ABSTRACT
Carotid artery stenosis (CAS) can cause ischemic stroke, and clinical intervention for CAS is critical clinically. The purpose of this study was to explore the expression changes of microRNA-486-5p in the serum of patients with CAS and its possible mechanism. Ninety-one cases with asymptomatic CAS were recruited, and serum levels of miR-486-5p were measured using RT-qPCR. The diagnostic ability was evaluated by drawing the receiver operating characteristic (ROC) curve. Human aortic endothelial cells (HAECs) were treated with oxidized low-density lipoprotein (oxLDL) to establish cell model, and cell proliferation and apoptosis were tested. The markers of cell inflammation and oxidative stress were detected via ELISA. The target gene was analyzed using bioinformatics analysis combined with luciferase reporting assay. CAS cases exhibited significantly low serum miR-486-5p levels in comparison with the control group and can identify asymptomatic CAS. Serum miR-486-5p manifested a negative correlation with the degree of carotid stenosis. Underexpression of miR-486-5p was also detected in ox-LDL treated HAECs. OxLDL treatment contributes to inflammatory response and oxidative stress of HAECs; however, these adverse impacts caused by ox-LDL were reversed by miR-486-5p upregulation. NFAT5 was confirmed to be the target gene of miR-486-5p in HAECs. MiR-486-5p serves as a promising biomarker for the early identification of CAS. Overexpression of miR-486-5p can prevent endothelial dysfunction, and the mechanism might be related to anti-inflammation and anti-oxidation via targeting NFAT5.
Graphical abstract
Introduction
Ischemic cerebrovascular disease is a kind of disease that severely affects life safety. It is characterized by high incidence, disability rate, fatality rate, and recurrence rate. Carotid artery stenosis (CAS) can reflect the degree of systemic arteriosclerosis and is one of the factors of ischemic stroke [Citation1]. The main cause of CAS is the dysfunction of endothelial cells caused by atherosclerotic plaque, and the apoptosis of vascular endothelial cells induced by oxidized low-density lipoprotein (ox-LDL) is a vital link leading to the lesion [Citation2]. It is demonstrated that age, hypertension, diabetes, and hyperlipidemia are all risk inducements for CAS [Citation3]. Clinical intervention for asymptomatic CAS patients can effectively reduce the risk of stroke, which has an important clinical significance.
In recent years, with the development of gene diagnostic technology, the role of microRNAs (miRNAs) in various diseases has attracted attention [Citation4–6]. Studies have shown that there are differential expression profiles of miRNAs in patients with CAS, suggesting the potentially important role of miRNAs in CAS [Citation7–9]. MiRNA is a kind of non-coding small molecule with 17 ~ 22 nucleotides in length, and its dysregulation is related to the occurrence and development of a variety of diseases [Citation10,Citation11]. It can bind to the 3’-untranslated regions of the specific target genes and then degrade or inhibit the mRNA post-translational levels of the target genes [Citation12]. Due to the stable expression of miRNA in peripheral blood, miRNA has become a biomarker of many diseases in recent years [Citation13–15]. MiR-486-5p is one of the important miRNAs with high abundance expression in the heart, downregulation of miR-486-5p has been detected in coronary atherosclerotic plaques via microarray experiments [Citation16]. In addition, significantly reduced miR-486-5p is detected in individuals with high blood pressure, which shows a significant association with the cardiovascular risk score [Citation17]. These results demonstrate the important influence of miR-486-5p on arteriosclerosis, which encourages us to explore its role in CAS.
In this study, a real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR) combined with bioinformatics analysis was used to investigate the expression changes of miR-486-5p in the serum of patients with CAS, and its clinical value was evaluated. In addition, human aortic endothelial cells (HAECs) were treated with oxidized low-density lipoprotein (oxLDL) to establish cell models, and the regulatory role of miR-486-5p in cell proliferation, apoptosis, cell inflammation, and oxidative stress was detected. It is attempted to lay a foundation for the further construction of the molecular regulatory network of miRNA in CAS.
