ABSTRACT
The aim of the present study was to observe the effects and mechianisms of melatonin on the proliferation and apoptosis of lung cancer (LC) cells. A549 cells were treated with a concentration gradient (0–100 μM) of melatonin for 24 hours, and cell viability was detected by XTT ((2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl) −2H-tetrazolium-5-carboxanilide)) colorimetry. Melatonin with a concentration of 50 μM was selected to interact with the LC cells for ten days, and then a colony formation assay was used to detect the proliferation of the LC cells. TUNEL (Terminal-deoxynucleoitidyl Transferase Mediated Nick End Labeling) staining was used to evaluate the amount of apoptosis in the two groups. Finally, Western blotting was used to detect the expression levels of related proteins in the p38MAP (mitogen-activated protein) signaling pathway. Meanwhile, another experiment, CCK-8 cell proliferation test, was conducted to detect the OD540 absorbance of LC cells at 24, 48, 72, and 96 hours. Melatonin inhibited the proliferation of LC cells in a concentration-dependent (5–100 μM) manner (P < 0.05), and inhibited the proliferation of LC cells in a time-dependent (0–96 hour) manner (P < 0.05). Melatonin (50 μM) could significantly inhibit the colony formation ability of LC cells (P < 0.05). The ratio of LC cells in the G0/G1 phase in the melatonin group increased, while the ratio of cells in the G2/M and S phase was significantly reduced (P < 0.05). Melatonin significantly promoted the apoptosis of LC cells (P < 0.05) and activate the phosphorylation of p38 (P < 0.05).
Introduction
Lung cancer (LC) is one of the most common primary malignant tumors. It has become a global public health problem as well as a great economic burden [Citation1,Citation2]. The incidence of LC in males is particularly high, with about 520,000 new cases each year worldwide [Citation3]. Although resection or transplantation is currently the most effective treatment for end-stage LC, its incidence and mortality have increased in recent decades. Due to its high recurrence and metastasis rates, the long-term prognosis and survival rate of patients with LC are not ideal [Citation4,Citation5]. Therefore, further insight into the molecular mechanism of the occurrence and development of LC is essential for early intervention and effective treatment.
Melatonin (N-acetyl-5-methoxytryptamine) is an indole compound, mainly secreted by the pineal gland of humans and mammals in response to darkness [Citation6]. In addition to the pineal gland, melatonin can be synthesized by the retina, gastrointestinal tract, skin, bone marrow, and lymphocytes [Citation7]. The synthesis and secretion of melatonin are regulated by the ‘master circadian clock’ located in the suprachiasmatic nucleus of the hypothalamus [Citation8]. Current research has revealed that melatonin is not only a hormone but also a cytoprotective agent involved in immune regulation, antioxidant processes, and hematopoietic functions [Citation9]. In addition, melatonin can exert anticancer properties through receptor-dependent and receptor-independent mechanisms. For example, melatonin can induce the apoptosis of breast cancer cells and inhibit the proliferation and angiogenesis of breast cancer cells [Citation10]. Melatonin can also exert anti-estrogenic effects and inhibit the activity of aromatase by regulating the ERα signaling pathway [Citation11]. However, the role of melatonin in LC has not yet been reported on.
In the present study, the effects of melatonin on the biological characteristics of LC cells, including proliferation and apoptosis, were observed, and a variety of molecular biology techniques were used to explore the potential molecular mechanism of the anti-LC effects of melatonin. The hypothesis is that melatonin can activate the p38 MAPK signaling pathway to inhibit the proliferation of LC cells and induce apoptosis. The aim of the present study was to observe the effects of melatonin on the proliferation and apoptosis of lung cancer (LC) cells and to explore the underlying mechanisms, so as to provide a clearer reference for the clinical treatment of LC.
Materials and methods
Materials and cells
LC cell line A549 was purchased from Shanghai Kanglang Biotechnology Co., Ltd., melatonin from the Sigma Company, fetal bovine serum from Biochrom, Germany, and RPMI 1640 (containing penicillin and streptomycin) culture medium from Gibco Biosynchronous Growth Company. The cells were cultured at 37°C.
