ABSTRACT
Alcoholic liver disease (ALD), with its increasing morbidity and mortality, has seriously and extensively affected the health of people worldwide. Caffeic Acid Dimethyl Ether (CADE) significantly inhibits alcohol-induced hepatic steatosis in vivo through AMP-activated protein kinase (AMPK) pathway, but its in-depth mechanism remains unclear. This work aimed to clarify further mechanism of CADE in improving hepatic lipid accumulation in ALD through the microRNA-378b (miR-378b)-mediated Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2)-AMPK signaling pathway. Here, we reported that the hepatic or serum triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), and aspartate transaminase (AST) levels were sharply escalated by ethanol while prominently decreased by CADE. Ethanol sharply up-regulated miR-378b expression while CADE effectively prevented the elevation of miR-378b in vivo. And treatment of CADE surely increased mRNA and protein expression of CaMKK2 as a kinase of AMPK and reduced lipid accumulation in the livers of alcohol-fed C57BL/6 mice. MiR-378b escalation exacerbated hepatic steatosis and inhibited CaMKK2-AMPK signaling, while miR-378b deficiency alleviated lipid accumulation and activated the CaMKK2 cascade. Furthermore, CADE alleviated the lipid deposition and reversed the disorder of CaMKK2-AMPK signaling pathway induced by miR-378b over-expression. However, knockdown of miR-378b eliminated the beneficial effect of CADE on lipid metabolism. In brief, our results showed that CADE ultimately improved hepatic lipid deposition by regulating the CaMKK2-AMPK signaling pathway through miR-378b.
Graphical abstract
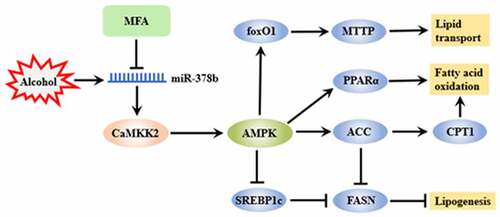
Highlights
Ethanol induced lipid deposition and high expression of miR-378b in vivo.
CADE decreased miR-378b expression and reduced lipid accumulation in vivo.
CADE reduced lipid deposition by regulating miR-378b.
CADE regulated multiple processes of lipid metabolism through miR-378b.
1. Introduction
Alcohol abuse is deemed as one of the largest threatening elements in the world and a major cause of many diseases, especially ALD that includes alcoholic steatosis, hepatitis, cirrhosis, and even a more serious form of liver cancer [Citation1]. In the absence of medications or other treatments, ALD can begin as steatosis of the liver and gradually progress to more serious forms of the disease, eventually leading to liver cancer [Citation2]. With growing researches, quantum leaps have been achieved in the mechanism of alcohol-induced hepatic steatosis in recent years. Hepatic steatosis induced by excessive alcohol results from imbalance of lipid metabolism charged upon increased fatty acid synthesis, decreased oxidation, increased input to the liver, and blunted export from the liver [Citation3]. For the moment, life-style changes, such as abstinence from alcohol might be the long-term goal and the most effective strategies of management for ALD, but this is not always practical or sufficient [Citation4]. Up to the present, clinical drugs for the treatment of ALD include S-adenosine methionine, glucocorticoids, pentoxifylline, propylthiouracil, etc., which directly or indirectly relieve ALD symptoms or give nutritional supports for patients to some extent [Citation5]. However, taking into consideration with therapeutic effect of these drugs and their adverse reactions, which have gradually been discovered, it is urgent to identify and excavate more potential therapeutic drugs in primary ALD.
CADE, also called as 4-dimethoxy cinnamic acid or methyl ferulic acid [Citation6], is a white acicular compound isolated from the rhizome of securidaca inappendiculata hassk, a kind of special chinese herbal medicine containing a variety of ingredients with potential medicinal value such as anti-inflammatory, analgesic, and immunodepressive effects [Citation7–9]. According to a previous reported study, 5–20 mg/kg of CADE significantly alleviated alcohol-induced hepatic steatosis, which was specifically manifested in the reduction of hepatic lipid, along with the remission of fat vacuoles in liver tissues [Citation10,Citation11]. In addition, a subsequent study reported that CADE improves alcohol-induced lipid accumulation by restoring activity of AMPK [Citation12]. However, the exact way in which CADE triggers AMPK activity has not been elucidated.