Materials and methods
Ninety-one cases with asymptomatic CAS and 87 healthy people were included, and serum miR-486-5p levels were detected using real-time quantitative polymerase chain reaction (RT-qPCR). HAECs were treated with oxLDL, and miR-486-5p levels were regulated in vitro. Cell proliferation and apoptosis were detected using a cell counting kit-8 (CCK-8) assay and a flow cytometry assay. Cell inflammation and oxidative stress-related factors were detected through an enzyme-linked immunosorbent assay (ELISA) assay. A double luciferase reporting assay was applied for the target gene analysis.
Study subjects and blood specimen collection
The CAS group consisted of 91 cases who underwent physical examination and were diagnosed with asymptomatic CAS via carotid artery ultrasound in the Department of Neurology of Renhe Hospital. The inclusion criteria are as follows: (1) aged more than 50 years old; (2) the degree of carotid artery stenosis was confirmed by carotid ultrasound examination to be >50%; (3) no history of ischemic stroke, focal neurological symptoms, transient ischemic attack or amaurosis was found by inquiring medical history; (4) was consciousness and cooperative with the physical examination; (5) the study was reviewed and approved by the Ethics Committee of Renhe Hospital [No. HIRB190017], and all subjects signed informed consent. The exclusion criteria are as follows: (1) with ischemic or hemorrhagic stroke; (2) accompanied with a malignant tumor; (3) accompanied with immune system diseases; (4) have a mental illness in the past and cannot cooperate with the examination; (5) accompanied with heart, liver, and renal dysfunction; and (6) family history of hereditary diseases. In addition, 87 healthy people who underwent physical examination in Renhe Hospital during the same period were selected as the control group. 10 ml elbow venous blood was taken from each participant and centrifuged at 3000 rpm for 20 min. The serum samples were collected and kept in the refrigerator at −80°C for use. 5 ml of blood was used for the detection of blood biochemical indexes, and the rest of the blood was used for the qRT-PCR.
Cell culture and transfection
Human aortic endothelial cells (HAECs) were provided by the American Type Culture Collection (ATCC), and endothelial cell medium (ECM) was used for the cell culture combined application of 5% fetal bovine serum (FBS). The cells were incubated under the circumstance of 5% CO2 at 37°C. When the cells grow to 90% confluency, the cell transfection was done to regulate the mRNA expression in cells. Sequences of miR-486-5p mimic or inhibitor or their negative controls (mimic-NC and inhibitor NC) were synthesized and provided by the Tiangen Biological Company and transiently transfected into HAECs using lipofectamine 2000. Then, the HAECs were treated with 20 µg/ml oxidized low-density lipoprotein (oxLDL, Guangzhou Yiyuan Biological Technology Co., Ltd., Guangzhou, China) for 48 h [Citation18].
RT-qPCR
TRIzol was applied to separate and extract total RNA, and Nanodrop 2000 was used to measure RNA concentration. QuantiMiR RT Kit (Systems Biosciences) and Script II Reverse transcriptase (Invitrogen) reverse transcription kit (Invitrogen) were used to transcribe the miRNA or mRNA into cDNA. The relative mRNA expression level was detected by 2× SYBR Green Master Mix (Applied Biosystems) in Applied Biosystems Model 7500 qRT-PCR amplifier. The relative expression of miR-486-5p and the nuclear factor of activated T cell 5 (NFAT5) was calculated using the 2 −ΔΔCt method via using U6 and GAPDH as the reference genes, respectively. All primers were designed and synthesized by Sangon (Shanghai, China) and the sequences were as follows: miR-486-5p, forward 5’-GAATTTGGAGTTTAGTTATAGTTTTTATT-3’ and reverse 5’-CCCAACACCACACACACCATACTA-3’; U6, forward 5’-CTCGCTTCGGCAGCACA-3’ and reverse 5’-AACGCTTCACGAATTTGCGT-3’; NFAT5, forward 5’-ACCCAGAGACCCTGACAACT-3’ and reverse 5’-TGAAACTGGGTAGCCTGCTG-3’; GAPDH, forward 5’-GGAGGGCCTCATGACCACCGT-3’ and reverse: 5’-CACATCTTCCCAGAGGGGCCGT-3’.
