ABSTRACT
Clear cell renal cell carcinoma, the most common type of renal cancer, is associated with poor survival. Ubiquitin-specific peptidase 2 regulates the molecular mechanisms of cancer cells. However, its mechanism in clear cell renal cell carcinoma remains unclear. Quantitative real-time polymerase chain reaction, enzyme-linked immunosorbent assay, and immunohistochemistry were performed to assess ubiquitin-specific peptidase 2 expression in human clear cell renal cell carcinoma samples. Ubiquitin-specific peptidase 2 was weakly expressed in clear cell renal cell carcinoma samples and associated with poor patient outcomes. Ubiquitin-specific peptidase 2 inhibition promoted clear cell renal cell carcinoma cell proliferation, migration, and invasion. Ubiquitin-specific peptidase 2 overexpression inhibited clear cell renal cell carcinoma cell proliferation, migration, and invasion in vitro and in vivo. RNA-sequencing showed significant changes in the epithelial-mesenchymal transition-related pathways following ubiquitin-specific peptidase 2 knockdown. Western blotting was performed to detect the protein expression levels. Expression of p-nuclear factor-κB p65, N-cadherin, Vimentin, and Snail, which were markedly increased, as well as E-cadherin, which was decreased following ubiquitin-specific peptidase 2 knockdown. Rescue experiments using the nuclear factor-κB inhibitor BAY 11–7082 revealed that the migration and invasion abilities and the expression of epithelial-mesenchymal transition pathway proteins were inhibited in both the short hairpin RNA (shRNA) for ubiquitin-specific peptidase 2 and shRNA for negative control groups. Ubiquitin-specific peptidase 2 is a potential biomarker to distinguish clear cell renal cell carcinoma patients from healthy individuals. Ubiquitin-specific peptidase 2-mediated inhibition of epithelial-mesenchymal transition in clear cell renal cell carcinoma cells is dependent on the nuclear factor-κB pathway.
Graphical abstract
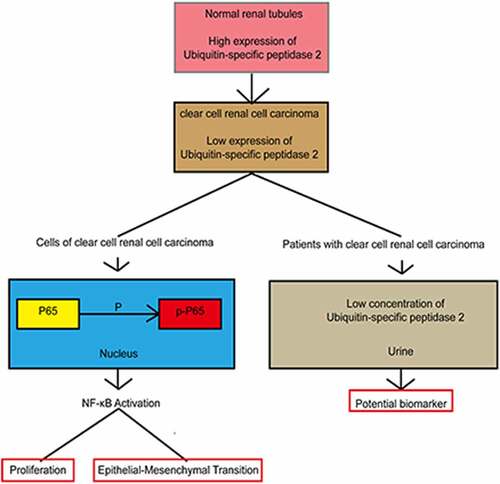
Introduction
Clear cell renal cell carcinoma is the most common type of renal cancer, making up over 70% of all cases of kidney cancer [Citation1]. The five-year cancer-specific survival rate of clear cell renal cell carcinoma is less than 70% [Citation2]. However, the specific mechanisms of clear cell renal cell carcinoma remain unclear. Ubiquitin-specific peptidase 2 was first described in a study on rat testis [Citation3]. Researchers showed that upregulation of ubiquitin-specific peptidase 2 in prostate cancer and ovarian carcinoma, leads to increased levels of deubiquitinated substrates [Citation4]. However, the mechanism of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma biological behavior has not been fully understood.
The migration and invasion abilities are important characteristics and biomarkers for malignant tumors associated with poor outcomes [Citation5]. Migration and invasion are mainly caused by epithelial-mesenchymal transition [Citation6], a process critical to tumorigenesis [Citation7].
In this study, our hypothesis was whether ubiquitin-specific peptidase 2 could be served as a potential biomarker to distinguish clear cell renal cell carcinoma patients from healthy volunteers. The goals and aims of this research were to investigate the role of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma cells via in vitro and in vivo experiments and explore the mechanism behind this phenomenon.
Methods
Patient and tissue samples
A total of 26 paired cancer and paracancerous tissue specimens and 57 urine samples from patients with clear cell renal cell carcinoma were collected [Citation8]. Moreover, we collected 27 urine samples from healthy volunteers. All samples were obtained from the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). The first part of the paired cancer tissue specimen was used for RNA extraction. Other paired cancer tissues were stored in formalin until further use for immunohistochemistry analysis. These patients had not been administered adjuvant anticancer therapy before surgery. All patients and volunteers provided written informed consent. The research protocol was approved by the Ethics committee of the First Affiliated Hospital of Zhengzhou University (The amendment-2019-148).
RNA sequencing
Four paired-cancer and paracancerous clear cell renal cell carcinoma tissue samples were subjected to mRNA-transcriptome sequencing using an LC-Bio Technologies instrument (Hangzhou, China) to identify differentially expressed genes. RNA-sequencing was performed to detect mutated pathways using short hairpin RNA (shRNA) for ubiquitin-specific peptidase 2 and shRNA for negative control in 786–0 cells.