AMPK, a classical node that regulates lipid metabolism processes [Citation13], can be activated by its upstream kinase liver kinase B1 (LKB1) and CaMKK2 [Citation14,Citation15]. Served as a direct upstream kinase of AMPK, CaMKK2 is extensively involved in hepatic lipid metabolism. S100 calcium-binding protein A16 (S100A16) is identified to be a novel adipogenic promoter in mouse livers and hepatocytes by reason of its capacity in hepatic lipid metabolism by regulating the CaMKK2-AMPK signaling pathway [Citation16]. Another research showed that tomatidine activates AMPK through CaMKK2 and reduced palmitate-induced lipid accumulation in human hepatocytes HepG2 [Citation17]. And more importantly, a study has confirmed that ethanol exhibits a considerable inhibition of CaMKK2, resulting in an apoptosis in hepatocytes [Citation18]. Summing up the above, considering the inhibitory effect of ethanol on itself, its function in regulating AMPK and its proven role in hepatic lipid metabolism, CaMKK2 is regarded as a new pointcut to further explore how CADE activates ethanol-inhibited AMPK signaling.
MicroRNA (miRNA), an endogenous, highly conserved, single stranded, and non-coding RNA of approximately 18–25 nucleoside, is considered as a crucial factor in conscription regulation involved in processes of hepatic lipid metabolism [Citation19]. Cadmium chloride inhibits sirtuin1 translation and reduces its protein expression by inducing the ascent of miR-34a transcription level, eventually leading to hepatic steatosis [Citation20]. MiR-3548 was engaged in the process of α-politic acid stimulating hepatic fat deposition and degeneration by targeting fatty acid syntheses (FASN) mRNA [Citation21]. The biological function of miRNA in hepatic lipid metabolism is an inspiration to expound the molecular mechanism of CADE improving alcohol-caused lipidosis from the perspective of gene regulation. A recent study indicated that miR-378b, which showed great activities to be involved in lipid regulation and systemic energy homeostasis [Citation22–24], is elevated by ethanol in C57BL/6 mice [Citation25]. Additionally, another research reported that miR-378b negatively regulated CaMKK2, which was served as a key regulator to balance energy both in cells and the whole organism, participating in lipid metabolism [Citation26]. These findings indicated that miR-378b/CaMKK2 signal has a great potential to be involved in the protective effect of CADE on lipid metabolism.
Although CADE has been demonstrated to improve lipid accumulation induced by ethanol, the underlying molecular mechanisms have not been clarified. Combined with the results mentioned above, a possibility was proposed that miR-378b might be involved in the procedure of CADE improving hepatic fat deposition by modulating CaMKK2. This work aimed to elucidate further molecular mechanism of CADE in improving hepatic lipid accumulation in ALD through the miR-378b-mediated CaMKK2-AMPK signaling pathway.
2. Materials and methods
2.1. Animals and protocols
Male C57BL/6 mice (age 6 weeks and weight 20–25 g) were obtained from Hunan SJA Laboratory Animal Co. Ltd. (Hunan, China). Animal procedures were all approved by the Institutional Ethics Committee of Guilin Medical University. After adaptive feeding on a liquid diet for one week, the mice mentioned above were randomly divided into a control group, an ALD model group and three CADE-treatment groups with different concentrations (20 mg·kg−1, 10 mg·kg−1, 5 mg·kg−1). The scheme to construct the mouse model of ALD referred to the previous approach with minor modifications [Citation12]. Briefly, the mice except for those in control group were all fed on a 5% alcoholic liquid diet for four weeks with daily changing and were gavaged with 31.5% ethanol (5 g·kg−1) once two weeks during the period. In addition, the CADE-treatment groups were supplied with CADE depending on the required dose once a day and lasted for two weeks (weeks 3–4). After 4 weeks for establishment of ALD model, all mice were euthanized after injection with 0.2 mL/10 g 1.25% avertin and their livers and bloods were collected for follow-up tests.