CCK-8 assay
CCK-8 assay was done to evaluate the cell viability of the cells. The cell viability was monitored for three consecutive days, and 10 µL of CCK-8 solution was added to each well at 0, 24, 48, and 72 h, respectively. After 2 hours of incubation at 37°C, the optical density (OD) value was measured at 450 nm with a microplate reader.
Flow cytometry assay
To detect cell apoptosis, the cells were stained with Annexin V-FITC/Propidium Iodide (PI) detecting kits (Nanjing KeyGen Biotech, Nanjing, China) by flow cytometry based on the manufacturer’s protocol. 1 mL of cell suspension was taken and centrifuged, precipitation was collected, and 5 μL Annexin V-FITC and PI were added, respectively, and incubated for 30 min in the dark. The cell apoptosis rate of different groups was measured by flow cytometry, and the experiments were repeated 3 times.
ELISA assay
After centrifugation, the supernatant of each cell group was collected. The concentration of interleukin-6 (IL-6), IL-1β, soluble intercellular adhesion molecule-1 (sICAM-1), superoxide dismutase (SOD), reactive oxygen species (ROS), and malondialdehyde (MDA) in the cell culture medium was determined according to the instructions of the enzyme-linked immunosorbent assay (ELISA) kit.
Double luciferase reporting assay
TargetScan was used for the target gene analysis, it was found that there were binding sites in the 3’-untranslated region (3’-UTR) of miR-486-5p and NFAT5. The wild type (Wt) and mutant type (Wt) NFAT5 luciferase reporter gene vectors were constructed, respectively. After the above two vectors were mixed with miR-486-5p mimic and miR-486-5p inhibitor, respectively, they were co-transfected into HAECs. After 48 hours of culture, the cells were collected and washed with phosphoric acid buffer (PBS) to detect luciferase activity.
Statistical analysis
Experimental data are expressed by mean ± standard deviation (SD). Spss 22.0 and GraphPad 7.0 software were used for statistical analysis. Student's t-test and one-way ANOVA analysis were used to assess the difference among different groups. The diagnostic ability was evaluated by drawing the receiver operating characteristic (ROC) curve, and the diagnostic sensitivity and specificity were calculated. The influence of clinical indicators on the occurrence of disease was detected by logistic regression analysis. P < 0.05 means the difference is statistically significant.
Results
Clinical indicators of the study population
Clinical indicators of the study groups are shown in . We discovered that the CAS patients possessed significantly high levels of low-density lipoprotein (LDL), systolic blood pressure (SBP), diastolic blood pressure (DBP), C-reactive protein (CRP), and soluble intercellular adhesion molecule-1 (sICAM-1) in contrast to the healthy people (P < 0.001). However, no significant difference emerged with respect to other indicators between the two groups (P > 0.05), including age, gender, body mass index (BMI), fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), and high-density lipoprotein (HDL).
Table 1. Comparison of indicators among the study population
Aberrant expression of miR-486-5p in CAS and its diagnostic ability analysis
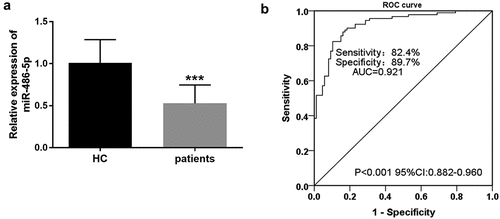
As shown in (), CAS cases exhibited significantly low serum miR-486-5p levels when compared with the control group (P < 0.001). Furthermore, we performed an ROC analysis to assess the diagnostic ability of miR-486-5p for CAS. As seen from , the area under the curve (AUC) of miR-486-5p was 0.921 (95% confidence interval (CI) = 0.882–0.960, P < 0.001). Then, the Youden index was performed to calculate the best cutoff value. The cutoff value of 0.692 carries the maximum Youden index, and the corresponding sensitivity and specificity were 82.4% and 89.7%, respectively.
Association of serum miR-486-5p with clinical indicators
Pearson’s correlation analysis was done for the correlation evaluation. shows a negative correlation of serum miR-486-5p with LDL (r = −0.521), SBP (r = −0.261), DBP (r = −0.272), CRP (r = −0.325), sICAM-1 (r = −0.424), as well as degree of carotid stenosis (r = −0.680). In view of the aberrant expression of miR-486-5p in CAS patients and the significant correlation with the degree of carotid stenosis, the logistic regression analysis was further performed to estimate its influence on CAS degree after adjusting other clinical factors. We can observe from that miR-486-5p had independent influence on the degree of carotid stenosis (odds ratio (OR) = 0.236, 95% CI = 0.079–0.704, P = 0.010).
