ABSTRACT
Studies have revealed the relationship between histone deacetylases (HDACs)/microRNAs (miRNAs) and sepsis, but little has ever investigated the mechanism of HDAC1/miR-124-5p in sepsis. Herein, we studied the impacts of HDAC1/miR-124-5p on myocardial damage of septic mice via regulating high-mobility group box chromosomal protein 1 (HMGB1). Septic mice were induced by cecal ligation and puncture. HDAC1, miR-124-5p and HMGB1 expression in myocardial tissues of septic mice were detected. Septic mice were injected with HDAC1 low expression-, miR-124-5p high expression- or HMGB1 low expression-related structures to observe cardiac function, inflammatory response, oxidative stress response, myocardial pathological changes and apoptosis in myocardial tissues of septic mice. The relationship of HDAC1/miR-124-5p/HMGB1 was verified. HDAC1 and HMGB1 expression were upregulated while miR-124-5p expression was decreased in myocardial tissues of septic mice. Restored miR-124-5p/depleted HDAC1 or HMGB1 recovered the cardiac function, improved cardiac function, inflammatory response, oxidative stress response, myocardial pathological changes and inhibit ed cardiomyocyte apoptosis in septic mice. HDAC1 bound to miR-124-5p which directly targeted HMGB1. This study suggests that down-regulated HDAC1 or up-regulated miR-124-5p recovers myocardial damage of septic mice via decreasing HMGB1.
GRAPHICAL ABSTRACT
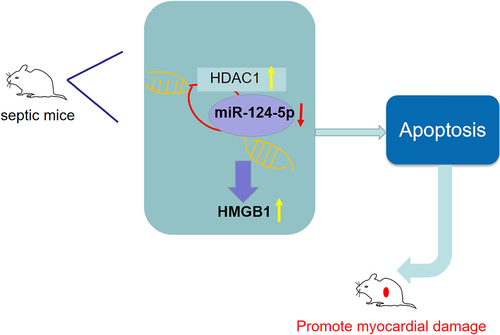
Introduction
Sepsis refers to potentially morbid organ dysfunction induced by maladjustment of the host response to infection, and featured by immune inhibition, early activation of inflammatory responses as well as coagulation [Citation1]. It is a major cause of mortality, morbidity, and healthcare for children in the world [Citation2]. The symptoms of sepsis patients vary according to fever, shock, and organ dysfunction [Citation3]. Severe sepsis can result in multiple organ dysfunction, among which cardiac dysfunction is a frequent complication [Citation4]. Multiple organ dysfunctions often happen and complicate sepsis, leading to a worse prognosis [Citation5]. Despite efforts made to ameliorate therapeutic interventions for sepsis, treatment outcomes remain unsatisfactory [Citation6]. The severe situation of sepsis treatment makes it necessary to further explore the mechanism of sepsis and find a new therapeutic strategy.
Histone deacetylase (HDAC) belongs to an epigenetic modifier and exerts crucially in modulating cell proliferation and differentiation [Citation7]. As a class I HDAC, HDAC1 is significant in multiple biological processes [Citation8]. A study has reported that HDAC suppressors represses pro- and anti-inflammatory mediator expression, which impedes sepsis progression [Citation9]. It has been reported that HDAC1 is implicated in myocardial tissues of septic rats [Citation10]. MicroRNAs (miRs) can modulate different target mRNAs expression in a sequence-specific manner [Citation11]. It has been presented that miR-124-5p regulates the inflammatory microenvironment and the reactive oxygen species (ROS) production to protect against cerebral I/R injury [Citation12]. Moreover, miR-124 is accounted for raised sepsis risk, and correlates with the deteriorative disease condition, higher inflammation, and reduced survival in sepsis patients [Citation13]. High-mobility group box chromosomal protein 1 (HMGB1) is a highly conserved widely expressed nuclear protein that participated in gene transcription and nucleosome stabilization [Citation14]. According to Walko III et al., it is documented that HMGB1 secretion is involved in sepsis [Citation15]. A previous study has displayed that HMGB1 interacts with lipopolysaccharide to mediate caspase-11-dependent pyroptosis in lethal sepsis [Citation16]. However, the role of miR-124-5p and HDAC1 in myocardial injury of septic mice has not yet been illustrated in combination.
As stated above, HDAC1, miR-124-5p, and HMGB1 are potentially involved in sepsis progression. However, the regulatory mechanism of HDAC1/miR-124-5p/HMGB1 axis and the interaction among them have not been fully explored. Based on previous references and current exploration, we aim to clarify the mechanism of HDAC1/miR-124-5p in myocardial injury in septic mice via regulating HMGB1 and hypothesize that HDAC1 worsens sepsis-induced myocardial injury through mediating miR-124-5p and HMGB1.
Materials and methods
Ethics statement
All animal experiments were performed in line with the Guide for the Care and Use of Laboratory Animal of the National Institutes of Health. The protocol was permitted by the Committee on the Ethics of Animal Experiments of the Affiliated Hospital of Youjiang Medical University for Nationalities.
Experiment animals and establishment of sepsis mouse models
Male C57BL/6 mice (8–10 weeks, 20–22 g) were bought from the animal center of Youjiang Medical University for Nationalities (Guangxi, China). Mice were placed at 24 ± 2°C with free access to drinking and eating and adaptively fed for 7 d under 12 h day-night cycles.