Bioinformatics analysis
Ubiquitin-specific peptidase 2 mRNA expression in clear cell renal cell carcinoma was analyzed to determine the stage, overall survival and disease-free survival for clear cell renal cell carcinoma patients using Gene Expression Profiling Interactive Analysis software (www.cancer-pku.cn) [Citation9]. Further, the Human Protein Atlas was used to search for data on the protein expression of ubiquitin-specific peptidase 2 [Citation10,Citation11]. STRING (functional protein association networks (string-db.org)) was used to identify relevant proteins and pathways involving ubiquitin-specific peptidase 2 [Citation12].
RNA isolation and quantitative real-time polymerase chain reaction
According to the research method of Cao, et al [Citation13], we extract RNA from tissue and cell line samples using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA was prepared using the Prime Script RT reagent kit with gDNA Eraser (Takara, Shiga, Japan). The quantitative real-time polymerase chain reaction was carried out by SYBR Green (Takara) to analyze the mRNA expression of ubiquitin-specific peptidase 2. We used GAPDH as the internal amplification control. Primer sequences were as follows: ubiquitin-specific peptidase 2 forward: 5′-AGTAGGATCGGGGATCTCTTTG-3′ and reverse: 5′-GAAGACCGTAGAACAGTAACCAC-3′; GAPDH forward: 5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse: 5′-ACCACCCTGTTGCTGTAGCCAA -3′.
Western blot analysis
As described by Xie et al [Citation14], all proteins were isolated by the RIPA buffer (Solarbio, Beijing, China) and the concentration of protein was tested using a BCA Protein Assay Kit (Solarbio). The proteins were subjected to 10% SDS-PAGE, followed by transfer of the separated proteins onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The main antibodies were ubiquitin-specific peptidase 2 (Proteintech, Rosemont, IL, USA, 1:1000), GAPDH (Proteintech, 1:5000), nuclear factor (NF)-κB p65 (Cell Signaling Technology, Danvers, MA, USA, 1:1000), phospho-NF-κB p65 (Cell Signaling Technology, 1:1000), N-cadherin (Proteintech, 1:5000), E-cadherin (Proteintech, 1:25,000), Vimentin (Proteintech, 1:5000), and Snail (Cell Signaling Technology, 1:1000). Secondary antibodies used were horseradish peroxidase-conjugated Affinipure Goat Anti-Rabbit IgG(H + L) and horseradish peroxidase-conjugated Affinipure Goat Anti-Mouse IgG(H + L) (Proteintech, 1:8000). Incubations with the antibodies were performed per the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
The concentration of ubiquitin-specific peptidase 2 in the urine samples from the clear cell renal cell carcinoma patients and healthy volunteers was measured using a Human ubiquitin-specific peptidase 2 enzyme-linked immunosorbent assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) [Citation15]. The urine samples were first centrifuged at 3000 rpm for 10 min, and the upper layer of the urine samples was collected. The upper layer was divided into 100 µl aliquots and then stored at -80°C until the experiments. For the enzyme-linked immunosorbent assay experiment, the upper layer was added to wells that have been pre-coated with primary antibodies. For each experimental well, recognition antigens labeled with horseradish peroxidase were added. After incubating for 30 min at 37°C, phosphate-buffered saline was used to wash each well. We used Varioskan Lux (Thermo Fisher Scientific) to measure the absorbance at 450 nm.
Immunohistochemistry analysis
As described by Li et al [Citation16], embedded tissues from clear cell renal cell carcinoma patients were incubated with the primary ubiquitin-specific peptidase 2 antibody described above (Proteintech, 10,392-1-AP, 1:200) overnight at 4°C and then washed with phosphate-buffered saline. Furthermore, the samples were incubated at 23°C with anti-rabbit secondary antibodies for 2 h. For the color reaction, 3,3′-N-diaminobenzidine tetrahydrochloride was used. The development of dark brown coloration was defined as a positive result.
Cell culture
The kidney cell line HK2 and three clear cell renal cell carcinoma cell lines 769-P, 786–0, and Caki-1 were obtained from the China Center for Type Culture Collection (Wuhan, China). The four cell lines were cultured in Roswell Park Memorial Institute 1640 (HyClone, Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum (HyClone) as well as 1% penicillin and streptomycin. The cells were cultured at 37°C with 5% CO2 in the incubator. Roswell Park Memorial Institute 1640 containing 10% serum and 2 µg/mL puromycin (Sigma, St. Louis, MO, USA) was used to culture the clear cell renal cell carcinoma cells transduced with lentivirus.
Knockdown and overexpression of ubiquitin-specific peptidase 2
The small interfering (Si) RNAs Si-ubiquitin-specific peptidase 2–1, Si-ubiquitin-specific peptidase 2–2, and Si-negative control were transfected into 769-P and 786–0 clear cell renal cell carcinoma cells using JetPRIME (Polyplus-transfection, New York, NY, USA) per the manufacturer’s instructions. All three small interfering RNAs were synthesized by GenePharma (Shanghai, China) [Citation17]. The two Si-ubiquitin-specific peptidase 2 sequences were: Si-ubiquitin-specific peptidase 2–1 (5′-3′): UCGCUGACGUGUACAGAUUUU and Si-ubiquitin-specific peptidase 2–2 (5′-3′): CCAGCAAGCUCACAACAUUUU. Additionally, a plasmid for overexpressing ubiquitin-specific peptidase 2 was transfected into the three clear cell renal cell carcinoma cell lines using JetPRIME. The plasmid vector and ubiquitin-specific peptidase 2 plasmid were synthesized by Genechem (Shanghai, China). To evaluate the function of ubiquitin-specific peptidase 2 in vivo, we constructed Caki-1 cells stably overexpressing ubiquitin-specific peptidase 2 by infection with lentivirus synthesized by Genechem. To explore the mechanism of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma cells, shRNA ubiquitin-specific peptidase 2 (sequence as ubiquitin-specific peptidase 2–1) for 769-P and 786–0 cells were constructed by Genechem.
