ABSTRACT
Tripartite motif-containing 21 (TRIM21) has been reported to have a cancer-promoting or anticancer effect in various tumors; however, its role in ovarian cancer (OC) remains to be elucidated. In this study, we explored the biological function of TRIM21 in OC progression and investigated the potential mechanisms. We found that TRIM21 was remarkably decreased in OC tissues and cell lines compared with adjacent-cancerous tissues and normal ovarian epithelium cell. Decreased expression of TRIM21 in OC patients was significantly correlated with shorter overall and disease-specific survival by The Cancer Genome Atlas database (TCGA) analysis. Functional assays revealed that TRIM21 inhibited the migration and invasion of OC cells; and that TRIM21 also obviously impaired cell proliferation by inhibiting cell cycle progression in vitro and in vivo. Taken together, our results suggest that TRIM21 may be a promising biomarker and target for OC diagnosis and treatment.
Graphical abstract

Introduction
Ovarian cancer is the most deadliest malignant tumor in gynecological patients [Citation1]. The standard therapy includes aggressive surgery, followed by chemotherapy or radiation [Citation2]. The 5-year survival rate of OC patients remains at 46%; because of the lack of appropriate methods for early detection, increased chemoresistance and limited surgical debulking [Citation3,Citation4]. Therefore, a novel early diagnostic and potential therapeutic target for OC needs to be identified.
TRIM21 belongs to the tripartite motif-containing (TRIM) family, which can act as an E3 ligase via its RING domain [Citation5,Citation6]. Initially, TRIM21 was mainly related to autoimmune diseases and participated in immune responses [Citation7–9]. Recently, an increasing number of studies have suggested that the differential expression of TRIM21 is positively or negatively with the development and prognosis of various tumors. It has been reported that lower TRIM21 expression indicates poorer prognosis in hepatocellular carcinoma and diffuse large B-cell lymphoma [Citation10,Citation11], while TRIM21 is elevated in human glioma, and is associated with poor prognosis [Citation12]. Nevertheless, it is unclear whether TRIM21 is expressed or functions in OC.
In the present study, we aim to explore the role of TRIM21 in OC. A tissue microarray (TMA) was used to explore the expression of TRIM21 in OC patients first. Then the TCGA database was used to analyze the connection between TRIM21 expression and prognosis. The results showed that TRIM21 could serve as an independent prognostic biomarker in OC. Subsequently, CCK8, apoptosis and cycle staining, migration, and invasion assays were used to analyze the effect of TRIM21 on the biological function of OC cells. The Western blot results show that TRIM21 restricts the proliferation of OC by regulating p21. TRIM21 promotes apoptosis by decreasing total caspase-3, total caspase-7 and total-PARP expression in OC cells. These data provide new insights into the mechanisms of OC tumorigenesis and support the potential value of TRIM21 as a diagnostic and therapeutic target in OC.
Materials and methods
TCGA database analysis TRIM21 expression and ovarian cancer survival
We used Xena (UCSC Xena: https://xenabrowser.net/) to primarily detect the prognostic effect of TRIM21 in the ovarian cancer dataset of the TCGA database. Then we use GraphPad software to perform statistical analysis on the downloaded dataset file and plot it including OS and DSS survival curve.
Tissue microarray (TMA) slides
There are 8 normal ovarian tissues, 20 adjacent normal ovarian tissues and 588 ovarian cancer tissues in the tissue microarray (TMA) slides, which were brought form Avilabio Biotechnology Ltd., Co (China, Shanxi).
Cell culture and cell treatment
IOSE-80 (IOSE), HO8910, OVCAR3, and SKOV3 were obtained from the cell bank of the Chinese Academy of Sciences, and were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were incubated in the humidified incubator with 37°C and 5% CO2.