Table 2. Correlation between miR-486-5p and clinical indicators
Table 3. Association of different variables with degree of carotid artery stenosis
The negative regulatory effect of miR-486-5p on the cell apoptosis, inflammation, and oxidative stress of HAECs
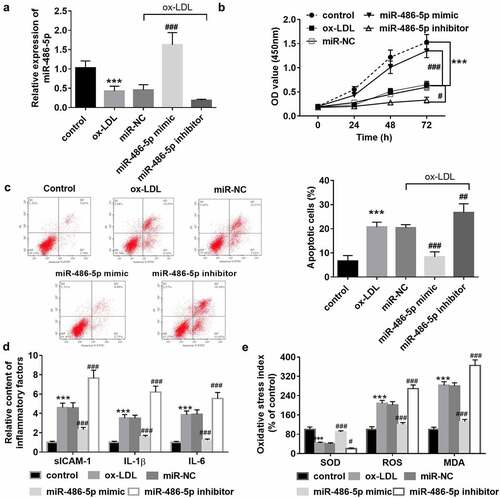
Ox-LDL was applied for the HAECs to mimic the endothelial dysfunction caused by atherosclerosis. After ox-LDL induction, the cell proliferation was suppressed (), while the cell apoptosis was promoted (). Also, markers of the inflammatory response () and oxidative stress () were successfully induced. In ox-LDL treated cells, underexpression of miR-486-5p was also detected, and it was consistent with its levels in clinical serum samples (). Then, cell transfection was performed for the miR-486-5p level regulation to explore the role of miR-486-5p in cell function. As observed from , the overexpression of miR-486-5p can promote HAEC proliferation, while suppressing cell apoptosis. Besides, ox-LDL induced release of inflammatory factors and oxidative stress indicators was reversed by miR-486-5p (). But miR-486-5p inhibitor transfection further exacerbated the cell apoptosis, inflammation, and oxidative stress of HAECs induced by ox-LDL ().
Figure 2. In ox-LDL treated cells, low levels of miR-486-5p were also detected, which was reversed by miR-486-5p mimic transfection (a). MiR-486-5p negatively regulated the HAECs apoptosis (b), inflammation (c) and oxidative stress (d) induced by ox-LDL. *** P < 0.001 (VS control group); ### P < 0.001 (VS ox-LDL group).
Target relationship confirmation between miR-486-5p and NFAT5
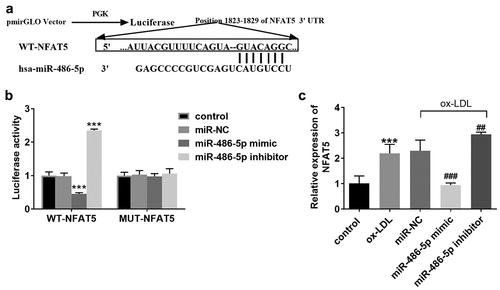
shows the target-binding sequence between miR-486-5p and NFAT5 through TargetScan analysis. The luciferase reporter assay showed that miR-486-5p mimic transfection led to the reduction of cell luciferase activity, whereas miR-486-5p inhibitor transfection contributed to the increase of luciferase activity (). The mRNA levels of NFAT5 were also detected in ox-LDL treated cells models transfected miR-486-5p mimic or not, and high expression of NFAT5 was detected in cell models, which was decreased by miR-486-5p mimic transfection ().
Figure 3. Target relationship confirmation between miR-486-5p and NFAT5. A. TargetScan showed a target binding sequence between miR-486-5p and NFAT5. B. The luciferase activity of HAECs was regulated by miR-486-5p mimic or inhibitor. C. The mRNA levels of NFAT5 in ox-LDL treated cells transfected with miR- miR-486-5p mimic or inhibitor. *** P < 0.001 (VS control group); ### P < 0.001 (VS ox-LDL group).