Cell proliferation, invasion, and migration assays
As described by Shen et al [Citation18], the Cell counting kit-8 and clone formation experiments were conducted to evaluate the proliferative abilities. For cell counting kit-8 experiments, 5,000 cells were seeded into each well of 96-well plates with six replicates for 769-P, 786–0, and Caki-1. Absorbance at 450 nm was measured using cell counting kit-8 assay (Dojindo, Kumamoto, Japan) at 12, 24, 48, and 72 h post-seeding. To analyze clone formation, 150 cells/well were seeded into each well of the 6-well plates. The cells were cultured for 2 weeks. According to the research method of Zhang, et al [Citation19], wound-healing experiments were performed to determine migratory ability of the cell. The cells were grown in a 6-well plate until reaching greater than 90% confluence. Images were captured under 200× magnification at 0, 24, and 48 h using the microscope. As described by Hong et al [Citation20], transwell chambers, coated with Matrigel (BD Bioscience, Franklin Lakes, NJ, USA), were used for the invasion assay. Cells (1 × 105) were seeded into the upper chamber with Roswell Park Memorial Institute 1640. The lower chamber contained 10% fetal bovine serum in Roswell Park Memorial Institute 1640. We randomly selected five images and counted the number of cells under 200× magnification with an IX71 microscope after 24 h.
Animal model
Animal experiments were conducted in accordance with the National Institutes of Health’s guide for the care and use of laboratory animals as well as with ARRIVE guidelines [Citation21]. The procedures were approved by the Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University. As described by Shen et al [Citation22], BALB/c nude mice (male, 5 weeks old) were randomly divided into two groups for in vivo experiments (n = 6 in each group). A subcutaneous injection in the right flank with 1 × 107 Caki-1(Lentivirus-vector and Lentivirus-Overexpression- ubiquitin-specific peptidase 2) cells was performed to investigate the tumor growth ability in vivo. The tumor size was measured every 3 days, and the tumor volume was calculated as [length × width 2]/2. Mice were sacrificed at 9 weeks after inoculation. The size and weight of tumors were measured.
Rescue experiments
The NF-κB inhibitor BAY 11–7082 from Peprotech (Rocky Hill, NJ, USA), was used for the rescue experiments [Citation23]. The migratory and invasive abilities were tested in vitro, with or without BAY 11–7082, for 786–0 and 769-P cells transfected with shRNA ubiquitin-specific peptidase 2 or shRNA Negative Control.
Statistical analysis
Statistical Product and Service Solutions software version 22.0 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. The data are presented as the mean ± standard deviation or mean rank. Group differences were analyzed using Student’s t-test or Mann-Whitney U test for nonparametric data. Significance was determined at a p-value <0.05.
Results
In this study, we hypothesized that ubiquitin-specific peptidase 2 could be a potential biomarker for clear cell renal cell carcinoma detection and clinical diagnosis. The results from our in vitro and in vivo research demonstrated that ubiquitin-specific peptidase 2 inhibits epithelial-mesenchymal transition in clear cell renal cell carcinoma metastasis by downregulating the NF-κB pathway.
Ubiquitin-specific peptidase 2 downregulation in clear cell renal cell carcinoma is associated with worse clinical stage and prognosis
To identify mRNAs important in clear cell renal cell carcinoma, mRNA-transcriptome sequencing was conducted. The threshold was set as log2 (fold-change) >1.5 or log2 (fold-change) <-1.5, p < 0.01. Ubiquitin-specific peptidase 2 (ENSG00000036672) was significantly downregulated in clear cell renal cell carcinoma tissues compared with in normal tissues ()).
Figure 1. Ubiquitin-specific peptidase 2 downregulation in clear cell renal cell carcinoma is associated with better clinical stage and prognosis. (a) The mRNA-transcriptome sequencing was used to identify mRNAs important in clear cell renal cell carcinoma between tumor tissues and normal tissues. The numbers of highly expressed genes (red) and lowly expressed genes (blue) were shown. (b) Gene Expression Profiling Interactive Analysis showed that the expression of ubiquitin-specific peptidase 2 in paracancerous tissues (gray) was higher than in tumor tissue (red). (c) The expression of ubiquitin-specific peptidase 2 significantly differed between patients with stage I and stage II shown using Gene Expression Profiling Interactive Analysis. (d), (e) Furthermore, Gene Expression Profiling Interactive Analysis revealed that higher expression of ubiquitin-specific peptidase 2 was positively correlated with a better clinical clear cell renal cell carcinoma prognosis including overall survival and disease-free survival. (f) Human Protein Atlas showed that ubiquitin-specific peptidase 2 was mainly enriched in the renal tubules. (g) The Human Protein Atlas showed that ubiquitin-specific peptidase 2 protein expression was higher in the kidney than in other normal tissues. (h) The group that showed a high expression of ubiquitin-specific peptidase 2 tended to have a higher survival probability for Human Protein Atlas. *p < 0.05. Abbreviations: KIRC, kidney renal cell carcinoma; HR, hazard ratio.