2.2 Adeno-associated virus administration
To regulate miR-378b levels in livers of mice, the pAAV-MCS consisting of amplified miR-378b sequences that were successfully constructed with molecular cloning technique were provided from Gene Pharma (Shanghai, China) and used for this work. Adeno-associated virus (AAV, serotype 9) was used to package recombinant AAV on account of high infection efficiency and stability [Citation27]. The packaged adeno-associated virus was called as AAV-miR-378b agomir or AAV-miR-378b antagomir, while the empty (untransformed) was named AAV-miR-378b agomir NC or AAV-miR-378b antagomir NC used as controls. To up-regulate or down-regulate miR-378b in vivo, the AAV was injected into the mice through tail vein immediately at a dose of 1 × 1011 vg per animal and injected again once a week later. Moreover, CADE-treatment group were extra provided with CADE (10 mg/kg) by oral gavage once daily for two consecutive weeks.
2.3 Histopathological analysis
Hematoxylin and eosin (H&E) staining was carried out roughly similar as previously claimed with slight difference [Citation11]. In brief, liver specimens harvested and fixed in 10% buffered formalin roughly 24 h were embedded in liquid paraffin and cut into 6 µm sections for H&E staining. Subsequently, pathological changes were observed in accordance with micrographs obtained from light microscopes (Olympus, Tokyo, Japan).
2.4 Biochemical analysis
The procedure to extract lipids from the liver is based on previously reported method [Citation12]. TG or TC concentrations in livers or serum were measured using commercially available diagnostic kits (Unitech Medical Electronics Co., Ltd., Guilin, China). Beyond that, ALT and AST levels were detected using Erba XL-600 automatic biochemical analyzer.
2.5 Western blot analysis
Total proteins in livers were extracted by the precooled RIPA agentia (Beyotime Bio Co., Nanjing, China). According to the manufacturer’s instructions [Citation28], the proteins of uniform weight were subjected to SDS-PAGE and attached onto PVDF membranes (Millipore Corp, Billerica, MA, USA) when proteins with different molecular weights have reached the points where they can be differentiated and separated according to the indication of protein ladder marker. After that, the membranes were sealed in 5% nonfat milk for 1 hour and then immunoblotted with antibodies. Finally, the protein bands were visualized using an enhanced chemiluminescence system (ECL) reagent (E002-100, 7Sea Biotech Co., Shanghai, China). Antibodies against β-actin, p-CaMKK2, CaMKK2, Peroxisome Proliferator Activated Receptor α (PPARα), Microsomal Triglyceride Transfer Protein (MTTP), FASN, Sterol Regulatory Element Binding Protein 1c (SREBP-1c), Carnitine Palmitoyl Transferase 1 (CPT1), Forkhead box other 1 (foxO1), and p-foxO1 were purchased from Affinity Biosciences (OH, USA). Antibodies against Acetyl-CoA Carboxylase (ACC), p-ACC, AMPK, and p-AMPK were purchased from Abcam Technology (Cambridge, UK).
2.6 RNA-seq data analysis
Total RNA covering miRNA in liver tissues was isolated and extracted using TRIzol (Gibco BRL). According to the manufacturer’s protocol [Citation29], one microgram of total RNA was used for reverse transcription with a FastQuant RT Kit (Sangon Biotech Inc., ShangHai, China). After amplified on the MJ PTC-200 PCR system (Bio-Rad, Hercules, CA, USA) in line with the primer sequences, target genes were identified using 1.5% agarose gel electrophoresis. Besides that, miRNA was reversely transcribed with miRNeasy Kit and detected by miRNA-Detection Kit (GeneCopoeia™, Guangzhou, China). As internal controls, the expression of U6 and β-actin were simultaneously quantified. Primer sequences were listed as follows: miR-378b (forward: ACACTCCAGCTGGGAGTGGACTTGGAGTCA; reverse: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCTTCTGA), U6 (forward: CTCGCTTCGGCAGCACA; reverse: AACGCTTCACGAATTTGCGT), CaMKK2 (forward CATGAACGGACGCTGCATCT; reverse ACAGTCCTGCATACCCGTGAT), β-actin (forward GACTCCTATGTGGGTGACGA; reverse ACGGTTGGCCTTAGGGTTCA). All Primers mentioned above were purchased from Sangon Biotech (Shanghai, China).
2.7 Statistical analyses
All data listed in the work are summarized including at least three replicates and expressed as the mean ± SD. In data comparison among groups, only P < 0.05 is considered as significant difference. Differences between means were evaluated by analysis of variance (ANOVA) and student’s t-test. Data analyses and figure preparation were carried out with GraphPad Prism 6.0 and Adobe Illustrator 2021.