Cell intervention
The LC cell line A549 was seeded in 96-well plates (50,000 cells/ml), and 48 hours later, the cells were treated with melatonin at different concentrations (0.1, 1, 5, 10, 20, 50, and 100 μM). After 24, 48, 72, and 96 hours of treatment, 50 ml XTT ((2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl) −2 H-tetrazolium-5-carboxanilide)) was added, and the cells were incubated at 37°C for two hours, then the absorbance was read at 450 [Citation5].
Western blot
The total protein of the LC cells in each dish was extracted after being treated with melatonin for 72 hours. After this, the culture solution in the culture medium was discarded, and the medium was washed three times with phosphate-buffered saline (PBS). Next, 1000 μl of lysate was added to each dish, and they were well shaken for 20 minutes. The cells at the bottom of the dishes were then scraped off with a brush and put into the prepared Eppendorf plastic tube. The collected cells were cleaved for approximately 15 seconds by ultrasonic laser, and after being left to stand for 15 minutes, they were then centrifuged for 0.5 hours (12,000 r/min). The supernatant was dispensed into the EP tube, and the bicinchoninic acid assay (BCA) was used to measure the protein concentration. After the protein concentration was measured using UV spectrophotometry, the protein in all the samples was adjusted to equalize the concentration, and the dispensed samples were put into the refrigerator at −80°C. With the extraction of the total protein, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis was performed, and the protein in the gel was then transferred into the Polyvinylidene Fluoride (PVDF) membrane, which was incubated with the primary antibody at 4°C overnight. In the morning goat anti-rabbit secondary antibody was added, and the PVDF membrane was incubated in the dark for one more hour. Finally, the protein bands were scanned and quantified by the Odyssey scanner, and reduced glyceraldehyde-phosphate dehydrogenase (GAPDH) was used to adjust the protein level [Citation6].
Colony formation assay
The cells in control group and Melatonin group were cultured to the logarithmic growth phase and digested into single-cell suspension with 0.25% trypsin, with the proportion of single cells reaching more than 95%. Then the cell suspension was inoculated into a 6-well plate, with about 500 cells per well plate at best. Then, 2 ml of RPMI 1640, a medium for cell culture, was added to each well, and the cells were left for ten days, during which time the solution was changed every 48 hours. Finally, the cells were fixed with formaldehyde and stained with crystal violet so that the number of cell clones in each well could be counted [Citation7].
Detection of cell apoptosis and cell cycle by flow cytometry
The LC cells in the logarithmic growth phase were digested with 0.25% trypsin EDTA to prepare suspension and inoculated on 6-well plates (i.e., each plate contains 6 wells). The apoptosis rate was detected according to the operating procedures of the cell apoptosis detection kit, Annexin V-FITC PI (Biyuntian Company), and the cell cycle kit, Annexin V-FITC PI (Biyuntian Company) [Citation8].
TUNEL staining
The cells on the climbing flake were immersed in fixing solution for one hour and washed three times with PBS. With the completion of penetration, the TUNEL reagents were prepared, and two negative controls were set up. Once staining was completed, the cells were observed under a fluorescence microscope, photos taken, and the cells counted [Citation9].
CCK-8 cell proliferation assay
The cells in the logarithmic growth phase were inoculated into the 96-well plate and cultured at 37°C and 5% CO2 concentration in an incubator at a constant temperature for 0, 24, 48, 72, and 96 hours. After the incubation period, the medium was discarded, and the chromogenic solution was prepared (in the dark) using a ratio of 1640 medium: CCK-8 = 10:1. Each well of the 96-well plate had 110 μ of the chromogenic solution added to it, and the cells were incubated at 37°C for a further two hours. The absorbance of cells incubated for 0, 24, 48, 72, and 96 hours was detected with the UV spectrophotometer (540 nm) [Citation10].