OC cells were planted in the 6 mm3 dishes for TRIM21 knockdown when the fusion rate reaches 30–40%. siLenFect reagent (Thermo Fisher Scientific Inc, USA) was used to transfect Small interfering RNAs (siRNAs, 50 nM) against human TRIM21 into the OC cells as previously described [Citation13]. All siRNAs were purchased from GenePharma Technology (Shanghai, China). The sequences of siRNAs were described as follows:
si-TRIM21-nc sense: UUCUCCGAACGUGUCA CGUTT;
si-TRIM21-#1 sense: GACUUCACCUGUUCU GUGATT;
si-TRIM21-#2 sense: CAGCACGCUUGACAA UGAUTT;
si-TRIM21-#3 sense:GGACCUGGAUAUUAC CUCUTT;
Lipofectamine 2000 (Life Technologies) was used for plasmid transfections, following the manufacturer’s instructions.
Generation of stable cells using lentivirus
The generation of stable cells using lentivirus was performed as previously described [Citation14]. Briefly, TRIM21 cDNA was cloned to the pCDH1-CMV-MSC-EF1-Puro vector. Lentiviruses were produced by co-transfecting HEK293T cells with one of the expression plasmids and the packaging plasmids (psPAX2 and pMD2.G). The supernatants were collected, filtered and concentrated. The concentrated viruses were used to infect SKOV3 and HO8910 cells. Stable transfection cell lines were selected with 2 mg/ml puromycin for 15 days.
Western blot analysis and antibodies
Western blot analysis was performed as previously described [Citation15]. The primary antibodies used in Western blot are described as follows: TRIM21 (11,039-1-AP, Proteintech), GAPDH (60,004-1-AP, Proteintech), p21 (10,355-1-AP, Proteintech), cyclin D1 (2978 T, Cell Signaling Technology), cyclin E2 (4132 T, Cell Signaling Technology), caspase-3 (9662s, Cell Signaling Technology), caspase-7 (9492P, Cell Signaling Technology), PARP (9542, Cell Signaling Technology).
Immunohistochemistry (IHC) and assessment of IHC
IHC assays were performed as previously described [Citation16]. Heat-induced epitope retrieval was performed with retrieval buffer (EDTA, pH 9.0 or citrate, pH 6.0). The primary antibodies used in IHC are described as follows: anti-TRIM21 antibody (11,039-1-AP, Proteintech) was used with 1:400 dilution, anti-Ki-67 antibody (ab51608, abcam) was used with 1:100 dilution, anti-p21 antibody (10,355-1-AP, Proteintech) was used with 1:100 dilution.
The detailed IHC assessment method of TMAs was performed as previously described [Citation13]. Briefly, the staining scores of TRIM21 were evaluated via combining the percentage of cells with the staining intensity and the IRS (immunoreactivity score, IRS) by three pathologists separately. The intensity of TRIM21 immunostaining was scored as 0–3 (0, negative; 1, weak; 2, moderate; 3, strong); the percentage of immunoreactivity cells was graded as 1 (0–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). Relied on the IRS, the level of TRIM21 expression was categorized as low (IRS: 0–6) and high (IRS: 8–12) expression.
Cell proliferation, migration, invasion, and wound healing assays
Cell Counting Kit-8 (Dojindo) was used to measure cell proliferation according to the manufacturer’s protocol.
Cell migration and invasion assay were performed as previously described [Citation17]. Briefly, the transwell filter inserts were coated with or without Matrigel (BD Biosciences, Mississauga, Canada), respectively, for invasion and migration assay. Cells were treated with serum starvation overnight and then 2 × 105 cells were seeded in the upper chamber. After incubated at 37°C with 5% CO2 for 24 and 36 h, the cells were fixed in 90% methanol and stained with crystal violet. The cells that had traversed were counted after drying.
The wound healing assay was performed as previously described [Citation13]. Briefly, Cells were planted in six-well plates and cultured overnight. Artificial scratches were made by a sterile 10-μl pipette tip on each well. PBS was used to wash away the suspended cells, and then added medium with 1% FBS to the six-well plates. Cell migration distance was photographed at 0 and 24 h under an inverted light microscope.