The sepsis mouse model was established by cecal ligation and puncture (CLP). Mice have fasted for 24 h before modeling. Mice were anesthetized with the intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg). After disinfection, a 2–3 cm incision was made longitudinally below the xiphoid process to expose the abdominal cavity. The cecum was isolated and ligated at half the distance between the distal end and the bottom of the cecum, and punctured twice with a 22-gauge needle. Then, the cecum was carefully put back into the abdominal cavity and the abdomen was sutured. For mice with sham operation, the incision was sutured, but puncture was not performed[Citation17].
Animal treatment
Septic mice were intramyocardially injected with HDAC1 siRNA, negative control (NC)-siRNA, miR-124-5p agomir, agomir NC, HMGB1 shRNA, sh-NC, si-HADC1 + miR-124-5p antagomir and si-HADC1 + overexpressed (oe)-HMGB1. All these constructs were purchased from Genechem Co., Ltd. (Shanghai, China). The injectable dose of miR-124-5p agomir and antagomir was 30 mg/kg and that of siRNA or plasmid was 100 ng [Citation18]. A model group was set (mice were intramyocardially injected with an equal volume of saline). After 24 h, the cardiac function factors left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were tested by VEVO 770 (Visual Sonics, Inc., Toronto, Canada) and 30-MHz ultrasonic probe (type: RMV707B).
Enzyme-linked immunosorbent assay (ELISA)
Mice (n = 8/group) were euthanized after 72 h of injection. The left carotid was separated and the blood was taken. The blood was centrifuged at 3000 r/min for 3 min to harvest the serum. HMGB1 (1:1000; Abcam, Cambridge, USA), tumor necrosis factor-α (TNF-α) (1:1000; Abcam), interleukin (IL)-6 (1:1000; Abcam), IL-1β (1:1000; Abcam), B-type natriuretic peptide (BNP) (1:1000; Abcam), cardiac Troponin-I (cTnI) (1:1000; Abcam) and ROS (1:1000; Abcam) were tested by the ELISA kit (ThermoFisher, Massachusetts, USA) [Citation19].
Detection of oxidative stress
The myocardial tissue of mice (n = 8/group) was prepared into protein homogenate with normal saline at 1:9. With the detection kits ((Nanjing Juli Institute of Biomedical Engineering, Nanjing, China), superoxide (SOD), glutathione peroxidase (GSH-Px) and malondiadehyde (MDA) were detected [Citation19].
Hematoxylin-eosin (HE) staining
Myocardial tissues of mice (n = 5/group) were fixed with 4% paraformaldehyde, embedded in paraffin and sectioned for staining by the HE staining kit (ThermoFisher).
Transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining
Apoptosis of cardiomyocytes of mice (n = 5/group) was measured by the TUNEL kit (Boehringer Mannheim, Mannheim, Germany). The specific steps were referred to a publication [Citation20].
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The total RNA extraction kit (Invitrogen, CA, USA) was utilized to extract the total RNA in myocardial tissues. The primers (Supplementary Table S1) were compounded by Invitrogen. U6 and β-actin served as the loading control. Revert Aid First Strand Complementary DNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) or Mir-X miRNA First-Strand Synthesis Kit (Takara, Japan) was used to reversely transcribe complementary DNA (cDNA) for mRNA or miRNA. The 2−ΔΔCt was used for quantitative analysis. Each reaction was run in triplicate.
Western blot analysis
The total protein in myocardial tissues was extracted, and the protein concentration was determined by the bicinchoninic acid method. The protein was transferred to the polyvinylidene fluoride membrane and added with blocking fluid (PBS containing 3% fetal bovine serum and 0.1% Tween-20) for 1 h. The membrane was probed with the corresponding dilution proportion (all 1:1000) of primary antibodies HDAC1, HMGB1 (Abcam), β-actin (Santa Cruz Biotechnology, CA, USA) and cleaved caspase-3 (Abcam) overnight, then re-probed with immunoglobulin G (Cell Signaling Technology) labeled by horseradish peroxidase. The blots were developed using enhanced chemiluminescence (ECL) Kit from Beyotime Institute of Biotechnology (China) and visualized using the ChemImager 5500 V2.03 software from Alpha Innotech (San Leandro, CA).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were implemented using Magna ChIP Assay kit (Millipore) following the manufacturer's instruction. In short, tissues were fixed with 1% formaldehyde and collected on ice with ChIP lysis buffer. Afterward, the DNA was sonicated and next, the supernatant was obtained and cultured with dynabeads protein G that had been conjugated with anti‐SP1, anti‐HDAC1 and anti‐acetylated histone antibodies. IgG was utilized as a negative control. ChIP‐derived DNA was quantified with qRT‐PCR with SYBR Green incorporation (Applied Biosystems) [Citation21].
Dual luciferase reporter gene assay
The target relationship between miR-124-5p and HMGB1 as well as the binding site of miR-124-5p and HMGB1 3ʹuntranslated region (3ʹUTR) were forecasted by bioinformatics software (http://www.targetscan.org/vert_72). TM The 3′UTR of HMGB1 containing the miR-124-5p binding sites was cloned into pMIR-Report Luciferase vector (Ambion, Austin, TX, USA) downstream of the firefly luciferase gene to generate the wild-type(WT)3′UTR luciferase reporter vector. Subsequently, the mutant-type (MUT) 3′UTR luciferase reporter vector was developed by the site-directed mutagenesis with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA). The 293 T cells were seeded onto 24-well plates. The cells reaching 70–80% confluence were co-transfected with WT or MUT 3′UTR-Luc reporter vector and miR-124-5p mimic by using Lipofectamine 2000 (Invitrogen). The pRL-CMV Renilla luciferase vector (Promega, Madison, WI, USA) was utilized to normalize the cell numbers and transfection efficiency. After an additional 48 h, the luciferase activity was detected by using the dual luciferase assay (Promega). Each reaction was run in triplicate.