3. Results
In this study, C57BL/6 mice were randomly divided into five groups: normal control group, ethanol group, and CADE-treatment groups with three dosages (20 mg·kg−1, 10 mg·kg−1, 5 mg·kg−1). The expression levels of miR-378b and CaMKK2 in liver tissues were tested to confirm whether miR-378b-CaMKK2 signaling is involved in the improvement of alcohol-induced lipid deposition by CADE. Subsequently, we explored the effect of miR-378b in the protective process of CADE on ethanol-induced hepatic steatosis by constructing miR-378b adeno associated virus delivered to ethanol-fed mice. The expression levels of miR-378b, CaMKK2-AMPK signal, downstream factors associated with lipid synthesis, lipid oxidation, and lipid transport in liver tissues were tested. We discovered a critical role of miR-378b in the regulation of lipid metabolism by CADE.
3.1 CADE reduced hepatic lipid accumulation in alcohol-fed mice
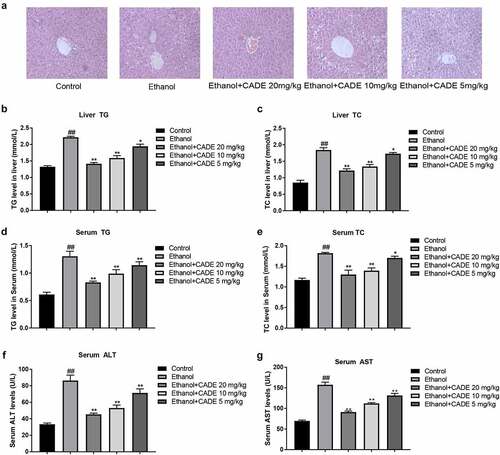
To verify the effect of CADE on lipid metabolism in vivo, mice were fed with alcohol-diet and/or treated with different doses of CADE. From the results of H&E staining, it could be seen that the alcohol-diet led to fat vacuoles, inordinate hepatocyte cords and obvious globular inflammation in the livers of alcohol-fed mice, whereas the treatment of CADE obviously improved hepatic pathological changes, lipid droplets and inflammatory lesions (). Afterward, we conducted biochemical analysis to detect ALT, AST, TG, and TC levels in livers or serum. The data displayed a memorably rise of serum ALT, serum AST, serum TG, serum TC, liver TG level and liver TC level in the ethanol-fed group when in comparison to the levels in NC group. However, we found that CADE in 5–20 mg/kg range was able to flatten these abnormally elevated indicators in a dose-dependent manner, indicating that CADE has the excellent protective effect on alcohol-induced injury to liver and lipid disorder ().
Figure 1. CADE reduced lipid accumulation in liver and serum of alcohol-fed mice. (a) Representative images of H&E staining (original scale = 1:10). (b) Liver TC levels. (c) Liver TC levels. (d) Serum TG levels. (e) Serum TC levels. (f) Serum ALT levels. (g) Serum AST levels. All data are expressed as means ± SD. ##p < 0.01 vs. the control group, and *p < 0.05 and **p < 0.01 vs. the ethanol group.
3.2 CADE decreased miR-378b expression and activates CaMKK2 in alcohol-fed mice
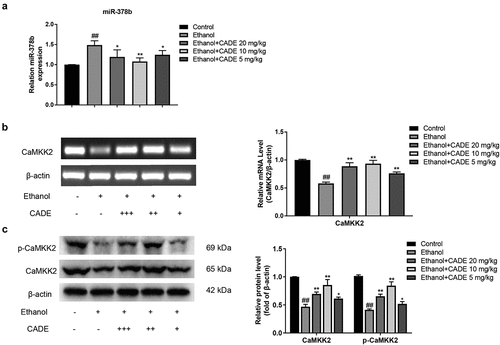
Subsequently, miR-378b expressions were verified by qRT-PCR, the results revealed that miR-378b was visibly risen in the alcohol-fed mice contrasted with control-diet mice, and the miR-378b expression was substantially decreased in the CADE-treated group in comparison to alcohol-fed group. Among the three doses we set, CADE at 10 mg/kg showed the best inhibitory effect for miR-378b (). Moreover, we assessed CaMKK2 mRNA and protein expressions by PCR and Western blot and found that they were dramatically inhibited by ethanol, whereas CADE effectively reversed the reduction of the CaMKK2 mRNA and protein expressions. Notably, 10 mg/kg CADE also showed the strongest activation of CaMKK2, which was similar to the inhibitory effect of miR-378b at the dose mentioned above. ().