Cell cycle analysis
Forty-eight hours after transfection, cells were collected, washed by PBS, and fixed in pro-cooled 70% ethanol at 4°C for at least 18 hours. Then the cells were washed twice with PBS, and then the cell cycle detection kit (Nanjing KeyGen Biotech, Inc.) was used to perform the cycle analysis.
Apoptosis assay
Annexin V-FITC/PI apoptosis detection kit (Nanjing KeyGen Biotech, Inc.) was used to perform apoptosis assay according to the manufacturer’s protocol.
Animal work
BALB/c nude mice (6–8 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animal experiments were approved by the Animal Care and Use Committee at Xuzhou Medical University (No. 202101A143). SKOV3 cells (1 × 107) with TRIM21 over-expression and control cells were injected subcutaneously into the flanks of mice. The tumor volume was calculated every week by using the formula V = a × (b × b)/2, where a is the largest and b is the smallest diameter. Twenty-five days later, the mice were killed and the tumors were weighted and processed for detecting the expression of TRIM21, p21 and Ki-67 by IHC.
Statistical analysis
Statistical analyses were conducted using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 (San Diego, California). One-way ANOVA was used for comparison among the different groups. The association between Trim21 and the clinicopathologic parameters of patients with OC was evaluated by chi-square test. Statistical differences between control and overexpression groups were analyzed by two-tailed Student’s t-test. Data were presented as mean squared error (SEM). p < 0.05 was considered statistically significant.
Results
TRIM21 is deregulated in OC patients and associated with poor prognosis
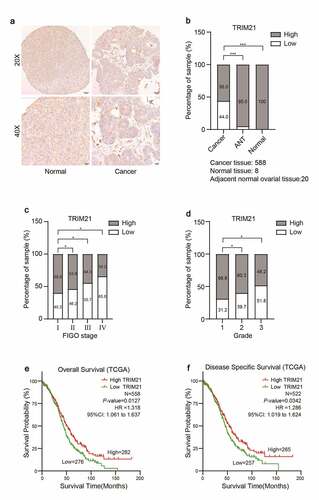
To investigate the potential role of TRIM21 in OC progression, immunohistochemistry (IHC) was performed to detect TRIM21 expression in ovarian cancer tissue microarray (TMA) slides. We found that the expression of TRIM21 was significantly decreased in OC tissues. TRIM21 was predominantly located in the cytoplasm (). We classified TRIM21 expression into low (immunoreactivity score (IRS): 0–6) or high (IRS: 8–12) groups. There was 100% high TRIM21 expression in normal tissues; and 19 cases of high expression (95%) in 20 adjacent tumor tissues, while there were only 329 (56%) cases of high expression in 588 ovarian cancer tissues (). Furthermore, we found that a higher International Federation of Gynecology and Obstetrics (FIGO) stage level was associated with lower TRIM21 expression; similarly, a higher OC grade was consistent with lower TRIM21 expression ().
Fisher’s exact test was used to examine the correlation between Trim21 expression and clinicopathological characteristics and to understand the clinical significance of Trim21 in OC. The clinicopathological characteristics of the patients are summarized in .
Table 1. Expression of TRIM21 and clinicopathological factors of ovarian cancer patients
We further used The Cancer Genome Atlas database (TCGA) to analyze the connection between TRIM21 expression and the survival period of OC patients. The results showed that in OC patients, lower TRIM21 expression was correlated with shorter overall survival (OS) and disease-specific survival (DSS) periods ().Our results confirmed that TRIM21 expression may serve as a potential prognostic factor in OC patients.