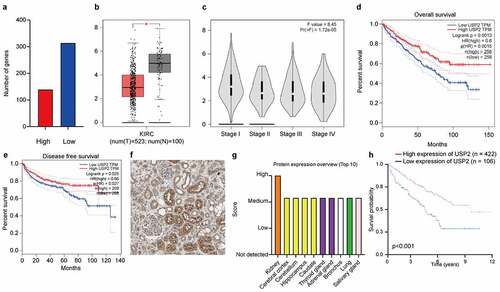
The results of Gene Expression Profiling Interactive Analysis showed that the ubiquitin-specific peptidase 2 expression in paracancerous tissues was higher than that in tumor tissue (p < 0.05; )). Further, the expression of ubiquitin-specific peptidase 2 significantly differed between patients with stage I and stage II clear cell renal cell carcinoma (p = 1.72e-05; )). Furthermore, Gene Expression Profiling Interactive Analysis revealed that higher expression of ubiquitin-specific peptidase 2 was positively correlated with a better clinical clear cell renal cell carcinoma prognosis, including overall survival and disease-free survival (p = 0.0013 and p = 0.025; ). The results of immunohistochemistry in the Human Protein Atlas showed that ubiquitin-specific peptidase 2 was mainly enriched in the renal tubules ()). The Human Protein Atlas showed that the protein expression level of ubiquitin-specific peptidase 2 was higher in the kidneys than in other normal tissues ()), and the group that showed a high expression of ubiquitin-specific peptidase 2 tended to have a higher survival probability for the Human Protein Atlas (p < 0.001; )).
The expression levels of ubiquitin-specific peptidase 2 were low in clear cell renal cell carcinoma tissues and cell lines, as detected using quantitative real-time polymerase chain reaction (p < 0.001; )), immunohistochemistry (p < 0.01; )), and Western blot ()). These results suggested that ubiquitin-specific peptidase 2 is downregulated in clear cell renal cell carcinoma tissues and cell lines. The immunohistochemistry results also showed that ubiquitin-specific peptidase 2 is mainly expressed in the renal proximal tubule ()).
Figure 2. Ubiquitin-specific peptidase 2 knockdown promoted clear cell renal cell carcinoma cell proliferation, migration, and invasion in vitro. (a) The expression of ubiquitin-specific peptidase 2 was low in clear cell renal cell carcinoma tissues detected using quantitative real-time polymerase. (b) The immunohistochemistry results showed that ubiquitin-specific peptidase 2 was richer in renal proximal tubule. (c) The concentrations of ubiquitin-specific peptidase 2 in urine samples of patients with different stages of clear cell renal cell carcinoma were lower than those of healthy volunteers. (d) The concentration of ubiquitin-specific peptidase 2 in urine samples of patients with clear cell renal cell carcinoma was significantly lower than that in healthy volunteers. (e) Protein expression of ubiquitin-specific peptidase 2 in 769-P, 786–0, and Caki-1 cells detected using Western blot. (f) The knockdown efficiency of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma cells shown using quantitative real-time polymerase chain reaction. (g), (h) The results of Cell Counting Kit-8 and clone formation assays showed that ubiquitin-specific peptidase 2 knockdown enhanced the proliferation rate of clear cell renal cell carcinoma cells. (i) Wound healing assays demonstrated that ubiquitin-specific peptidase 2 knockdown promoted greater migration of clear cell renal cell carcinoma cells. Three groups, Si-negative control and Si-ubiquitin-specific peptidase 2–1 for 786–0, Si-ubiquitin-specific peptidase 2–1 for 769-P were healed in 48 hours. (j) Invasion assays revealed that ubiquitin-specific peptidase 2 knockdown promoted the invasive ability of clear cell renal cell carcinoma cells. **p < 0.01, and ***p < 0.001.
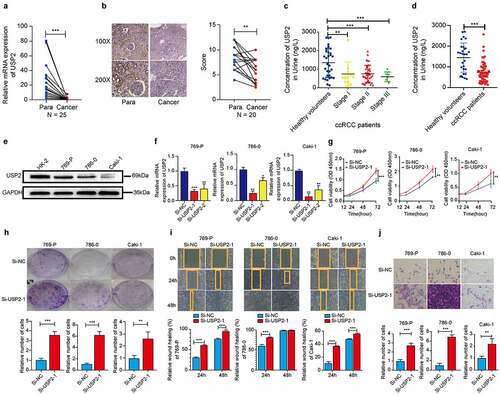
The findings suggested that ubiquitin-specific peptidase 2 concentrations may differ in urine samples from clear cell renal cell carcinoma patients and healthy volunteers. Therefore, Enzyme-linked immunosorbent assay was performed to detect the concentration of ubiquitin-specific peptidase 2 in urine samples. The results showed that the concentration of ubiquitin-specific peptidase 2 in patients with clear cell renal cell carcinoma was significantly lower than that in healthy volunteers (p < 0.001; ) and ). The cutoff value was 1,549 ng/L, and the area under receiver operating characteristic curve was 0.784.