Figure 2. Effect of CADE on miR-378b-CaMKK2 signal. (a) The expression level of miR-378b in liver tissues. (b) CaMKK2 mRNA expression. (c) Protein expression levels of CaMKK2 and p-CaMKK2. All data are expressed as means ± SD. ##p < 0.01 vs. the control group, and *p < 0.05 and **p < 0.01 vs. the ethanol group.
3.3 CADE alleviated lipid disorders in C57BL/6 mice through miR-378b
In order to explore the role of miR-378b in CADE’s improvement of alcohol-induced hepatic adipose degeneration in vivo, we injected AAV-miR-378b-agomir or AAV-miR-378b-antagomir including their corresponding negative controls (NCs) into C57BL/6 mice via tail vein, and then CADE was used to intervene on this basis. Based on the results presented before, dose of 10 mg/kg CADE decreased miR-378b the most prominently in the liver of ethanol-fed mice, along with activating the CaMKK2 signaling, so our subsequent experiments used the same conditions.
As shown, miR-378b was up-regulated in the liver of mice injected with AAV-miR-378b-agomir while down-regulated by AAV-miR-378b-antagomir, compared with mice in their corresponding NC groups, respectively. Concurrently, CADE reduced miR-378b-agonist-induced miR-378b over-expression, but nearly had no effect after injection with AAV-miR-378b-antagomir (). Compared with the mice in control group, mice injected with AAV-miR-378b-agomir displayed a large number of diffuse fat vacuoles accompanied by extracellular cord disorder and inflammatory filtration in the liver, which were distinctly reduced or alleviated after CADE intervention. Beyond that, the mice injected with AAV-miR-378b-antagomir exhibited a normal globular and radiating hepatic cords in liver that were basically unaffected by CADE ().
Figure 3. MiR-378b played a role in the improvement of lipid disorders in C57BL/6 mice by CADE. (a) The expression level of miR-378b in livers of mice (original scale = 1:10). (b) Representative images of H&E staining of liver sections. (c) Serum TG and TC levels. (d) Liver TG levels. (e) Serum ALT/AST levels. All data are expressed as the mean ± SD of at least three separate experiments. #p < 0.05, ##p < 0.01 vs. NC group of AAV-miR-378b-agomir or antagomir. *p < 0.05, **p < 0.01 vs. AAV-miR-378b-agomir or antagomir.
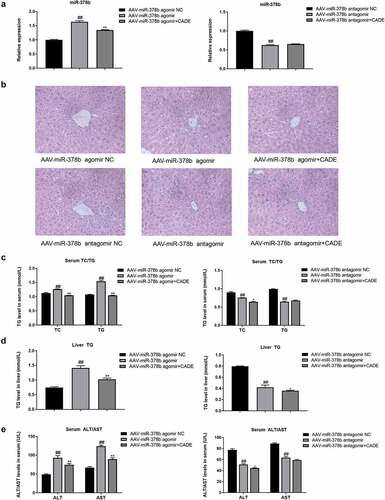
To further illuminate whether CADE modulated miR-378b to protect liver, we determined serum TG/TC, liver TG/TC, serum ALT and AST levels in the collected mouse liver and serum. When miR-378b was up-regulated by miR-378b-agomir, these levels were increased conspicuously, while loss of miR-378b lowered the mentioned levels in liver and serum of mice. Furthermore, CADE exhibited a fat-reducing function which was significantly weakened or almost disappeared when injected with AAV-miR-378b-antagomir ().
3.4 MiR-378b participated in the process in which CADE activated the CaMKK2-AMPK-ACC signaling pathway to improve hepatic steatosis in C57BL/6 mice
Next, we further explored the role of miR-378b on CADE in regulating the CaMKK2-AMPK signaling pathway in vivo. PCR results demonstrated that the up-regulation of miR-378b resulted in a decrease of CaMKK2 mRNA, while CADE had an opposite effect to miR-378b over-expression, although it could not completely neutralize the effect for CaMKK2 caused by miR-378b over-expression. In addition, the mRNA level of CaMKK2 was increased by miR-378b reduction, while the level remained basically unchanged under continued CADE treatment ().