(A) Representative immunohistochemistry images of TRIM21 protein expression in normal ovarian and ovarian cancer tissues. (B) The percentage of TRIM21 staining intensities in ovarian cancer, adjacent normal ovarian tissues and normal tissue. (C) Analysis of TRIM21 expression according to FIGO stage in ovarian cancer. (D) Analysis of TRIM21 expression according to the ovarian cancer Grade. (E) The decreased TRIM21 is associated with poor overall survival (OS) in patients with OC predicted by TCGA database. (F) The decreased TRIM21 is associated with poor disease-specific survival (DSS) in patients with OC predicted by TCGA database. *p < 0.05, **p < 0.01, ***p < 0.001.
TRIM21 suppress OC cells growth in vitro
We detected the expression of TRIM21 in different OC cell lines using Western blotting to further investigate the potential biological function of TRIM21 in OC. Compared with the normal ovarian epithelial cell line (IOSE), ovarian cancer cell lines (HO8910, OVCAR3, and SKOV3) had lower TRIM21 expression (), which is consistent with the reduced expression in OC tissues.
Figure 2. TRIM21 is decreased in OC cells and impairs the proliferation ability in vitro. (a) The expression level of TRIM21 in normal ovarian epithelial cell line (IOSE) and ovarian cancer cells was detected by Western blotting. (b) The quantification of the relative protein level of TRIM21 in different ovarian cell lines and normal ovarian epithelial cell line (IOSE). (c-d) The efficiency of TRIM21 knockdown and overexpression in SKOV3 and HO8910 cells were detected by Western blot. The quantification of the relative protein level of TRIM21 was quantified. (e-f) CCK8 assays were used to assess the effect of TRIM21 deficiency and TRIM21 overexpression on cell proliferation in SKOV3 and HO8910 cells. (g-h) TRIM21 over-expression distinctly suppressed the ability of clone formation both in SKOV3 and HO8910 cells. All experiments were performed in triplicate in triplicate. Data are shown as mean ± standard deviations. *p < 0.05, **p < 0.01, ***p < 0.001.
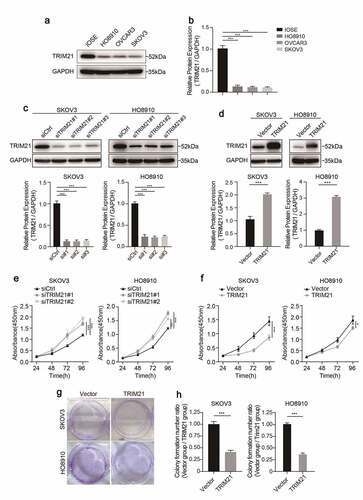
TRIM21 was further knocked down or overexpressed by RNAs (siRNAs) or TRIM21 overexpressing plasmids to investigate the biological function of TRIM21 in HO8910 and SKOV3 cells. The results revealed that TRIM21 expression was significantly reduced when TRIM21 siRNA was transfected (), while TRIM21 expression was significantly upregulated in cells transfected with the TRIM21 overexpression plasmid (), as determined by Western blotting.
Given that lower TRIM21 expression was associated with worse survival, we wondered whether TRIM21 accelerates tumor progression by promoting tumor cell proliferation. The CCK-8 cell assay showed that when TRIM21 was knocked down, cell proliferation was drastically increased in both SKOV3 and HO8910 cells (). Consistently, when TRIM21 was over-expressed, cells had impaired proliferation ability compared with the control group ().
We further performed colony formation assays to validate the inhibitory effect of TRIM21 on cell proliferation. The results showed that colony formation was significantly impaired when SKOV3 and HO8910 cells were transfected with the TRIM21 overexpression plasmid (). These results indicate that TRIM21 impaired the proliferation ability of OC cells in vitro.
TRIM21 restricts the migration and invasion of OC cells
Considering that peritoneal metastasis is a common issue in the progression of OC [Citation18], Transwell assays were performed to investigate whether TRIM21 may suppress migration and invasion in OC cells in vitro.