Discussion
CAS is a common atherosclerotic disease that commonly occurs in middle-aged and elderly people [Citation19]. The current screening methods for CAS include digital subtraction angiography (DSA), transcranial Doppler ultrasound (TCD), magnetic resonance angiography (MRA), CT angiography (CTA), etc. In recent years, miRNA, as a novel biomarker, has been widely reported in various human diseases [Citation20]. In CAS, aberrant expression of several miRNAs has been detected, such as miR-128-3p, miR-638, and miR-200c [Citation10,Citation21,Citation22]. In the current study, asymptomatic CAS cases were enrolled, and the cases showed elevated levels of DBP, LDL, CRP, and ICAM-1. The findings reflected that CAS patients may be complicated with dyslipidemia and abnormal blood pressure, inflammatory reaction, and vascular endothelial injury, which may also be the inducements of CAS [Citation23]. In addition, it was also found that the development of CAS is accompanied by the downregulation of serum miR-486-5p levels. And serum miR-486-5p was revealed to be an independent influence factor for the degree of CAS via logistic regression analysis.
MiR-486-5p has been revealed to have protective effects in cardiovascular and cerebrovascular diseases [Citation17,Citation24]. In patients with high blood pressure, significantly reduced miR-486-5p has been detected, which shows a close association with the cardiovascular risk score [Citation17]. In addition, decreased exosomal miR-486-5p is related to antiangiogenesis in refractory intracranial atherosclerosis [Citation24]. Moreover, microarray experiments also show the downregulation of miR-486-5p in coronary atherosclerotic plaque [Citation16]. Consistently, the analysis results of clinical data in the current study proposed that serum miR-486-5p was negatively correlated with the levels of DBP, LDL, CRP, and ICAM-1 in CAS cases, suggesting its potential influence on the vascular endothelial injury, which supported its potential role in the progress of CAS. Furthermore, the ROC curve revealed its diagnostic ability to identify CAS cases from healthy controls.
Subcutaneous deposition and oxidative modification of LDL are the initial events of atherosclerosis [Citation25]. OxLDL can induce endothelial cell apoptosis and promote the secretion of inflammatory factors and adhesion factors, leading to the accumulation of cholesterol in macrophages and the conversion of cholesterol into foam cells, ultimately causing the formation of atherosclerotic plaques. Endothelial cell damage is the initiating pathological basis of arteriosclerosis, and runs through the whole process of arteriosclerosis development. The dysfunction of vascular endothelial cells and activation of the inflammatory response ultimately cause the occurrence of CAS [Citation3]. In the current study, HAECs were recruited for cell function experiments and treated with oxLDL. The results showed that oxLDL-induced HAEC apoptosis was weakened by increased miR-486-5p, reflecting its protective effect against vascular endothelial cell injury. Vascular endothelial cell injury promotes the release of intracellular adhesion factors and then induces the transformation of macrophages into foam cells [Citation26]. The in vitro experimental results demonstrated that miR-486-5p can also inhibit the release of ICAM-1 in HAECs. Besides its regulatory role in the apoptosis of vascular endothelial cells, the present findings also demonstrated the influence of miR-486-5p on inflammation and oxidative stress. It is known that inflammation and oxidative stress are the main factors that induce vascular endothelial cell dysfunction. According to the present findings, oxLDL treatment contributed to inflammatory response and oxidative stress of HAECs; however, theses adverse impact caused by ox-LDL was reversed by miR-486-5p overexpression. In the previous studies, miR-486-5p has been widely reported to regulate inflammation and oxidative stress in several diseases, which was consistent with the findings in HAECs [Citation27,Citation28]. The present findings led us to hypothesize that miR-486-5p can prevent vascular endothelial dysfunction by inhibiting inflammation and oxidative stress, thus inhibiting the process of CAS.