Statistics analysis
SPSS 22.0 software was used for data analysis. The measurement data were expressed by means ± standard deviations. Data were tested by Shapiro-Wilk test as normally distributed, so the data comparison between the control and Melatonin groups was conducted with a t-test. P < 0.05 was considered statistically significant.
Results
We found that melatonin was able to activate the p38 MAPK signaling pathway to inhibit the proliferation of LC cells and induce apoptosis. Therefore, melatonin could become a new drug for the treatment of LC.
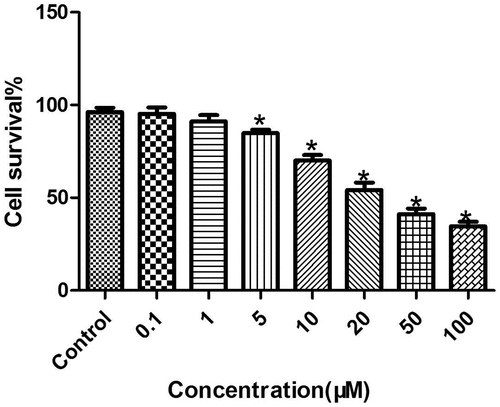
Melatonin inhibition of the proliferation of LC cells in a concentration-dependent manner
First, the LC cell lines were treated with different concentrations of melatonin (0.1, 1, 5, 10, 20, 50, and 100 μM) for 72 hours, and the proliferation of each group was then detected by the XTT test. The results were as follows (): At concentrations of 10, 20, 50, and 100 μM, melatonin significantly inhibited the proliferation of LC cells, and the inhibition of cancer cells was concentration-dependent (P < 0.05), but at concentrations of 0.1 and 1 μM, melatonin had no effect on the proliferation of LC cells (P > 0.05). Therefore, melatonin at a concentration of 50 μM was selected for verification in the subsequent experiments.
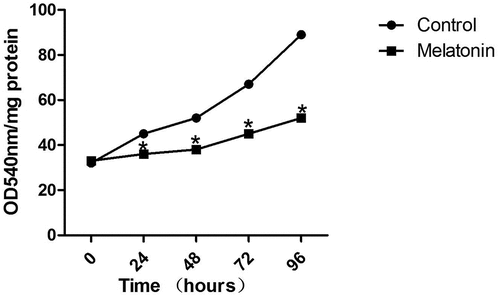
Melatonin inhibition of the proliferation of LC cells in a time-dependent manner
As shown in , compared with the control group (A549 cells which only had the solvent DMSO added to them), the proliferation ability of LC cells treated with melatonin (50 μM) for 24, 48, 72, and 96 hours was significantly decreased (P < 0.05). Therefore, a stimulation duration of 72 hours and a concentration of 50 μM of melatonin were chosen for the stimulation experiments on LC cells.
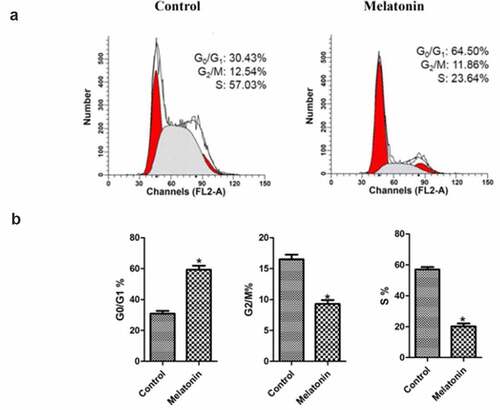
The effect of melatonin on the cell cycle of LC cells
As demonstrated in , after adding melatonin, the cell cycle of LC cells changed significantly. In the melatonin group, the ratio of LC cells in the G0/G1 increased significantly (P < 0.05), while the ratio of LC cells in the G2/M phase and S phase cells decreased significantly (P < 0.05). These results indicated that melatonin could significantly inhibit the cell cycle of LC cells.