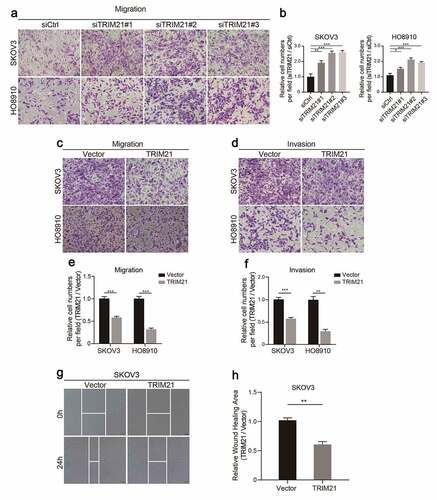
The results showed that TRIM21 deficiency promoted cell migration in SKOV3 and HO8910 cells (). As expected, TRIM21 overexpression impaired the migration and invasion ability of HO8910 and SKOV3 cells (). Moreover, wound healing assays also confirmed that TRIM21 overexpression restricted cell migration and invasion (). These data show that TRIM21 delays the malignant progression of OC in vitro.
Figure 3. TRIM21 restricts OC cells migration and invasion in vitro. (a) Representative images of migration in TRIM21deficiency SKOV3 and HO8910 cells. (b) Relative migration fold changes in TRIM21 deficient SKOV3 and HO8910 cells. (c-d) Representative images of migration and invasion in TRIM21 overexpression SKOV3 and HO8910 cells. (e-f) Relative migration and invasion fold changes in TRIM21 overexpression SKOV3 and HO8910 cells. (g-h) Representative images and relative wound closure rate fold changes in TRIM21 overexpressed SKOV3 cells. *p < 0.05, **p < 0.01, ***p < 0.001.
TRIM21 deficiency inhibits OC cell apoptosis and the cell cycle by regulating p21 expression
In view of our present findings that, TRIM21 deficiency promotes the proliferation of OC cells, we further explored the effect and underlying mechanism of TRIM21 on apoptosis and cell cycle progression in OC cells.
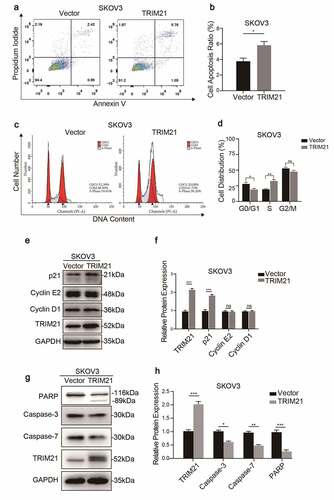
Flow cytometric analysis showed that TRIM21 overexpression increased the apoptosis rate in SKOV3 cells (). Taking the effect of apoptosis on cell proliferation into consideration, the influence of TRIM21 on cell cycle was further examined. The results revealed that the percentage of cells in the G0/G1 phase was decreased and the distribution of cells in S phase was increased when TRIM21 was overexpressed in SKOV3 cells ().
TRIM21 can regulate the expression of p53, Bcl-2 and GSK3β to induce cell proliferation, migration, and apoptosis [Citation12,Citation19,Citation20]. We evaluated the expression of key regulators in the cycle phase transition and apoptosis at the protein level to explore the potential mechanism of TRIM21 in the phase transition in OC cells. We found that TRIM21 overexpression increased the expression of p21, but had no effect on the expression of cyclin D1 and cyclin E2 (). Moreover, total caspase-3, total caspase-7, and total-PARP, the main apoptosis factors, were decreased when TRIM21 was overexpressed (). These data suggested that TRIM21 may impair cell proliferation by inducing cycle arrest and promoting apoptosis.
(A-B) Apoptosis was detected by Flow cytometry and quantified when TRIM21 was overexpressed in SKOV3 cells. (C-D) The cell cycle was detected by Flow cytometry and quantified when TRIM21 was overexpressed in SKOV3 cells. (E-F) Western blot was used to detect cyclically related proteins in TRIM21 overexpressed SKOV3 cells. The quantification of the relative protein level was quantified. (G-H) Western blot was used to detect apoptosis-related proteins in TRIM21 overexpressed SKOV3 cells. The quantification of the relative protein level was quantified. *p < 0.05, **p < 0.01, ***p < 0.001.