Figure 4. MiR-378b mediated the activation of CADE on the CaMKK2-AMPK signaling pathway in vivo. (a, b) mRNA expression levels of CaMKK2. (c, d) Western blot analysis of the protein expression of CaMKK2.
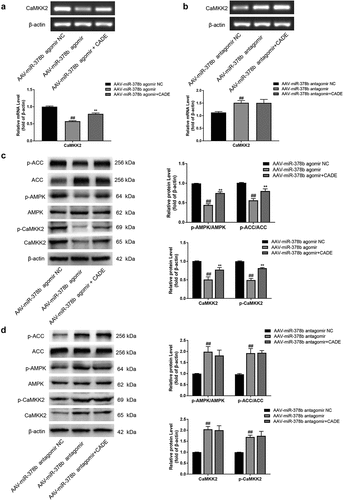
p-CaMKK2, protein ratios of p-AMPK/AMPK and p-ACC/ACC. All data are expressed as the means ± SD of at least three separate experiments. #p < 0.05, ##p < 0.01 vs. NC group of AAV-miR-378b agomir or antagomir. *p < 0.05, **p < 0.01 vs. AAV-miR-378b agomir or antagomir.
Results of western blot showed that over-expression of miR-378b declined CaMKK2 protein level, thereby reducing the ratios of p-AMPK/AMPK and p-ACC/ACC, and CADE partially reversed this effect. On the other hand, suppression of miR-378b caused a high expression of CaMKK2 protein and arouse AMPK and ACC activities. Simultaneously, the regulation of CADE on CaMKK2, AMPK, and ACC protein levels or activities could not be reflected after the miR-378b silence ().
To sum up, miR-378b participated in the process in which CADE activated the CaMKK2-AMPK-ACC signaling pathway to improve hepatic steatosis in C57BL/6 mice.
3.5. CADE regulates lipid synthesis, decomposition, and transport through miR-378b in C57BL/6 mice
Subsequently, we examined the protein expressions of several other factors related to lipid metabolism including lipid synthesis, decomposition, and lipid transport. Further experiment showed that up-regulation of miR-378b notably promoted the up-trends of FASN and SREBP-1c protein levels, but led to significant decreases of PPARα and CPT1 protein expression, along with the declines of MTTP protein level and p-foxO1/foxO1 ratio. However, silence of miR-378b displayed an opposite effect. At the same time, CADE reversed the abnormal expression of these lipid metabolic factors caused by miR-378b over-expression, but this reversal influence eliminated when miR-378b was inhibited by miR-378b antagomir ().
Figure 5. CADE regulates lipid synthesis, decomposition and transport through miR-378b in C57BL/6 mice. (a,b) Western blot analysis of the protein expressions of SREBP-1c, FASN, PPARα, CPT1, MTTP and protein ratio of p-foxO1/foxO1 when the mice were injected with AAV-miR-378b agomir and antagomir. All data are expressed as the means ± SD of at least three separate experiments. #p < 0.05, ##p < 0.01 vs. NC group of AAV-miR-378b agomir or antagomir. *p < 0.05, **p < 0.01 vs. AAV-miR-378b agomir or antagomir.
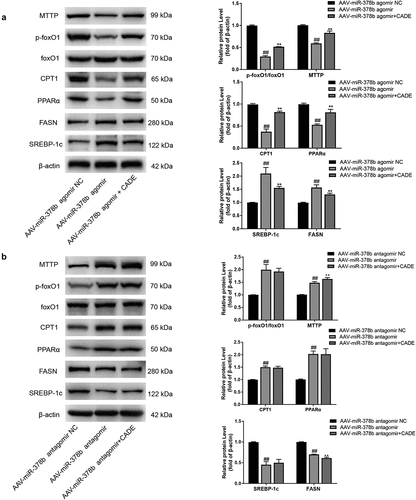
On basis of these experimental results, we concluded that miR-378b played a regulatory role in CaMKK2 cascade and mediated the effect of CADE on lipid metabolism in vivo.