Table 1. The prediction of ubiquitin-specific peptidase 2 between clear cell renal cell carcinoma patients and healthy volunteers.
Ubiquitin-specific peptidase 2 inhibited the proliferation, migration, and invasion of clear cell renal cell carcinoma cells
Caki-1 exhibited the lowest expression of ubiquitin-specific peptidase 2, whereas 769-P and 786–0 showed relatively higher expression levels ()). These three cell lines were all used to perform loss- and gain-of-function experiments.
Two independent ubiquitin-specific peptidase 2-specific siRNAs and Si-negative control were produced and transfected into 769-P, 786-O, and Caki-1 cells to reduce ubiquitin-specific peptidase 2 expression. Si-ubiquitin-specific peptidase 2–1 showed a stronger effect in these cell lines compared with Si-ubiquitin-specific peptidase 2–2 according to quantitative real-time polymerase chain reaction ()). Therefore, Si-ubiquitin-specific peptidase 2–1 was used for subsequent experiments. The results of the Cell Counting Kit-8 and clone formation assays showed that ubiquitin-specific peptidase 2 knockdown enhanced the proliferation rate of 769-P, 786-O, and Caki-1 cells (). Wound healing assays demonstrated that ubiquitin-specific peptidase 2 knockdown promoted greater migration ability of clear cell renal cell carcinoma cells ()). Invasion assays revealed that ubiquitin-specific peptidase 2 knockdown promoted the invasive ability of clear cell renal cell carcinoma cells ()).
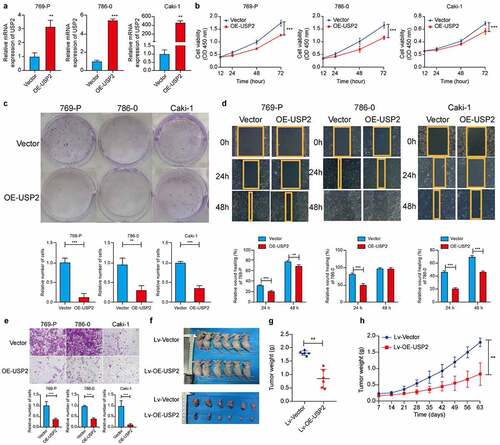
Further, we explored the role of ubiquitin-specific peptidase 2 overexpression in the three clear cell renal cell carcinoma cell lines. Efficiency was measured using quantitative real-time polymerase chain reaction ()). The proliferative abilities shown by the Cell Counting Kit-8 and clone formation assays indicated that cell proliferation was inhibited by ubiquitin-specific peptidase 2 overexpression in all three cell lines (). Ubiquitin-specific peptidase 2 overexpression prevented the migration and invasion ability of clear cell renal cell carcinoma cells (). These data showed that overexpression of ubiquitin-specific peptidase 2 downregulates the metastatic ability of clear cell renal cell carcinoma cells.
Figure 3. Ubiquitin-specific peptidase 2 overexpression suppressed clear cell renal cell carcinoma cells proliferation in vitro and in vivo. (a) The over-expression efficiency of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma cells shown using quantitative real-time polymerase chain reaction. (b, c) The results of Cell Counting Kit-8 and clone formation assays showed that ubiquitin-specific peptidase 2 overexpression suppressed the proliferation rate of clear cell renal cell carcinoma cells. (d) Wound healing assays demonstrated that ubiquitin-specific peptidase 2 overexpression inhibited migration ability of clear cell renal cell carcinoma cells. Both Vector and stably overexpressing ubiquitin-specific peptidase 2 groups for 786–0 cells healed at 48 hours. (e) Invasion assays revealed that ubiquitin-specific peptidase 2 overexpression prevented the invasive ability of clear cell renal cell carcinoma cells. (f) The size of tumor was significantly inhibited in the stably overexpressing ubiquitin-specific peptidase 2 vector group than in the lentivirus vector group. (g) Tumors in ubiquitin-specific peptidase 2 overexpression group were relatively lighter in weight. (h) Tumor growth curves for the lentivirus-Vector group and lentivirus-overexpression-ubiquitin-specific peptidase 2 group. Cells in the Vector and overexpression-ubiquitin-specific peptidase 2 groups were transfected with plasmid. The lentivirus-Vector and lentivirus-overexpression- ubiquitin-specific peptidase 2 groups were transduced with lentivirus. **p < 0.01 and, ***p < 0.001.
Overexpression of ubiquitin-specific peptidase 2 suppressed metastasis of clear cell renal cell carcinoma cells in vivo
Caki-1 cells, stably overexpressing ubiquitin-specific peptidase 2 and lentivirus vector, were subcutaneously injected into male BALB/c nude mice. A subsequent reduction in the tumor size was observed (). Tumors in the ubiquitin-specific peptidase 2 overexpression group weighed relatively lower ()). These results demonstrate that ubiquitin-specific peptidase 2 inhibited Caki-1 cell proliferation both in vivo and in vitro.