RNA immunoprecipitation (RIP) assay
RIP assay, performed with EZ-Magna RIP kit (Millipore), was utilized to analyze the relationship between HMGB1 and miR-124-5p. The cells transfected with miR-124-5p mimic or mimic NC were lysed in a lysis buffer and reacted with magnetic beads conjugated with anti-Ago2 and anti-IgG (Abcam). The co-precipitated RNA was checked by RT-qPCR [Citation22]. Each reaction was run in triplicate.
Statistical analysis
All data were analyzed by GraphPad Prism 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 21.0 software (IBM, NY, USA). Measurement data were indicated as mean ± standard deviation. Comparisons between two groups of data were conducted by t test, while those among multiple groups by analysis of variance (ANOVA) and Tukey post hoc tests. P value < 0.05 was indicative of statistically significant difference.
Results
Induction of sepsis in mice
We used CLP to prepare a sepsis mouse model and the success of modeling was assessed. Firstly, cardiac function was tested to be severely impaired, which was manifested by decreased LVEF and LVFS values in mice after modeling ().
Figure 1. Induction of sepsis in mice. a. LVEF value in mice with sepsis; b. LVFS value of mice with sepsis; c. Serum HMGB1 level of mice with sepsis; D. Serum BNP level of mice with sepsis; e. Serum cTnI level of mice with sepsis; f. Serum TNF-α, IL-6 and IL-1β levels of mice with sepsis; g. Serum ROS level of mice with sepsis; h. SOD activity in myocardial tissues of mice with sepsis; i. MDA content in myocardial tissues of mice with sepsis; j. GSH-Px in myocardial tissues of mice with sepsis; k. HE staining of myocardial tissues of mice with sepsis; l. TUNEL staining of myocardial tissues of mice with sepsis; m. TNF-α, IL-6 and IL-1β and apoptosis-related factors Bcl-2, Bax and Caspase-3 mRNA expression levels in myocardial tissues of mice with sepsis; n. cleaved Caspase-3 protein level in myocardial tissues of mice with sepsis. The data were all measurement data, and represented by the mean ± standard deviation; * P < 0.05 vs. the sham group.
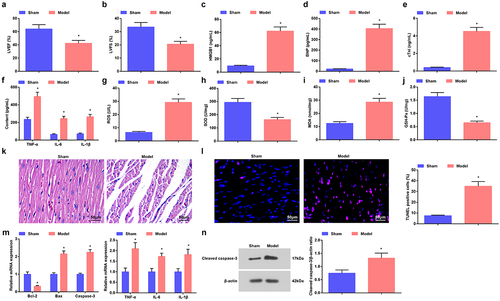
Then, ELISA method detected the factors in the serum of mice and found that HMGB1, BNP, cTnI, TNF-α, IL-6 and IL-1β levels were increased in mice treated with CLP ().
Oxidative stress injury of myocardial tissues in mice showed that in mice accepted CLP, ROS and MDA contents were increased, while SOD and GSH-Px activities were impaired ().
HE staining showed that the myocardial tissues of mice with sham operation were uniformly stained, the myocardial fibers were arranged regularly, and there was no inflammatory cell infiltration in the intermuscular spaces. Mice treated with CLP showed disordered myocardial tissues, blurred horizontal striations, myocardial degeneration and dissolution, broken myocardial fibers, myocardial rupture, and a lot of inflammatory cell infiltration in the intermuscular space ().
TUNEL staining manifested that the number of positive cells was increased (). The mRNA expression levels of related genes in myocardial tissues was examined by RT-qPCR, which suggested that TNF-α, IL-6 and IL-1β levels were augmented, while Bcl-2 mRNA was reduced, and Bax and Caspase-3 mRNA levels were increased in myocardial tissues of mice after modeling (); the protein levels of cleaved Caspase-3 were also elevated as reflected by Western blot assay ().
The phenomenons revealed that the sepsis mouse model was successfully induced.
Raised miR-124-5p ameliorates sepsis in mice
It is indicated that overexpression of miR-124 reduces doxorubicin-induced cardiac injury-caused oxidative stress and apoptosis [Citation23]. Detected by RT-qPCR, we found that miR-124-5p level was reduced in septic mice (). To comprehend the mechanism of miR-124-5p on myocardial injury in septic mice, we injected miR-124-5p agomir into septic mice, and it was verified that miR-124-5p level was raised ().