Figure 3. Effect of melatonin on cell cycle of LC cells. Control: the blank control group; Melatonin: the melatonin group. (a): the distribution of cells in each treatment group. (b): bar graphs with errors bars obtained from 3 replicates per cell cycle growth phase. *Compared with the control group, there existed a statistical significance, P < 0.05.
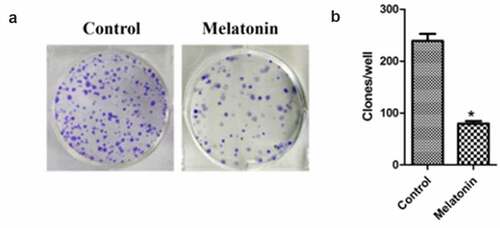
Melatonin inhibition of the colony formation ability of LC cells
The colony formation assay was used to detect the proliferation of LC cell lines. The results are illustrated in . The clone number of LC cells in the melatonin group (64.82 ± 6.32) was significantly lower than that in the control group (263.35 ± 11.46) (P < 0.05).
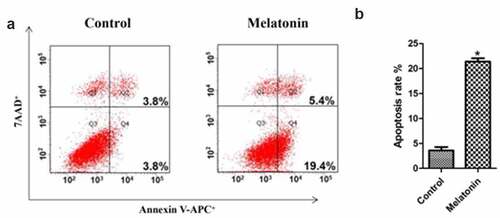
The promotion of the apoptosis of LC cells by melatonin
The results of flow cytometry () clearly showed that melatonin promoted the apoptosis of LC cells, the apoptosis rates in the control group and the melatonin group being 4.66 ± 0.34, and 24.78 ± 2.11, respectively (P < 0.05).
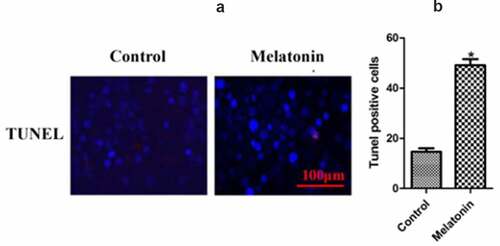
TUNEL staining in cells in the two groups
In order to detect the apoptosis of each group more intuitively, TUNEL staining was used to stain the cells in each group, and the TUNEL positive cells were counted. The results () show that the apoptosis rates in the control group and the melatonin group were 15.34 ± 0.77 and 44.98 ± 1.32, respectively (P < 0.05).
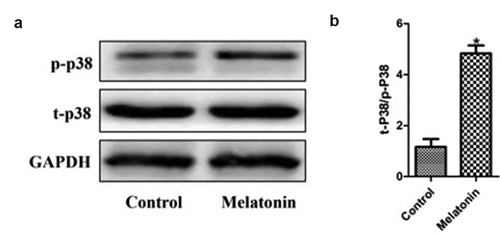
The effect of melatonin on the p38 MAPK signal pathway in LC cells
Considering the important role of the p38 MAPK signaling pathway in tumor biology, we hypothesized whether the effect of melatonin on the biological behavior of LC cells might depend on the p38 MAPK signaling pathway. Western blot was used to detect the activation of p38 phosphorylation in the two groups of cells. The results () indicated that melatonin could activate the phosphorylation of p38 (P < 0.05). These results suggested that the p38 MAPK signaling pathway might play an important role in the anti-proliferation and apoptosis of LC cells induced by melatonin.
Discussion
This study found that Melatonin induces cell apoptosis through p38 MAPK signaling pathway, thus inhibits the proliferation of LC cells and it could be a new drug for the treatment of LC.
LC is one of the most common malignant tumors. The pathogenesis is complex, involving many abnormal changes in genes and proteins [Citation12]. Studies have shown that the main causes of LC include smoking and drinking [Citation13]. Currently, many studies have been devoted to finding the pathogenic genes and diagnostic markers of LC, such as the tumor size and various differentially expressed genes in the tissues of primary LC [Citation14]. Although diagnosis and treatment strategies have greatly improved, the overall prognosis of LC is still very poor [Citation15]. Therefore, it is of great significance to find the key genes, proteins, or RNA that can lead to the occurrence and development of LC.