TRIM21 exerts a suppressive effect on OC growth in vivo
The same number of TRIM21-overexpressing SKOV3 cells (OE-TRIM21 group) or vector SKOV3 cells (Vector group) () was transplanted into nude mice subcutaneously to explore the effect of TRIM21 on tumorigenesis in vivo.
Figure 5. TRIM21 inhibits tumorigenesis of OC in vivo. (a) The overexpression efficiency of TRIM21 in SKOV3 stable cells was detected. (b-d) 1 × 107 TRIM21 overexpressed or vector SKOV3 cells and Matrigel (Corning; 1:1 ratio) were subcutaneously injected into the abdominal flanks of each mice. n = 6 for each group. Tumor weight and volume were recorded to assess the effect of TRIM21 overexpressed SKOV3 cells on the xenograft model. (e) Representative images of IHC-based correlation between TRIM21, Ki-67, p21 expression in the tumor xenografts. (f) Quantification of TRIM21 staining intensity in xenograft tumors. (g-h) Ki-67 and p21 positive cells were quantified in xenograft tumors. *p < 0.05, **p < 0.01, ***p < 0.001.
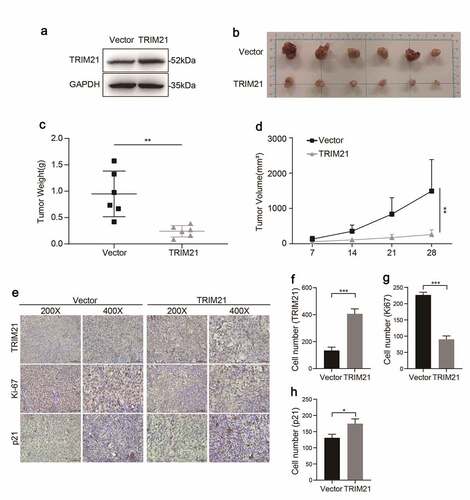
The results showed that the OE-TRIM21 group showed an impaired tumor growth capacity, with smaller tumor volumes and smaller tumor weights than the Vector group (). The expression of TRIM21, and p21 and the nuclear cell proliferation marker Ki67 in the OE-TRIM21 group and Vector group were further analyzed by IHC. The representative images () showed that the OE-TRIM21 group had a stronger staining intensity of TRIM21 () with fewer Ki-67 positive cells than the Vector group ().
Furthermore, consistent with the in vitro results, we found that the OE-TRIM21 group had more p21-positive cells than the Vector group (). Taken together, these results showed that TRIM21 might inhibit tumor growth by regulating p21 expression in vitro and in vivo.
Discussion
TRIM21 plays important roles in autophagy and innate immunity [Citation21], more and more studies have reported a strong relationship between TRIM21 and tumorigenesis. In this study, we found that TRIM21 is decreased in ovarian cancer tissues by TMAs, and reduced TRIM21 expression is correlated with higher disease stage and worse OS, and DSS, suggesting that TRIM21 plays an important role in the progression of OC.
Increased antiproliferative proteins, such as p21 and p53 are indispensable in tumor development [Citation22,Citation23]. TRIM21 destabilizes p53 by ubiquitinating GMPS or HuR [Citation24,Citation25] and induces cell apoptosis by decreasing the expression of Bcl-2 and GSK3β [Citation19,Citation20]. In this study, we found that TRIM21 overexpression inhibited cell proliferation, migration, and invasion and could lead to significant promotion of cell cycle arrest of ovarian cells. Mechanistically, TRIM21 overexpression led to the accumulation of p21, but had no effect on the expression of cyclin D1 or cyclin E2. Furthermore, the apoptosis factors, total caspase-3, total caspase-7 and total PARP were also decreased when TRIM21 was overexpressed.