Figure 2. Raised miR-124-5p ameliorates sepsis in mice. a/b. miR-124-5p expression in myocardial tissues of septic mice after up-regulating miR-124-5p; c. LVEF value in mice with sepsis after up-regulating miR-124-5p; d. LVFS value of mice with sepsis after up-regulating miR-124-5p; e. Serum HMGB1 level of mice with sepsis after up-regulating miR-124-5p; f. Serum BNP level of mice with sepsis after up-regulating miR-124-5p; g. Serum cTnI level of mice with sepsis after up-regulating miR-124-5p; h. Serum TNF-α, IL-6 and IL-1β levels of mice with sepsis after up-regulating miR-124-5p; I. Serum ROS level of mice with sepsis after up-regulating miR-124-5p; j. SOD activity in myocardial tissues of mice with sepsis after up-regulating miR-124-5p; k. MDA content in myocardial tissues of mice with sepsis after up-regulating miR-124-5p; l. GSH-Px in myocardial tissues of mice with sepsis after up-regulating miR-124-5p; m. HE staining of myocardial tissues of mice with sepsis after up-regulating miR-124-5p; n. TUNEL staining of myocardial tissues of mice with sepsis after up-regulating miR-124-5p; o. TNF-α, IL-6, IL-1β, Bcl-2, Bax and Caspase-3 mRNA expression levels in myocardial tissues of mice with sepsis after up-regulating miR-124-5p; p. cleaved Caspase-3 protein level in myocardial tissues of mice with sepsis after up-regulating miR-124-5p. The data were all measurement data, and represented by the mean ± standard deviation; # P < 0.05 vs. the sham group; * P < 0.05 vs. the agomir NC group.
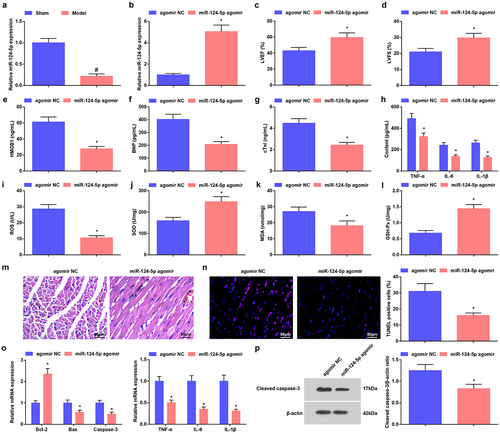
Results collected from experiments showed that LVEF and LVFS were enhanced, HMGB1, BNP, cTnI, TNF-α, IL-6, IL-1β, ROS and MDA contents were decreased while SOD and GSH-Px activities were facilitated by up-regulating miR-124-5p in septic mice ().
Further analysis of miR-124-5p effects displayed that miR-124-5p agomir treatment mitigated myocardial tissue injury (), decreased TUNEL-positive cells (), reduced inflammatory factors TNF-α, IL-6 and IL-1β levels, elevated Bcl-2 but reduced Bax and Caspase-3 mRNA levels and decreased protein levels of cleaved Caspase-3 ().
The findings suggested that elevating miR-124-5p improved myocardial injury in septic mice.
Reduced HDAC1 or HMGB1 attenuates sepsis in mice
Changes in the deacetylase activity of HDAC1 in cardiomyocyte mitochondria affect the maximum rate of oxygen consumption of cardiomyocyte mitochondria [Citation24]. HMGB1 is up-regulated in myocardial tissues, imposing an impact on the myocardial inflammatory injury [Citation25]. In septic mice, HDAC1 and HMGB1 levels were both augmented in myocardial tissues ( and ). We injected si-HADC1 or sh-HMGB1 into septic mice, and verified the interference efficacy of the plasmids by Western blot ().
Figure 3. Reduced HDAC1 attenuates sepsis in mice. a-d. HDAC1 expression in myocardial tissues of septic mice; e. LVEF value in mice with sepsis after down-regulating HDAC1; f. LVFS value of mice with sepsis after down-regulating HDAC1; g. Serum HMGB1 level of mice with sepsis after down-regulating HDAC1; h. Serum BNP level of mice with sepsis after down-regulating HDAC1; i. Serum cTnI level of mice with sepsis after down-regulating HDAC1; j. Serum TNF-α, IL-6 and IL-1β levels of mice with sepsis after down-regulating HDAC1; k. Serum ROS level of mice with sepsis after down-regulating HDAC1; L. SOD activity in myocardial tissues of mice with sepsis after down-regulating HDAC1; m. MDA content in myocardial tissues of mice with sepsis after down-regulating HDAC1; n. GSH-Px in myocardial tissues of mice with sepsis after down-regulating HDAC1; o. HE staining of myocardial tissues of mice with sepsis after down-regulating HDAC1; p. TUNEL staining of myocardial tissues of mice with sepsis after down-regulating HDAC1; q. TNF-α, IL-6, IL-1β, Bcl-2, Bax and Caspase-3 mRNA expression levels in myocardial tissues of mice with sepsis after down-regulating HDAC1; r. cleaved Caspase-3 protein level in myocardial tissues of mice with sepsis after down-regulating HDAC1. The data were all measurement data, and represented by the mean ± standard deviation; # P < 0.05 vs. the sham group; * P < 0.05 vs. the si-NC group.