4. Discussions
In this present work, we investigated the effects of CADE on lipid metabolism in alcohol-fed C57BL/6 mice and the role of miR‑378b in this process. Results obtained from animal experiments proved that ethanol up-regulated the expression of miR-378b and inhibited CaMKK2 mRNA and protein levels, conclusively stimulating fat deposition in mice livers. Amelioration of fat deposition in livers by CADE was accompanied by the suppression of miR-378b expression and elevation of CaMKK2. In addition, miR-378b reversely regulated CaMKK2, because over-expression of miR-378b led to a decrease of CaMKK2 while the loss of miR-378b resulted in the increase of CaMKK2. Moreover, over-expression of miR-378b caused liver lipid disturbance, which presented the opposite effect to CADE. Nevertheless, knockdown of miR-378b improved lipid accumulation, and miR-378b knockout significantly eliminated the beneficial effect of CADE on lipid metabolism. These findings implied that CADE may ultimately improve lipid deposition by regulating the CaMKK2-AMPK signaling pathway through miR-378b.
It is well established that excessive alcohol consumption placed a premium on liver disease including alcoholic fatty liver disease, hepatitis, cirrhosis and even liver cancer [Citation30]. Alcohol-induced hepatic steatosis, as the initial stage of ALD, is mainly manifested by the accumulation of large fat vacuoles in the liver and the elevated levels of liver TG and TC, which directly affects the occurrence and development of ALD. As shown in a previous research, CADE had an obvious inhibitory effect on alcohol-induced endogenous lipid production in liver by activating AMPK and foxO1 pathways, but the deeper molecular mechanisms have not been elucidated [Citation12]. As the upstream of AMPK, CaMKK2 directly regulates AMPK expression. The role of CaMKK2 in lipid metabolism seems to differ in different diseases. Knockout of CaMKK2 in primary hepatocytes inhibits AMPK activity and exhibits a visible increase in de novo lipogenesis [Citation31]. However, systemic inhibition of CaMKK2 has been shown to improve lipid disorders caused by a high-fat diet [Citation32]. This can be caused by the distribution of CaMKK2 in different tissues and the diverse diet compositions. For example, mutation of CaMKK2 in mice on a low-fat diet resulted in increased lipid accumulation while mutant CaMKK2 showed reduced hepatic steatosis in mice on a high-fat diet [Citation33]. In order to clarify the effect of alcoholic diet on CaMKK2 and whether CaMKK2 is related to the protective effect of CADE on liver, we constructed animal models of ALD and detected the expression of CaMKK2 in livers. In this study, lipid deposition is represented in the livers of alcohol-fed mice, along with the decreased levels of CaMKK2 protein and mRNA. In addition, we found that oxidative stress also induced inhibition of CaMKK2 [Citation34], suggesting that ROS produced by alcohol may be one of the multiple causes of CaMKK2 down-regulation. Furthermore, CADE significantly improved lipid accumulation and alleviated the inhibition of alcohol on CaMKK2. Thus, we pointed out that CADE might regulate lipid metabolism by activating CaMKK2, an upstream factor of AMPK, and we proposed for the first time that CaMKK2 might be involved in alcohol-induced hepatic steatosis, which provided a direction for the prevention and treatment of ALD.
MiRNAs, as important transcriptional regulatory factors, have been proved to be involved in various biological processes of lipid metabolism. It has been found that during the differentiation of 3T3-L1 preadipocytes into mature adipocytes, miRNA-16-5p was obviously over-expressed, and over-expression of miRNA-16-5p sharply brought about the accumulation of fat droplet accumulation [Citation35]. Another result declared that miR-130b has a potential role in enhancing the assembly of very low-density lipoprotein (VLDL) and secretion of glycerol metabolism-labeled triglyceride by sharply stimulating MTTP expression and TG mobilization [Citation36]. Notably, a recent study alleged that miR-378b is up-regulated by alcohol in the liver and is involved in alcohol-induced hepatic insulin resistance by targeting p110 and insulin receptor [Citation25]. Subsequently, through bio-informatics target-prediction by StarBase, we found that miR-378b has multiple potential-binding sites with several factors in lipid metabolism signaling, such as CaMKK2, PPARα, and foxO1, symbolizing the importance of miR-378b for lipid metabolism. Additionally, some results have been elucidated that HCC cells and mice with liver fibrosis have down-regulated miR-378b levels [Citation37,Citation38]. However, up-regulation of miR-378b has been reported in obese mice deprived of anaerobic exercise [Citation39]. In this work, our results showed that ethanol induced