It should be noted that intraperitoneal metastasis is not an issue that can be ignored during OC progression. Dynamic epithelial-to-mesenchymal phenotype (EMT) changes were triggered when tumor cells detached form the primary OC tumor, and EMT could also promote chemotherapy resistance [Citation18,Citation26]. In this study, we found that TRIM21 overexpression impaired the migration and invasion of OC cells (); however, more studies are needed to confirm the specific mechanism by which TRIM21 participates in the progression of EMT.
Surgical debulking, chemotherapy, and radiation are the standard therapies for OC patients [Citation2]. Immunotherapy with checkpoint blockade has transformed cancer treatment of many tumor types, but there is not yet any immunotherapy for ovarian cancer due to the heterogeneity of the tumor microenvironment (TME). It has been reported that intratumoral T cell infiltration is associated with better overall and progression-free survival in OC [Citation27,Citation28]. TRIM21 is a ubiquitin E3 ligase that has been reported to participate in numerous biological processes, including the immune response, cell metabolism, and cancer development [Citation29]. Cancer cells often prefer glycolysis to satisfy their increased needs for metabolic reprogramming [Citation30]. We recently found that TRIM21 could decrease the protein stability of HIF-1α through the ubiquitination-proteasome pathway and inhibit glycolysis, which inhibited renal cell carcinoma growth and metastasis [Citation13]. Whether TRIM21 reshapes metabolism and affects the infiltration of T cells in OC should be further confirmed.
Conclusion
In conclusion, our study shows that TRIM21 is decreased in ovarian cancer and may function as an independent prognostic factor. We found that TRIM21 inhibits cell proliferation via induction of p21-mediated cell cycle progression and promotes apoptosis in OC in vivo and in vitro. These findings provide new insights into TRIM21 as a new target in prognosis and therapy for patients with OC.
Highlight
TRIM21 is deregulated in ovarian cancer patients
TRIM21 functions as an independent prognostic factor in ovarian cancer patients
TRIM21 restricts the proliferation, migration, and invasion of OC cells
TRIM21 deficiency inhibits the cell cycle through regulating p21 expression
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20180989), the National Postdoctoral Research Funds of China (2019M651971 and 2021T140577), the Scientific Research Project of Jiangsu Province Maternal and child Health Care Association (FYX202023).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
- Kostova I, Mandal R, Becker S, et al. The role of caspase-8 in the tumor microenvironment of ovarian cancer. Cancer Metastasis Rev. 2021;40(1):303–318.
- Ghoneum A, Afify H, Salih Z, et al. Role of tumor microenvironment in the pathobiology of ovarian cancer: insights and therapeutic opportunities. Cancer Med. 2018;7(10):5047–5056.
- Brasseur K, Gévry N, Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. 2017;8(3):4008–4042.
- Wada K, Kamitani T. Autoantigen Ro52 is an E3 ubiquitin ligase. Biochem Biophys Res Commun. 2006;339(1):415–421.
- Kiss L, Zeng J, Dickson CF, et al. A tri-ionic anchor mechanism drives Ube2N-specific recruitment and K63-chain ubiquitination in TRIM ligases. Nat Commun. 2019;10(1):4502.
- Ben-Chetrit E, Chan EK, Sullivan KF, et al. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988;167(5):1560–1571.
- Ben-Chetrit E, Fox RI, Tan EM. Dissociation of immune responses to the SS-A (Ro) 52-kd and 60-kd polypeptides in systemic lupus erythematosus and Sjögren’s syndrome. Arthritis Rheum. 1990;33(3):349–355.
- Rhodes DA, Isenberg DA. TRIM21 and the Function of Antibodies inside Cells. Trends Immunol. 2017;38(12):916–926.
- Ding Q, He D, He K, et al. Downregulation of TRIM21 contributes to hepatocellular carcinoma carcinogenesis and indicates poor prognosis of cancers. Tumour Biol. 2015;36(11):8761–8772.