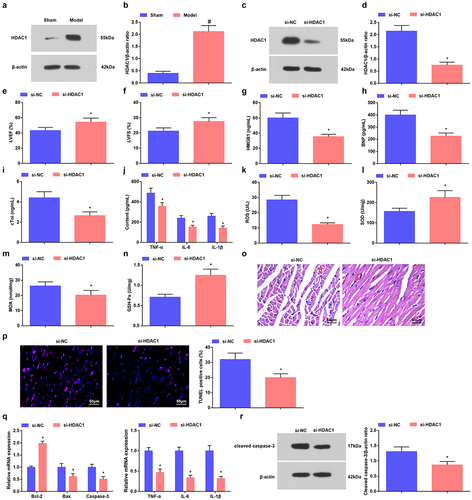
Figure 4. Reduced HDAC1 or HMGB1 attenuates sepsis in mice. a-d. HMGB1 expression in myocardial tissues of septic mice after down-regulating HMGB1; e. LVEF value in mice with sepsis after down-regulating HMGB1; f. LVFS value of mice with sepsis after down-regulating HMGB1; g. Serum HMGB1 level of mice with sepsis after down-regulating HMGB1; h. Serum BNP level of mice with sepsis after down-regulating HMGB1; i. Serum cTnI level of mice with sepsis after down-regulating HMGB1; j. Serum TNF-α, IL-6 and IL-1β levels of mice with sepsis after down-regulating HMGB1; k. Serum ROS level of mice with sepsis after down-regulating HMGB1; l. SOD activity in myocardial tissues of mice with sepsis after down-regulating HMGB1; m. MDA content in myocardial tissues of mice with sepsis after down-regulating HMGB1; n. GSH-Px in myocardial tissues of mice with sepsis after down-regulating HMGB1; o. HE staining of myocardial tissues of mice with sepsis after down-regulating HMGB1; p. TUNEL staining of myocardial tissues of mice with sepsis after down-regulating HMGB1; q. TNF-α, IL-6, IL-1β, Bcl-2, Bax and Caspase-3 mRNA expression levels in myocardial tissues of mice with sepsis after down-regulating HMGB1; r. cleaved Caspase-3 protein level in myocardial tissues of mice with sepsis after down-regulating HMGB1. The data were all measurement data, and represented by the mean ± standard deviation; # P < 0.05 vs. the sham group; * P < 0.05 vs. the sh-NC group.
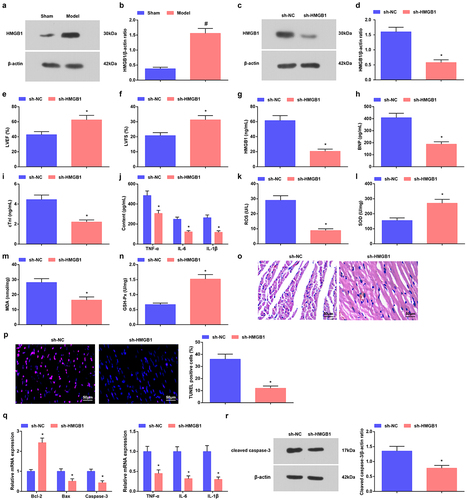
Next, in septic mice interfered with si-HDAC1 or sh-HMGB1, the cardiac function was recovered, myocardial injury, inflammation, and oxidative stress were improved, and apoptosis of cardiomyocytes was suppressed (, ). In addition, in septic mice interfered with si-HDAC1 or sh-HMGB1, there showed reduced inflammatory factors TNF-α, IL-6 and IL-1βlevels, elevated Bcl-2 but reduced Bax and Caspase-3 mRNA levels and decreased protein levels of cleaved Caspase-3 ().
Therefore, the cardioprotective effects of down-regulated HDAC1 or HMGB1 were evidenced in mice with sepsis.
HDAC1 binds to miR-124-5p; miR-124-5p directly targets HMGB1
Also, we studied the interaction between miR-124-5p, HDAC1 and HMGB1. In ChIP assay, we observed that HDAC1 was recruited specifically over IgG at the miR-124-5p promoter (). In RT-qPCR, we tested an enhanced level of miR-124-5p after silencing HADC1 ().
Figure 5. HDAC1 binds to miR-124-5p; miR-124-5p directly targets to HMGB1. a. The interaction between HDAC1 and miR-124-5p in ChIP assay; b. miR-124-5p expression after down-regulating HDAC1; c. The predicted binding site between miR-124-5p and HMGB1; d. The targeting relationship between miR-124-5p and HMGB1; e. The targeting relationship between miR-124-5p and HMGB1 in RIP assay; f. HMGB1 expression after up-regulating miR-124-5p. The data were all measurement data, and represented by the mean ± standard deviation; * P < 0.05 vs. the si-NC group; # P < 0.05 vs. the agomir NC group.
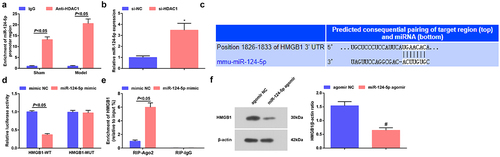
The website RNA22 showed that miR-124-5p could target HMGB1 (). Dual-luciferase reporter gene assay displayed that () miR-124-5p overexpression decreased the luciferase activity of HMGB1-WT vector, but did not significantly change the luciferase activity of HMGB1–MUT vector, indicating that miR-124-5p could directly bind to the 3ʹUTR of HMGB1. Also, we discovered in RIP assay that the ectopic expression of miR-124-5p augmented the enrichment of AGO2 at HMGB1 mRNA () and examined inWestern blot that HMGB1 level was decreased after the elevation of miR-124-5p ().
Thus, HDAC1 binding to miR-124-5p, and miR-124-5p targeting HMGB1 were proved.
Inhibition of miR-124-5p or elevation of HMGB1 mitigates silenced HDAC1-mediated protections on septic mice
We performed rescue experiments and eventually revealed that miR-124-5p antagomir and oe-HMGB1 weakened the effects of si-HDAC1 on mice with sepsis ().