Apoptosis is mediated by the death receptor pathway (exogenous) or the chondrial pathway (endogenous), which can clear away the damaged cells and maintain homeostasis. In the two pathways, the ADP-ribose polymerase 1 (PARP1) was cleaved, and caspases 3/7 were activated in response to the DNA injury stress in cells [Citation16]. Therefore, targeting programmed cell death by regulating apoptosis and autophagy has become one of the promising methods of cancer treatment [Citation17]. The proteins of the Bcl-2 family are the regulatory factors of apoptosis. Many members of the Bcl-2 family, such as Bax and Bak, which are important pathways of caspase-mediated cell death, can regulate apoptosis [Citation18]. The ratio of Bcl-2/Bax is an important determinant of apoptosis [Citation19]. The abnormal expression of the p38 MAPK signaling pathway, which can promote the survival and proliferation of cancer cells, has been observed in many kinds of tumors [Citation20–22]. The MAPK family, including ERK, JNK, and p38, mediates tumor signals in various tissues [Citation23]. For example, in the LC tissue, flavonoids can regulate the G0/G1 arrest and apoptosis of LC cells and reduce the expression of cyclin E1, CDK2, and CDK4 in LC cells. Some studies have found that the anti-LC mechanism of flavonoids may be mediated by activating the p38 MAPK phosphorylation and inhibiting JNK–MAPK phosphorylation [Citation24]. In another clinical study, the data of patients with LC were collected prospectively. The expression levels of phosphorylated p38 (p-p38) and JNK (p-JNK) were measured by enzyme-linked immunosorbent assay (ELISA), and the correlation between the expression levels, clinicopathological characteristics, and overall survival rate was analyzed. The results showed that the level of p38 phosphorylation was closely correlated with the tumor size and the number of satellite tumors in patients with LC (P < 0.05). The prognosis of patients with low p-p38 and high p-JNK levels was significantly worse (P < 0.05) [Citation25]. These studies indicated that the p38 MAPK could effectively prevent the occurrence and development of LC.
As an endogenous hormone synthesized by the human body, melatonin has a variety of pharmacological protective effects. In A549 LC cells, melatonin can regulate the activity of MMP-9 and MMP-2, thus affecting the deposition of the LC cell-matrix and playing a role in the anti-metastasis of LC cells [Citation26]. In addition, melatonin has also been shown to have antiangiogenic effects by inhibiting the transcriptional activation of a vascular endothelial growth factor (VEGF) and, ultimately, inhibiting the vascularization of LC cells [Citation27]. One in vivo study showed that melatonin could inhibit the metastasis of LC by targeting the Twist [Citation28]. In the present study, it was demonstrated for the first time that melatonin could inhibit the proliferation of LC cells and promote the apoptosis of LC cells by activating the phosphorylation level of p38 MAPK. However, there were some limitations to the present study: 1) No animal experiment was designed to verify the results.; and, 2) only one cell line was used.
Conclusion
In conclusion, the results of the present study suggested that melatonin might have anti-LC properties, and the mechanism might be correlated with the activation of p38 MAPK in LC cells.
Highlights
Melatonin induces cell apoptosis through p38 MAPK signaling pathway.
Melatonin inhibits the proliferation of LC cells.
Melatonin could be a new drug for the treatment of LC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–473.
- Romaszko AM, Doboszyńska A. Multiple primary lung cancer: a literature review. Adv Clin Exp Med. 2018 May;27(5):725–730.
- Šutić M, Vukić A, Baranašić J, et al. Diagnostic, predictive, and prognostic biomarkers in non-small cell lung cancer (NSCLC) management. J Pers Med. 2021;11(11):1102.
- Pallis AG, Syrigos KN. Lung cancer in never smokers: disease characteristics and risk factors. Crit Rev Oncol Hematol. 2013 Dec;88(3):494–503.
- Cheung CHY, Juan HF. Quantitative proteomics in lung cancer. J Biomed Sci. 2017;24(1):37.