Ubiquitin-specific peptidase 2 inhibited epithelial-mesenchymal transition in clear cell renal cell carcinoma cells
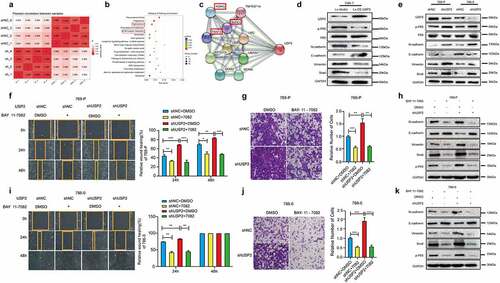
The results of RNA-seq sequencing showed that tight junction and extracellular matrix receptor interaction, epithelial-mesenchymal transition-related pathways, were changed remarkably (). Western blot results showed that the protein expression of N-cadherin, Vimentin, and Snail were negatively correlated with ubiquitin-specific peptidase 2 protein expression in Caki-1 cells ()), 769-P and 786-O ()). Protein expression of E-cadherin was positively correlated with ubiquitin-specific peptidase 2 protein expression in all three cell lines ().
Figure 4. Ubiquitin-specific peptidase 2 inhibited epithelial-mesenchymal transition in clear cell renal cell carcinoma cells by downregulating nuclear factor κB pathway. (a) Pearson correlation between shRNAs for negative control and ubiquitin-specific peptidase 2 in 786–0 cells. (b) Tight junction and extracellular matrix (ECM)-receptor interaction, epithelial-mesenchymal transition-related pathways, were changed remarkably. (c) The results of String showed that ubiquitin-specific peptidase 2 was related to NF-κB pathway gene as inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma (IKBKG), receptor-interacting protein kinase 1 (RIPK1), and TNF receptor associated factors 2(TRAF2). (d) The validation of the lentivirus vector and lentivirus for stably overexpressing ubiquitin-specific peptidase 2 in Caki-1, and the related genes expression for both NF-κB and epithelial-mesenchymal transition pathways. (e) The validation of shRNA negative control and shRNA ubiquitin-specific peptidase 2 in 769-P and 786–0 cells, along with the related genes expression for both NF-κB and epithelial-mesenchymal transition pathways. (f), (g) The migration and invasion abilities of 769-P cells were inhibited by BAY 11–7082 treatment in the shRNA ubiquitin-specific peptidase 2 group and shRNA negative control group. (h) The related gene expression of NF-κB and epithelial-mesenchymal transition pathways are shown, with or without BAY 11–7082. (i), (j) The migration and invasion abilities of 786–0 cells were inhibited by BAY 11–7082 treatment in the shRNA ubiquitin-specific peptidase 2 group and shRNA negative control group. (k) The related protein expression of NF-κB and epithelial-mesenchymal transition pathways are shown, with or without BAY 11–7082. *p < 0.05, **p < 0.01, and ***p < 0.001.
Ubiquitin-specific peptidase 2 inhibits epithelial-mesenchymal transition in clear cell renal cell carcinoma metastasis by downregulating the NF-κB Pathway
STRING results showed that ubiquitin-specific peptidase 2 was related to the NF-κB pathway according to inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma (IKBKG), receptor-interacting protein kinase 1 (RIPK1), and TNF receptor associated factors 2(TRAF2) ()). Western blot results showed that the NF-κB pathway was activated when ubiquitin-specific peptidase 2 was knocked down in 786-O and 769-P cells ()). The activation of the NF-κB pathway was decreased when ubiquitin-specific peptidase 2 was overexpressed in Caki-1 cells ()).
The rescue experiment results showed that both the migration and invasion abilities of 769-P () and 786–0 ()) cells were inhibited by BAY 11–7082 treatment in the shRNA ubiquitin-specific peptidase 2 and shRNA negative control groups. Protein expression of N-cadherin, Vimentin and Snail increased, whereas E-cadherin expression decreased following BAY 11–7082 treatment in both the shRNA ubiquitin-specific peptidase 2 and shRNA negative control groups (). Hence, ubiquitin-specific peptidase 2 inhibits epithelial-mesenchymal transition of clear cell renal cell carcinoma by downregulating the NF-κB pathway.
Discussion
Recently, researchers have mainly focused on the clinical features of clear cell renal cell carcinoma, such as the low rate of early-stage diagnosis and resistance to chemotherapy [Citation24]. We showed that expression of ubiquitin-specific peptidase 2 is reduced in both clear cell renal cell carcinoma cell line as well as tissues and urine samples, which was consistent with the results of previous studies [Citation25]. However, the potential of ubiquitin-specific peptidase 2 as a biomarker for clear cell renal cell carcinoma diagnosis and the mechanisms of this phenomenon have not been thoroughly investigated. On the other hand, we further evaluated ubiquitin-specific peptidase 2 knockdown in vitro and overexpressed ubiquitin-specific peptidase 2 in a xenograft mouse model to confirm the role of ubiquitin-specific peptidase 2 in clear cell renal cell carcinoma, which provided more support for our hypothesis [Citation26].
The research of biomarker in cancer has become a promising point and more meaningful than only in vitro experiments [Citation27,Citation28]. Given that urine sample collection is noninvasive compared to the collection of blood samples [Citation29–31]. Furthermore, since ubiquitin-specific peptidase 2 expression is mainly localized to the renal proximal tubule, we collected urine samples from both patients with clear cell renal cell carcinoma and healthy volunteers for clinical biomarker analysis [Citation32,Citation33]. The concentration of ubiquitin-specific peptidase 2 in urine samples from both patients with clear cell renal cell carcinoma and healthy volunteers showed a significant difference. Our results suggested that ubiquitin-specific peptidase 2 is a potential biomarker for distinguishing patients with clear cell renal cell carcinoma from healthy individuals.