- Brauner S, Zhou W, Backlin C, et al. Reduced expression of TRIM21/Ro52 predicts poor prognosis in diffuse large B-cell lymphoma patients with and without rheumatic disease. J Intern Med. 2015;278(3):323–332.
- Zhao Z, Wang Y, Yun D, et al. TRIM21 overexpression promotes tumor progression by regulating cell proliferation, cell migration and cell senescence in human glioma. Am J Cancer Res. 2020;10(1):114–130.
- Chen X, Li Z, Yong H, et al. Trim21-mediated HIF-1α degradation attenuates aerobic glycolysis to inhibit renal cancer tumorigenesis and metastasis. Cancer Lett. 2021;508:115–126.
- Li Z, Wang D, Lu J, et al. Methylation of EZH2 by PRMT1 regulates its stability and promotes breast cancer metastasis. Cell Death Differ. 2020;27(12):3226–3242.
- Li Z, Wang D, Chen X, et al. PRMT1-mediated EZH2 methylation promotes breast cancer cell proliferation and tumorigenesis. Cell Death Dis. 2021;12(11):1080.
- Hou P, Li L, Chen F, et al. PTBP3-mediated regulation of ZEB1 mRNA stability promotes epithelial-mesenchymal transition in breast cancer. Cancer Res. 2018;78(2):387–398.
- Li Z, Hou P, Fan D, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24(1):59–71.
- Li Y, Fei H, Lin Q, et al. ZEB2 facilitates peritoneal metastasis by regulating the invasiveness and tumorigenesis of cancer stem-like cells in high-grade serous ovarian cancers. Oncogene. 2021;40(32):5131–5141.
- Jauharoh SN, Saegusa J, Sugimoto T, et al. SS-A/Ro52 promotes apoptosis by regulating Bcl-2 production. Biochem Biophys Res Commun. 2012;417(1):582–587.
- Gao X, Xu F, Zhang HT, et al. PKCα-GSK3β-NF-κB signaling pathway and the possible involvement of TRIM21 in TRAIL-induced apoptosis. Biochem Cell Biol. 2016;94(3):256–264.
- Jin J, Meng X, Huo Y, et al. Induced TRIM21 ISGylation by IFN-β enhances p62 ubiquitination to prevent its autophagosome targeting. Cell Death Dis. 2021;12(7):697.
- Collin G, Huna A, Warnier M, et al. Transcriptional repression of DNA repair genes is a hallmark and a cause of cellular senescence. Cell Death Dis. 2018;9(3):259.
- Zhu D, Shi C, Jiang Y, et al. Cisatracurium inhibits the growth and induces apoptosis of ovarian cancer cells by promoting lincRNA-p21. Bioengineered. 2021;12(1):1505–1516.
- Reddy BA, van der Knaap JA, Bot AG, et al. Nucleotide biosynthetic enzyme GMP synthase is a TRIM21-controlled relay of p53 stabilization. Mol Cell. 2014;53(3):458–470.
- Guha A, Ahuja D, Das Mandal S, et al. Integrated Regulation of HuR by Translation Repression and Protein Degradation Determines Pulsatile Expression of p53 Under DNA Damage. iScience. 2019;15:342–359.
- Loret N, Denys H, Tummers P, et al. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers (Basel). 2019;11(6):838.
- Bou-Tayeh B, Miller ML. Ovarian tumors orchestrate distinct cellular compositions. Immunity. 2021;54(6):1107–1109.
- Desbois M, Udyavar AR, Ryner L, et al. Integrated digital pathology and transcriptome analysis identifies molecular mediators of T-cell exclusion in ovarian cancer. Nat Commun. 2020;11(1):5583.
- Wang F, Zhang Y, Shen J, et al. The ubiquitin E3 ligase TRIM21 promotes hepatocarcinogenesis by suppressing the p62-keap1-Nrf2 antioxidant pathway. Cell Mol Gastroenterol Hepatol. 2021;11(5):1369–1385.
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233.