Figure 6. Inhibition of miR-124-5p or elevation of HMGB1 mitigates silenced HDAC1-mediated protections on septic mice. a. HMGB1 expression in myocardial tissues of septic mice in rescue experiments; b. LVEF value in mice with sepsis in rescue experiments; c. LVFS value of mice with sepsis in rescue experiments; d. Serum HMGB1 level of mice with sepsis in rescue experiments; e. Serum BNP level of mice with sepsis in rescue experiments; f. Serum cTnI level of mice with sepsis in rescue experiments; G. Serum TNF-α, IL-6 and IL-1β levels of mice with sepsis in rescue experiments; h. Serum ROS level of mice with sepsis in rescue experiments; i. SOD activity in myocardial tissues of mice with sepsis in rescue experiments; J. MDA content in myocardial tissues of mice with sepsis in rescue experiments; k. GSH-Px in myocardial tissues of mice with sepsis in rescue experiments; l. HE staining of myocardial tissues of mice with sepsis in rescue experiments ; m. TUNEL staining of myocardial tissues of mice with sepsis in rescue experiments; n. TNF-α, IL-6, IL-1β, Bcl-2, Bax and Caspase-3 mRNA expression levels in myocardial tissues of mice with sepsis in rescue experiments; o. cleaved Caspase-3 protein level in myocardial tissues of mice with sepsis in rescue experiments;. The data were all measurement data, and represented by the mean ± standard deviation;* P < 0.05 vs. the si-HDAC1 group.
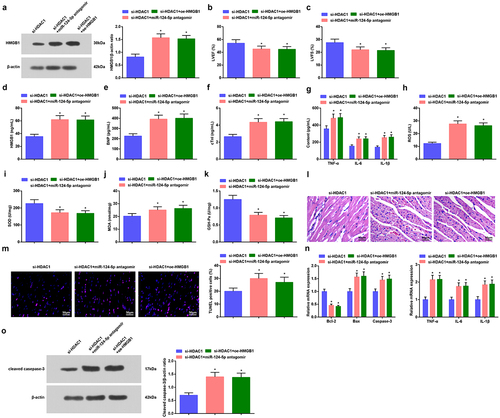
Those findings implied that HDAC1 promoted HMGB1 by inhibiting miR-124-5p, and worsened myocardial damage in septic mice.
Discussion
Sepsis is a fatal, expensive, and worldwide disease induced by the infection that causes systemic inflammatory response syndrome [Citation26]. As previously explored, HDAC1 is involved in autophagy in the myocardial tissues of septic mice [Citation10]. Another study has revealed that miR-124 is related to sepsis risk and survival of patients with sepsis [Citation27]. It has been presented that myocardial HMGB1 translocation participates in severe sepsis [Citation28]. The current study was designed to explore the mechanism of HDAC1 in myocardial injury in septic mice via regulating miR-124-5p and HMGB1.
Our study has revealed that HDAC1 level was raised in myocardial tissues in septic mice. A recent study has promoted that HDAC1 expression is enhanced in the myocardial tissues in sepsis [Citation10]. Another study has presented that HDAC1 protein expression is markedly elevated in myocardial infarction rats [Citation29]. Additionally, we also found that depletion of HDAC1 raised LVEF and LVFS levels, SOD and GSH-Px activities and Bcl-2 mRNA expression as well as reduced serum BNP, cTnI, TNF-α, IL-6 and IL-10 levels, ROS and MDA contents as well as Bax and Caspase-3 mRNA expression and cleaved Caspase-3 protein expression. It has been indicated in a study that that scavenging ROS and depletion of HDAC1 mitigate the apoptosis of HMEC-1 cells in high glucose [Citation30]. A study has reported that down-regulated HDAC1 restrains inflammation as reflected by inhibiting inducible nitric oxide synthase and TNF-α contents [Citation31]. A study has shown that lowly expressed HDAC1 attenuates invasion and accelerates apoptosis in non-small cell lung cancer cells [Citation32]. Also, selectively down-regulating mitochondrial HDAC1 is helpful for the attenuation of myocardial ischemia/reperfusion injury [Citation24].
Subsequently. It was predicted that HDAC1 bound to miR-124-5p. MiR-124-5p was down-regulated in septic mice and restoration of miR-124-5p recovered cardiac function, attenuated myocardial injury, oxidative stress and inflammatory responses, as well as reduced apoptosis of cardiomyocytes. It is reported that miR-124 expression is depressed in patients with sepsis in comparison to healthy controls [Citation33]. Similarly, miR-124-5p content is revealed to decrease in high-grade gliomas and colorectal cancer [Citation34,Citation35]. A study has purported that up-regulating miR-124 notably inhibits ROS production, TNF-α and IL-6 production, and cell apoptosis while raises SOD activity in the cerebral I/R injury [Citation12]. Another study has manifested that the cell apoptosis and oxidative stress are lightened via restoring miR-124, featured by reduced activity of MDA and enhanced activity of SOD [Citation23]. A study has reported that restoration of miR-124-3p suppresses the hypoxia/reoxygenation-induced cell apoptosis and decreases TNF-α, IL-6 and IL-1β levels [Citation36].