Ubiquitin-specific peptidase 2 has shown promise for cancer therapy, especially in the treatment of ErbB2-positive breast cancer, targeting ubiquitin-specific peptidase 2-SKP2 axis and bladder cancer [Citation34–36]. Through our experiments, the results showed that ubiquitin-specific peptidase 2 negatively regulated the migration and invasion abilities in clear cell renal cell carcinoma cell lines. Additionally, ubiquitin-specific peptidase 2 was found to negatively regulate the epithelial-mesenchymal transition pathway by RNA sequencing results. A previous study reported that NF-κB is strongly involved in controlling carcinogenesis and positively regulating tumor proliferation [Citation37,Citation38]. Further research suggested that activation of NF-κB can promote prostate cancer metastasis [Citation39]. Epithelial-mesenchymal transition, which is positively regulated by NF-κB, also regulate and affect clear cell renal cell carcinoma migration and invasion through ubiquitin-specific peptidase 2 as shown in rescue experiments [Citation40]. The results revealed that ubiquitin-specific peptidase 2 downregulated NF-κB to inhibit epithelial-mesenchymal transition in clear cell renal cell carcinoma.
Conclusion
In conclusion, this research showed that ubiquitin-specific peptidase 2 was downregulated in clear cell renal cell carcinoma by RNA sequencing, bioinformatics and tissue validation. The results from both in vitro and in vivo showed that ubiquitin-specific peptidase 2 inhibited epithelial-mesenchymal transition by downregulating the NF-κB pathway. Our findings suggest that ubiquitin-specific peptidase 2, in urine samples, might be a potential biomarker for clear cell renal cell carcinoma detection and clinical diagnosis.
Supplemental Material
Download MS Word (470.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s)
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Klatte T, Stewart GD. Renal cell carcinoma: standards and controversies. World J Urol. 2018;36(12):1889–1890.
- Berglund A, Amankwah EK, Kim YC, et al. Influence of gene expression on survival of clear cell renal cell carcinoma. Cancer Med. 2020;9(22):8662–8675.
- Lin H, Keriel A, Morales CR, et al. Divergent N-terminal sequences target an inducible testis deubiquitinating enzyme to distinct subcellular structures. Mol Cell Biol. 2000;20(17):6568–6578.
- Chuang SJ, Cheng SC, Tang HC, et al. 6-Thioguanine is a noncompetitive and slow binding inhibitor of human deubiquitinating protease USP2. Sci Rep. 2018;8(1):3102.
- Zhang LN, Zhang DD, Yang L, et al. Roles of cell fusion between mesenchymal stromal/stem cells and malignant cells in tumor growth and metastasis. FEBS J. 2021;288(5):1447–1456.
- Yan X, Han D, Chen Z, et al. RUNX2 interacts with BRG1 to target CD44 for promoting invasion and migration of colorectal cancer cells. Cancer Cell Int. 2020;20:505.
- Zhang T, Zhao L, Zhang T, et al. Curcumin negatively regulates cigarette smoke-induced renal cell carcinoma epithelial-mesenchymal transition through the ERK5/AP-1 pathway. Onco Targets Ther. 2020;13:9689–9700.
- Chen W, Wang J, Wang X, et al. Knockdown of hypoxia-inducible factor 1-alpha (HIF1α) interferes with angiopoietin-like protein 2 (ANGPTL2) to attenuate high glucose-triggered hypoxia/reoxygenation injury in cardiomyocytes. Bioengineered. 2022;13(1):1476–1490.
- Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102.
- Davra V, Saleh T, Geng K, et al. Cyclophilin A inhibitor debio-025 targets Crk, reduces metastasis, and induces tumor immunogenicity in breast cancer. Mol Cancer Res. 2020;18(8):1189–1201.
- Kumar S, Lu B, Davra V, et al. Crk tyrosine phosphorylation regulates PDGF-BB-inducible Src activation and breast tumorigenicity and metastasis. Mol Cancer Res. 2018;16(1):173–183.
- Qin G, Wang X, Ye S, et al. NPM1 upregulates the transcription of PD-L1 and suppresses T cell activity in triple-negative breast cancer. Nat Commun. 2020;11(1):1669.
- Cao S, Zhang S. Forkhead-box C1 attenuates high glucose-induced trophoblast cell injury during gestational diabetes mellitus via activating adenosine monophosphate-activated protein kinase through regulating fibroblast growth factor 19. Bioengineered. 2022;13(1):1174–1184.
- Xie W, Sun Y, Zeng Y, et al. Comprehensive analysis of PPPCs family reveals the clinical significance of PPP1CA and PPP4C in breast cancer. Bioengineered. 2022;13(1):190–205.
- Xu L, Shao F. Sitagliptin protects renal glomerular endothelial cells against high glucose-induced dysfunction and injury. Bioengineered. 2022;13(1):655–666.
- Li H, Wang N, Xu Y, et al. Upregulating microRNA-373-3p promotes apoptosis and inhibits metastasis of hepatocellular carcinoma cells. Bioengineered. 2022;13(1):1304–1319.