NFAT5 is the main transcription factor activated by elevated osmotic pressure in mammalian cells, which can protect cells from hypertonic stimulation. NFAT5 plays an important role in inflammatory diseases and autoimmune function [Citation29]. NFAT5 can promote arterial smooth muscle cell proliferation and motility in vitro, thus preventing atherosclerosis [Citation30]. In addition, NFAT5 can also induce vascular endothelial cell apoptosis and inflammatory response, further contributing to the formation of atherosclerosis and CAS [Citation31,Citation32]. As previous evidence reported, NFAT5 is a target gene of miR-486-5p [Citation33]. In the current study, the prediction of the binding between miR-486-5p and NFAT5 was done using bioinformatics analyses and verified through the dual-luciferase reporter assay. Furthermore, NFAT5 was overexpressed in ox-LDL treated HAECs, and the levels were downturned by increased miR-486-5p. The underlying mechanism was raised that miR-486-5p might be involved in the progress of CAS via targeting NFAT5.
Conclusion
In conclusion, findings from the current study revealed that miR-486-5p was aberrantly underexpressed in CAS, and it might be able to be a promising biomarker for the early diagnosis of CAS. MiR-486-5p can prevent endothelial dysfunction, and the mechanism might be related to anti-inflammation and anti-oxidative via targeting NFAT5. This study provides potential therapeutic targets and ideas for the prevention and treatment of endothelial dysfunction and CAS.
Supplemental Material
Download Zip (402 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Zhu B, Zhang L, Cheng XP, et al. The association between metabolic syndrome and asymptomatic carotid artery stenosis in menopausal women: a cross-sectional study in a Chinese population. Ther Clin Risk Manag. 2018;14:2183–2188.
- Zhou S, Li Z, Liu P, et al. Donepezil prevents ox-LDL-Induced attachment of thp-1 monocytes to human aortic endothelial cells (HAECs). Chem Res Toxicol. 2020 Apr 20 33(4):975–981.
- Zhang L, Zeng Y, Qi J, et al. A cynomolgus monkey model of carotid atherosclerosis induced by puncturing and scratching of the carotid artery combined with a high-fat diet. Exp Ther Med. 2018 Jul;16(1):113–120.
- Sassi Y, Avramopoulos P, Ramanujam D, et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun. 2017 Nov 20 8(1):1614.
- Du K, Zhao C, Wang L, et al. MiR-191 inhibit angiogenesis after acute ischemic stroke targeting VEZF1. Aging (Albany NY). 2019 May 7 11(9):2762–2786.
- Li B, Liu X, Wu G, et al. MicroRNA-934 facilitates cell proliferation, migration, invasion and angiogenesis in colorectal cancer by targeting B-cell translocation gene 2. Bioengineered. 2021 Dec;12(2):9507–9519.
- Yan Z, Wang H, Liang J, et al. MicroRNA-503-5p improves carotid artery stenosis by inhibiting the proliferation of vascular smooth muscle cells. Exp Ther Med. 2020 Nov;20(5):85.
- Barbalata T, Moraru OE, Stancu CS, et al. MiR-223-3p levels in the plasma and atherosclerotic plaques are increased in aged patients with carotid artery stenosis; association with HDL-related proteins. Mol Biol Rep. 2021 Aug 19;10.1007/s11033-021-06636-y
- Zhang T, Liu R. Dysregulation of miR-637 serves as a diagnostic biomarker in patients with carotid artery stenosis and predicts the occurrence of the cerebral ischemic event. Bioengineered. 2021 Dec;12(1):8658–8665.
- Farina FM, Hall IF, Serio S, et al. miR-128-3p is a novel regulator of vascular smooth muscle cell phenotypic switch and vascular diseases. Circ Res. 2020 Jun 5 126(12):e120–e135.
- Su Y, Yuan J, Zhang F, et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019 May 7 10(5):365.
- Xun G, Ma M, Li B, et al. miR-138 and miR-193 target long non-coding RNA UCA1 to inhibit cell proliferation, migration, and invasion of lung cancer. Thorac Cancer. 2020 Sep;11(9):2681–2689.
- Shi Y, Liu Z. Serum miR-92a-1 is a novel diagnostic biomarker for colorectal cancer. J Cell Mol Med. 2020 Aug;24(15):8363–8367.
- Xu P, Xin J, Song L, et al. Serum miR-133 as a potential biomarker in acute cerebral infarction patients. Clin Lab. 2020 Oct 1;66(15). 10.7754/Clin.Lab.2019.190933.