Moreover, our study demonstrated that HMGB1 was targeted and mediated by miR-124-5p, and we also demonstrated that silencing of HMGB1 exerted similar effects of restored miR-124-5p and depleted HDAC1 on septic mice. Having been suggested in a former work, HMGB1 level is dramatically raised in the myocardium in severe sepsis [Citation28]. An important finding purports that circulating HMGB1 expression is heightened in lipopolysaccharides-injected mice [Citation37]. A recent research has discovered that miR-103a-3p-mediated inhibition of HMGB1 could reduce inflammation and lung and liver tissue apoptosis, improve survival of mice with sepsis [Citation38]. It has been elucidated previously that hederacolchiside-E can reduce the production of HMGB1, thus attenuating tissue injury in septic mice [Citation39]. In addition, suppressed HMGB1 also interacts wtith miR-129-5p to protect mice from inflammation and acute kidney injury that induced by sepsis [Citation40]. Additionally, the protective action of miRNA-mediated HMGB1 suppression has been witnessed in sepsis, as mirrored by renal and hepatic function recovery and inflammation relief [Citation41–43]. Moreover, Liu et al. have discovered that the mRNA expression of HMGB1 was negatively accommodated by the variation of miR-381-3p levels in sepsis, yet the concrete regulatory efficiency of HMGB1 was not investigated in this study [Citation44]. Comparatively, our research fully manifested that HMGB1 depletion contributed to relieving myocardial injury in sepsis mice.
Conclusion
Taken together, our study underscores that down-regulated HDAC1 or up-regulated miR-124-5p could recover myocardial injury of septic mice and attenuate the level of inflammatory factors via inhibiting HMGB1, and it may have potentially important therapeutic implications in the treatment of sepsis. However, the role of HDAC1 and miR-124-5p in myocardial injury of sepsis needs further exploration.
Highlights
A novel report on the role of HDAC1/miR-124-5p/HMGB1 in sepsis.
Raised HDAC1/HMGB1 and reduced miR-124-5p are found in myocardial tissues of sepsis.
Restored miR-124-5p/depleted HDAC1 diminishes inflammatory factors in serum of sepsis.
Depleted HDAC1/restored miR-124-5p relieves myocardial damage of sepsis mice.
This study is essential for finding new therapeutic targets for sepsis.
Supplemental Material
Download MS Word (14.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Xu Y, Shao B. Circulating long noncoding RNA ZNFX1 antisense RNA negatively correlates with disease risk, severity, inflammatory markers, and predicts poor prognosis in sepsis patients. Medicine (Baltimore). 2019;98(9):e14558.
- Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–e106.
- Chen JX, Xu X, Zhang S. Silence of long noncoding RNA NEAT1 exerts suppressive effects on immunity during sepsis by promoting microRNA-125-dependent MCEMP1 downregulation. IUBMB Life. 2019;71(7):956–968.
- Wei JL, Wu CJ, Chen JJ, et al. LncRNA NEAT1 promotes the progression of sepsis-induced myocardial cell injury by sponging miR-144-3p. Eur Rev Med Pharmacol Sci. 2020;24(2):851–861.
- Jia P, Wu X, Dai Y, et al. MicroRNA-21 is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit Care Med. 2017;45(7):e703–e710.
- Chen K, Shi X, Jin Y, et al. High lncRNA MEG3 expression is associated with high mortality rates in patients with sepsis and increased lipopolysaccharide-induced renal epithelial cell and cardiomyocyte apoptosis. Exp Ther Med. 2019;18(5):3943–3947.
- Woo SR, Lee HJ, Oh SJ, et al. Stabilization of HDAC1 via TCL1-pAKT-CHFR axis is a key element for NANOG-mediated multi-resistance and stem-like phenotype in immune-edited tumor cells. Biochem Biophys Res Commun. 2018;503(3):1812–1818.
- Wei D, Lu T, Ma D, et al. Synergistic activity of imatinib and AR-42 against chronic myeloid leukemia cells mainly through HDAC1 inhibition. Life Sci. 2018;211:224–237.
- von Knethen A, Brune B. Histone deacetylation inhibitors as therapy concept in sepsis. Int J Mol Sci. 2019;20(2):346.
- Shi X, Liu Y, Zhang D, et al. Valproic acid attenuates sepsis-induced myocardial dysfunction in rats by accelerating autophagy through the PTEN/AKT/mTOR pathway. Life Sci. 2019;232:116613.
- Herdoiza Padilla E, Crauwels P, Bergner T, et al. mir-124-5p regulates phagocytosis of human macrophages by targeting the actin cytoskeleton via the ARP2/3 complex. Front Immunol. 2019;10:2210.
- Wu Y, Yao J, Feng K. miR-124-5p/NOX2 axis modulates the ROS production and the inflammatory microenvironment to protect against the cerebral I/R injury. Neurochem Res. 2020;45(2):404–417.
- Zheng JJ, Du XJ, Wang HP, et al. Long non-coding RNA 00152 promotes cell proliferation in cervical cancer via regulating miR-216b-5p/HOXA1 axis. Eur Rev Med Pharmacol Sci. 2019;23(9):3654–3663.
- Lee W, Ku SK, Park EJ, et al. Exendin-4 inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation. 2014;37(5):1876–1888.
- Walko TD 3rd, Di Caro V, Piganelli J, et al. Poly(ADP-ribose) polymerase 1-sirtuin 1 functional interplay regulates LPS-mediated high mobility group box 1 secretion. Mol Med. 2015;20(1):612–624.
- Deng M, Tang Y, Li W, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49(4):740–753. e7.
- Yin J, Shen Y, Si Y, et al. Knockdown of long non-coding RNA SOX2OT downregulates SOX2 to improve hippocampal neurogenesis and cognitive function in a mouse model of sepsis-associated encephalopathy. J Neuroinflammation. 2020;17(1):320.