- Ren W, Liu G, Chen S. Melatonin signaling in T cells: functions and applications. J Pineal Res. 2017;62(3):e12394.
- Hardeland R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res. 2017;62(1):e12377.
- Reiter RJ, Rosales-Corral SA, Tan DX. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18(4):843.
- Martínezcampa C, Menéndezmenéndez J, Alonsogonzález C. What is known about melatonin, chemotherapy and altered gene expression in breast cancer. Oncol Lett. 2017;13(4):2003–2014.
- Asghari MH, Moloudizargari M, Ghobadi E. Melatonin as a multifunctional anti-cancer molecule: implications in gastric cancer. Life Sci. 2017;185:38–45.
- Yang CY, Lin CK, Tsao CH. Melatonin exerts anti-oral cancer effect via suppressing LSD1 in patient-derived tumor xenograft models. Oncotarget. 2017;8(20):33756–33769.
- Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin North Am. 2019 Sept;31(3):303–313.
- Guo LW, Lyu ZY, Meng QC, et al. A risk prediction model for selecting high-risk population for computed tomography lung cancer screening in China. Lung Cancer. 2021 Dec;163:27–34.
- Heimbach JK, Kulik LM, Finn RS. AASLD guidelines for the treatment of lung cancer. Hepatology. 2017;67(1):358–380.
- Ally A, Balasundaram M, Carlsen R. Comprehensive and integrative genomic characterization of lung cancer. Cell. 2017;169(7):1327.
- Peña‐Blanco A, García-Sáez AJ. Bax, Bak and beyond: mitochondrial performance in apoptosis. Febs J. 2017;285(3):416–431.
- Ashkenazi A, Fairbrother WJ, Leverson JD. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273.
- Askew K, Li K, Olmos-Alonso A. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain:. Cell Rep. 2017;18(2):391–405.
- Baar MP, Brandt RMC, Putavet DA. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169(1):132–147.
- Liu Z, Zheng Q, Chen W. Chemosensitizing effect of Paris Saponin I on Camptothecin and 10-hydroxycamptothecin in lung cancer cells via p38 MAPK, ERK, and Akt signaling pathways. Eur J Med Chem. 2017;125:760–769.
- Hui H, Chang R, Zhang T. ATM mediates DAB2IP-deficient bladder cancer cell resistance to ionizing radiation through the p38MAPK and NF-κB signaling pathway. Mol Med Rep. 2017;16(2):1216–1222.
- Utaipan T, Athipornchai A, Suksamrarn A. Isomahanine induces endoplasmic reticulum stress and simultaneously triggers p38 MAPK-mediated apoptosis and autophagy in multidrug-resistant human oral squamous cell carcinoma cells. Oncol Rep. 2017;37(2):1243.
- Inselman A, Handel MA. Mitogen-activated protein kinase dynamics during the meiotic G2/MI transition of mouse spermatocytes1. Biol Reprod. 2017;71(2):570–578.
- Zanoaga O, Braicu C, Jurj A, et al. Progress in research on the role of flavonoids in lung cancer. Int J Mol Sci. 2019 Sept;20(17):4291.
- Huang XH, Wang Y, Hong P, et al. Benzethonium chloride suppresses lung cancer tumorigenesis through inducing p38-mediated cyclin D1 degradation. Am J Cancer Res. 2019 Nov;9(11):2397–2412.
- Gurunathan S, Jeyaraj M, Kang M-H, et al. Melatonin enhances palladium-nanoparticle-induced cytotoxicity and apoptosis in human lung epithelial adenocarcinoma cells A549 and H1229. Antioxidants. 2020 Apr;9(4):357.
- Cheng J, Yang HL, Gu CJ, et al. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int J Mol Med. 2019 Feb;43(2):945–955.
- Chao CC, Chen PC, Chiou PC, et al. Melatonin suppresses lung cancer metastasis by inhibition of epithelial-mesenchymal transition through targeting to twist. Clin Sci. 2019 Mar;133(5):709–722.