- Liu G, Lei Y, Luo S, et al. MicroRNA expression profile and identification of novel microRNA biomarkers for metabolic syndrome. Bioengineered. 2021 Dec;12(1):3864–3872.
- Berkan O, Arslan S, Lalem T, et al. Regulation of microRNAs in coronary atherosclerotic plaque. Epigenomics. 2019 Sep;11(12):1387–1397.
- Santamaria-Martos F, Benitez I, Pinilla L, et al. MicroRNA profile of cardiovascular risk in patients with obstructive sleep apnea. Respiration. 2020;99(12):1122–1128.
- Zhang B, Zhang Y, Li R, et al. Knockdown of circular RNA hsa_circ_0003204 inhibits oxidative stress and apoptosis through the miR-330-5p/Nod2 axis to ameliorate endothelial cell injury induced by low-density lipoprotein. Cent Eur J Immunol. 2021;46(2):140–151.
- Zeng X, Zhu H, Liu W, et al. Electrocardiogram-based r wave pulse wave index for assessment of carotid atherosclerosis. Med Sci Monit. 2020 Jan 16;26:e919606.
- Binderup HG, Madsen JS, Heegaard NHH, et al. Quantification of microRNA levels in plasma - impact of preanalytical and analytical conditions. PLoS One. 2018;13(7):e0201069.
- Luque A, Farwati A, Krupinski J, et al. Association between low levels of serum miR-638 and atherosclerotic plaque vulnerability in patients with high-grade carotid stenosis. J Neurosurg. 2018 Jul 27 131(1):72–79.
- Magenta A, Sileno S, D’Agostino M, et al. Atherosclerotic plaque instability in carotid arteries: miR-200c as a promising biomarker. Clin Sci (Lond). 2018 Nov 30 132(22):2423–2436.
- Wang Y, Song X, Li Z, et al. Long non-coding RNAs in coronary atherosclerosis. Life Sci. 2018 Oct 15;211:189–197.
- Jiang H, Toscano JF, Song SS, et al. Differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antiangiogenesis. Sci Rep. 2019 Dec 19 9(1):19429.
- Sorokin AV, Kotani K, Elnabawi YA, et al. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis. Circ Res. 2018 Nov 9 123(11):1244–1254.
- Li F, Guo X, Chen SY. Function and therapeutic potential of mesenchymal stem cells in atherosclerosis. Front Cardiovasc Med. 2017;4:32.
- Chai X, Si H, Song J, et al. miR-486-5p inhibits inflammatory response, matrix degradation and apoptosis of nucleus pulposus cells through directly targeting foxo1 in intervertebral disc degeneration. Cell Physiol Biochem. 2019;52(1):109–118.
- Chang Q, Ji M, Li C, et al. Downregulation of miR4865p alleviates LPSinduced inflammatory injury, oxidative stress and apoptosis in chondrogenic cell ATDC5 by targeting NRF1. Mol Med Rep. 2020 Sep;22(3):2123–2131.
- Lee N, Kim D, Kim WU. Role of NFAT5 in the immune system and pathogenesis of autoimmune diseases. Front Immunol. 2019;10:270.
- Su F, Shi M, Zhang J, et al. MiR-223/NFAT5 signaling suppresses arterial smooth muscle cell proliferation and motility in vitro. Aging (Albany NY). 2020 Dec 28 12(24):26188–26198.
- Xie X, Huang C, Xu D, et al. Elevation of hypertonicity induced protein NFAT5 promotes apoptosis of human umbilical vein endothelial cells through the NFkappaB pathway. Mol Med Rep. 2021 Mar;23(3). 10.3892/mmr.2021.11823.
- Ma P, Zha S, Shen X, et al. NFAT5 mediates hypertonic stress-induced atherosclerosis via activating NLRP3 inflammasome in endothelium. Cell Commun Signal. 2019 Aug 20 17(1):102.
- Duan YR, Chen BP, Chen F, et al. LncRNA lnc-ISG20 promotes renal fibrosis in diabetic nephropathy by inducing AKT phosphorylation through miR-486-5p/NFAT5. J Cell Mol Med. 2021 Jun;25(11):4922–4937.