- Metzig M, Nickles D, Falschlehner C, et al. An RNAi screen identifies USP2 as a factor required for TNF-α-induced NF-κB signaling. Int J Cancer. 2011;129(3):607–618.
- Shen E, Zhang J, Lu Y. DEP domain containing 1B (DEPDC1B) exerts the tumor promoter in hepatocellular carcinoma through activating p53 signaling pathway via kinesin family member 23 (KIF23). Bioengineered. 2022;13(1):1103–1114.
- Zhang J, Zhang Y, Li L, et al. Pregnancy-associated plasma protein-A (PAPPA) promotes breast cancer progression. Bioengineered. 2022;13(1):291–307.
- Hong X, Mao L, Xu L, et al. Prostate-specific membrane antigen modulates the progression of prostate cancer by regulating the synthesis of arginine and proline and the expression of androgen receptors and Fos proto-oncogenes. Bioengineered. 2022;13(1):995–1012.
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLOS Biol. 2010;8(6):e1000412.
- Shen J, Wang M, Li F, et al. Homeodomain-containing gene 10 contributed to breast cancer malignant behaviors by activating Interleukin-6/Janus kinase 2/Signal transducer and activator of transcription 3 pathway. Bioengineered. 2022;13(1):1335–1345.
- Pustchi SE, Avci NG, Akay YM, et al. Astrocytes decreased the sensitivity of glioblastoma cells to temozolomide and Bay 11–7082. Int J Mol Sci. 2020;21(19):7154.
- Li P, Xiao J, Zhou B, et al. SYNE1 mutation may enhance the response to immune checkpoint blockade therapy in clear cell renal cell carcinoma patients. Aging (Albany, NY). 2020;12(19):19316–19324.
- Meng X, Xiong Z, Xiao W, et al. Downregulation of ubiquitin-specific protease 2 possesses prognostic and diagnostic value and promotes the clear cell renal cell carcinoma progression. Ann Transl Med. 2020;8(6):319.
- Zhu F, Duan W, Zhong C, et al. The protective effects of dezocine on interleukin-1β-induced inflammation, oxidative stress and apoptosis of human nucleus pulposus cells and the possible mechanisms. Bioengineered. 2022;13(1):1399–1410.
- Feng M, Dong N, Zhou X, et al. Myosin light chain 9 promotes the proliferation, invasion, migration and angiogenesis of colorectal cancer cells by binding to Yes-associated protein 1 and regulating Hippo signaling. Bioengineered. 2022;13(1):96–106.
- Kubiliūtė R, Žukauskaitė K, and Žalimas A, et al. Clinical significance of novel DNA methylation biomarkers for renal clear cell carcinoma. J Cancer Res Clin Oncol. 2021. DOI:10.1007/s00432-021-03837-7.
- Murdocca M, Torino F, Pucci S, et al. Urine LOX-1 and volatilome as promising tools towards the early detection of renal cancer. Cancers (Basel). 2021;13(16):4213.
- Arendowski A, Ossoliński K, Ossolińska A, et al. Serum and urine analysis with gold nanoparticle-assisted laser desorption/ionization mass spectrometry for renal cell carcinoma metabolic biomarkers discovery. Adv Med Sci. 2021;66(2):326–335.
- Huang H, Cui G, Tang H, et al. Relationships between plasma expression levels of microRNA-146a and microRNA-132 in epileptic patients and their cognitive, mental and psychological disorders. Bioengineered. 2022;13(1):941–949.
- Lee CH, Motzer RJ, Glen H, et al. Correlative serum biomarker analyses in the phase 2 trial of lenvatinib-plus-everolimus in patients with metastatic renal cell carcinoma. Br J Cancer. 2021;124(1):237–246.
- Pastore AL, Palleschi G, Silvestri L, et al. Serum and urine biomarkers for human renal cell carcinoma. Dis Markers. 2015;2015:251403.
- Zhang J, Liu S, Li Q, et al. The deubiquitylase USP 2 maintains ErbB2 abundance via counteracting endocytic degradation and represents a therapeutic target in ErbB2-positive breast cancer. Cell Death Differ. 2020;27(9):2710–2725.
- Zhang F, Zhao Y, Sun Y. USP 2 is an SKP2 deubiquitylase that stabilizes both SKP2 and its substrates. J Biol Chem. 2021;297(4):101109.
- Liu XQ, Shao XR, Liu Y, et al. Tight junction protein 1 promotes vasculature remodeling via regulating USP2/TWIST1 in bladder cancer. Oncogene. 2021;41:502–514.
- D’Orazi G, Cordani M, Cirone M. Oncogenic pathways activated by pro-inflammatory cytokines promote mutant p53 stability: clue for novel anticancer therapies. Cell Mol Life Sci. 2021;78(5):1853–1860.
- Zhang LM, Su LX, Hu JZ, et al. Epigenetic regulation of VENTXP1 suppresses tumor proliferation via miR-205-5p/ANKRD2/NF-kB signaling in head and neck squamous cell carcinoma. Cell Death Dis. 2020;11(10):838.
- Jadli M, Thakur K, Aggarwal N, et al. Delineating role of NF-κB and interacting cytokines during prostate cancer-induced osteoclastogenesis. J Cell Biochem. 2021;122(2):259–276.
- Liu W, Wang H, Bai F, et al. IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-κB. Cell Prolif. 2020;53(3):e12776.