- Pan J, Alexan B, Dennis D, et al. microRNA-193-3p attenuates myocardial injury of mice with sepsis via STAT3/HMGB1 axis. J Transl Med. 2021;19(1):386.
- Zhou YX, Han WW, Song DD, et al. Effect of miR-10a on sepsis-induced liver injury in rats through TGF-beta1/Smad signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(2):862–869.
- Yang ZW, Chen JK, Ni M, et al. Role of Kir6.2 subunits of ATP-sensitive potassium channels in endotoxemia-induced cardiac dysfunction. Cardiovasc Diabetol. 2013;12(1):75.
- Tang C, Hu J. HDAC1-mediated microRNA-124-5p regulates NPY to affect learning and memory abilities in rats with depression. Nanoscale Res Lett. 2021;16(1):28.
- Chen X, Song D. LPS promotes the progression of sepsis by activation of lncRNA HULC/miR-204-5p/TRPM7 network in HUVECs. Biosci Rep. 2020;40(6). DOI:10.1042/BSR20200740.
- Liu Y, Li Y, Ni J, et al. MiR-124 attenuates doxorubicin-induced cardiac injury via inhibiting p66Shc-mediated oxidative stress. Biochem Biophys Res Commun. 2020;521(2):420–426.
- Herr DJ, Baarine M, Aune SE, et al. HDAC1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury. J Mol Cell Cardiol. 2018;114:309–319.
- Jia P, Wu N, Jia D, et al. Downregulation of MALAT1 alleviates saturated fatty acid-induced myocardial inflammatory injury via the miR-26a/HMGB1/TLR4/NF-kappaB axis. Diabetes Metab Syndr Obes. 2019;12:655–665.
- Zhang CC, Niu F. LncRNA NEAT1 promotes inflammatory response in sepsis-induced liver injury via the Let-7a/TLR4 axis. Int Immunopharmacol. 2019;75:105731.
- He F, Zhang C, Huang Q. Long noncoding RNA nuclear enriched abundant transcript 1/miRNA-124 axis correlates with increased disease risk, elevated inflammation, deteriorative disease condition, and predicts decreased survival of sepsis. Medicine (Baltimore). 2019;98(32):e16470.
- Ha T, Xia Y, Liu X, et al. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2011;301(3):H848–55.
- Lin C, Wei D, Xin D, et al. Ellagic acid inhibits proliferation and migration of cardiac fibroblasts by down-regulating expression of HDAC1. J Toxicol Sci. 2019;44(6):425–433.
- Zhu X, Lei Y, Tan F, et al. Vaccarin protects human microvascular endothelial cells from apoptosis via attenuation of HDAC1 and oxidative stress. Eur J Pharmacol. 2018;818:371–380.
- Choi HW, Shin PG, Lee JH, et al. Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-kappaB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int J Mol Med. 2018;41(2):1103–1109.
- Zhang L, Bu L, Hu J, et al. HDAC1 knockdown inhibits invasion and induces apoptosis in non-small cell lung cancer cells. Biol Chem. 2018;399(6):603–610.
- Jiang Q, Cheng L, Ma D, et al. FBXL19-AS1 exerts oncogenic function by sponging miR-431-5p to regulate RAF1 expression in lung cancer. Biosci Rep. 2019;39(1). DOI:10.1042/BSR20181804.
- Chen Q, Lu G, Cai Y, et al. MiR-124-5p inhibits the growth of high-grade gliomas through posttranscriptional regulation of LAMB1. Neuro Oncol. 2014;16(5):637–651.
- Jinushi T, Shibayama Y, Kinoshita I, et al. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancer via targeting of SMC4. Cancer Med. 2014;3(6):1544–1552.
- Liang YP, Liu Q, Xu GH, et al. The lncRNA ROR/miR-124-3p/TRAF6 axis regulated the ischaemia reperfusion injury-induced inflammatory response in human cardiac myocytes. J Bioenerg Biomembr. 2019;51(6):381–392.
- Hong G, Zheng D, Zhang L, et al. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic Biol Med. 2018;123:125–137.
- Sun S, Li W, Ma X, et al. Long Noncoding RNA LINC00265 promotes glycolysis and lactate production of colorectal cancer through regulating of miR-216b-5p/TRIM44 axis. Digestion. 2020;101(4):391–400.
- Lee W, Choi HJ, Sim H, et al. Barrier protective functions of hederacolchiside-E against HMGB1-mediated septic responses. Pharmacol Res. 2020;163:105318.
- Huang X, Hou X, Chuan L, et al. miR-129-5p alleviates LPS-induced acute kidney injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int Immunopharmacol. 2020;89(Pt A):107016.
- Ma XF, Qin J, Guo XH. MiR-181-5p protects mice from sepsis via repressing HMGB1 in an experimental model. Eur Rev Med Pharmacol Sci. 2020;24(18):9712–9720.
- Mei L, He M, Zhang C, et al. Paeonol attenuates inflammation by targeting HMGB1 through upregulating miR-339-5p. Sci Rep. 2019;9(1):19370.
- Zhu C, Chen T, Liu B. Inhibitory effects of miR-25 targeting HMGB1 on macrophage secretion of inflammatory cytokines in sepsis. Oncol Lett. 2018;16(4):5027–5033.
- Liu J, Yang Y, Lu R, et al. MicroRNA-381-3p signatures as a diagnostic marker in patients with sepsis and modulates sepsis-steered cardiac damage and inflammation by binding HMGB1. Bioengineered. 2021;12(2):11936–11946.
