang H, Wu D, Li H, et al. Downregulation of microRNA-448 inhibits IL-1beta-induced cartilage degradation in human chondrocytes via upregulation of matrilin-3. Cell Mol Biol Lett. 2018;23:7.
- Park KW, Lee KM, Yoon DS, et al. Inhibition of microRNA-449a prevents IL-1beta-induced cartilage destruction via SIRT1. Osteoarthritis Cartilage. 2016;24:2153–2161.
- Yang B, Kang X, Xing Y, et al. Effect of microRNA-145 on IL-1beta-induced cartilage degradation in human chondrocytes. FEBS Lett. 2014;588:2344–2352.
- Chen S, Li B. MiR-128-3p post-transcriptionally inhibits WISP1 to suppress apoptosis and inflammation in human articular chondrocytes via the PI3K/AKT/NF-kappaB signaling pathway. Cell Transplant. 2020;29:963689720939131.
- Hu S, Liu YM, Chen C, et al. MicroRNA-708 prevents ethanol-induced hepatic lipid accumulation and inflammatory reaction via direct targeting ZEB1. Life Sci. 2020;258:118147.
- de Barrios O, Sanchez-Moral L, Cortes M, et al. ZEB1 promotes inflammation and progression towards inflammation-driven carcinoma through repression of the DNA repair glycosylase MPG in epithelial cells. Gut. 2019;68(12):2129–2141.
- Lai Z, Cao Y. Plasma miR-200c-3p, miR-100-5p, and miR-1826 serve as potential diagnostic biomarkers for knee osteoarthritis: randomized controlled trials. Medicine (Baltimore). 2019;98(51):e18110.
- Wu H, Zhang M, Li W, et al. Stachydrine attenuates IL-1β-induced inflammatory response in osteoarthritis chondrocytes through the NF-κB signaling pathway. Chem Biol Interact. 2020;326:109136.
- Piao S, Du W, Wei Y, et al. Protectin DX attenuates IL-1β-induced inflammation via the AMPK/NF-κB pathway in chondrocytes and ameliorates osteoarthritis progression in a rat model. Int Immunopharmacol. 2020;78:106043.
- Li Z, Liu B, Zhao D, et al. Protective effects of Nebivolol against interleukin-1β (IL-1β)-induced type II collagen destruction mediated by matrix metalloproteinase-13 (MMP-13. Cell Stress Chaperones. 2017;22(6):767–774.
- Yao Y, Li X, Cheng L, et al. Circular RNA FAT atypical cadherin 1 (circFAT1)/microRNA-525-5p/spindle and kinetochore-associated complex subunit 1 (SKA1) axis regulates oxaliplatin resistance in breast cancer by activating the notch and Wnt signaling pathway. Bioengineered. 2021;12(1):4032–4043.
- Zhang CC, Li Y, Feng XZ, et al. Circular RNA circ_0001287 inhibits the proliferation, metastasis, and radiosensitivity of non-small cell lung cancer cells by sponging microRNA miR-21 and up-regulating phosphatase and tensin homolog expression. Bioengineered. 2021;12:414–425.
- Zhang H, Liu S, Tang L, et al. Long non-coding RNA (LncRNA) MRPL23-AS1 promotes tumor progression and carcinogenesis in osteosarcoma by activating Wnt/β-catenin signaling via inhibiting microRNA miR-30b and upregulating myosin heavy chain. Bioengineered. 2021;12:162–171.
- Jiang L, Ge W, Cui Y, et al. The regulation of long non-coding RNA 00958 (LINC00958) for oral squamous cell carcinoma (OSCC) cells death through absent in melanoma 2 (AIM2) depending on microRNA-4306 and Sirtuin1 (SIRT1) in vitro. Bioengineered. 2021;12(1):5085–5098.
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126.
- Zhong G, Long H, Ma S, et al. miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. 2019;226:164–172.
- Li ZC, Han N, Li X, et al. Decreased expression of microRNA-130a correlates with TNF-alpha in the development of osteoarthritis. Int J Clin Exp Pathol. 2015;8:2555–2564.
- Wu L, Zhang G, Guo C, et al. MiR-128-3p mediates TNF-alpha-induced inflammatory responses by regulating Sirt1 expression in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2020;521:98–105.
- Pan T, Chen R, Wu D, et al. Alpha-Mangostin suppresses interleukin-1beta-induced apoptosis in rat chondrocytes by inhibiting the NF-kappaB signaling pathway and delays the progression of osteoarthritis in a rat model. Int Immunopharmacol. 2017;52:156–162.
- Neuhold LA, Killar L, Zhao W, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44.
- Vandewalle C, Comijn J, De Craene B, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578.
- Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487.
- Okumura N, Minamiyama R, Ho LT, et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Lab Invest. 2015;95(11):1291–1304.
- Wang X, Xu Y, Zhu YC, et al. LncRNA NEAT1 promotes extracellular matrix accumulation and epithelial-to-mesenchymal transition by targeting miR-27b-3p and ZEB1 in diabetic nephropathy. J Cell Physiol. 2019;234:12926–12933.
- Matsui S, Zhou L, Nakayama Y, et al. MiR-200b attenuates IL-6 production through IKKbeta and ZEB1 in human gingival fibroblasts. Inflamm Res. 2018;67(11–12):965–973.