adipose disorder in the liver, while indeed elevating miR-378b, which was consistent with previously reported findings. For the phenomenon that miR-378b has different manifestations in different liver disorders, factors relevant to lifestyle interventions, such as feeding duration, dietary composition, exercise patterns, and degree of liver damage may explain these observed contradictions. Meanwhile, the regulation of miRNA on diseases is a complex system, and miRNA not only plays a regulatory role independently, but also overlaps and interacts with other factors. Next, in order to investigate whether the improvement of CADE on ethanol-stimulated lipid disorder is related to the abnormal increase of ethanol-induced miR-378b, we constructed the ALD model in vivo combined with CADE therapy, and measured the expression of miR-378b. The results showed that ethanol induced the increase of miR-378b in livers of mice while CADE neutralized the fat disorder induced by ethanol and reduced miR-378b expression. Collectively, the regulation of CADE on miR-378b and the correlation between miR-378b and lipid level provide us an inspiration that the improvement effect of CADE on alcohol-induced hepatic adipose deposition may be related to miR-378b. This was confirmed by over-expression and knockdown of miR-378b in vivo. CADE reduced miR-378b-induced lipid deposition by lowering miR-378b. Surprisingly, although CADE did not decrease miR-378b expression when the mice were injected with AAV-miR-378b-antagomir, it still partially reduced serum TC, liver TG, and ALT levels in mice. The complex regulatory pattern of the body and the multi-target of drugs may explain this phenomenon.
Extensive studies have demonstrated that alcohol participated in a variety of hepatic lipid metabolic pathways, including lipid uptake novo lipogenesis, decomposition, and transport [Citation40]. In alcohol-fed rodents, ethanol suppresses AMPK activity but increases hepatocellular lipid accumulation through the activation of a series of lipid synthesis-related factors, such as ACC, FASN, SREBP-1c, and ChREBP [Citation41]. Simultaneously, ethanol also impairs fatty acid catabolism through the destroy of mitochondrial β-oxidation by inhibiting activities of PPARα and CPT1 [Citation42]. Additionally, it has been reported that alcohol impedes the extra-hepatic secretion of lipid in liver through the low-phosphorylation of foxO1 and the inhibition of MTTP, thus promoting the accumulation of fat in liver [Citation43,Citation44]. Based on the confirmation above that miR-378b and CaMKK2 may be related to the lipid reduction effect of CADE, we further investigated the role of miR-378b in CADE improvement of these multiple lipid metabolism processes. More specifically, we up-regulated or down-regulated miR-378b by adeno-associated virus in mice combined with CADE intervention, and detected CaMKK2 and downstream lipid synthesis, decomposition, and transport signals. In our experiment, miR-378b negatively regulated CaMKK2 and AMPK-ACC signaling. Additionally, miR-378b positively regulated FASN, SREBP-1c, and reversely regulated the CPT1, PPARα, MTTP, and phosphorylation levels of foxO1. CADE not only alleviated the ethanol-induced up-regulation of miR-378b, improved liver steatosis, but also inhibited the disorder of lipid metabolism caused by miR-378b over-expression. Meanwhile, suppression of miR-378b reduced-fat deposition and abrogated the inhibitory effect of CADE on lipid. In brief, miR‑378b mediated the protective effects of CADE in ethanol-induced fatty liver lesions. MiR-378b and CADE have so many regulatory functions in lipid synthesis, decomposition and transport, indicating that they play great roles in promoting mechanism research and drug development of ALD. In addition, it is worth mentioning that several studies have shown that MTTP activator reduced hepatic lipids, but resulted in another question due to accumulation of lipids in the plasma synchronically [Citation45]. Our evidence indicated that CADE improved the hepatic levels of MTTP for fatty acid transport, reduced plasma lipids as well at the same time. Therefore, CADE has a great potential to be developed as a drug to reduce fatty degeneration of liver without causing plasma lipid disorders.
In summary, CADE attenuates lipid generation, facilitates lipid decomposition and transport via activating the CaMKK2-AMPK pathway by inhibiting miR-378b, ultimately alleviating liver steatosis. Given the findings in this study, we make it clear that CADE has a great capacity in the prevention and treatment of ALD, and miR-378b is a potential targeted therapeutic target. However, there are some deficiencies or limitations in this study which needs to be further improved. On the one hand, the absence of excavation on the targeted relationships among miR-378b and PPARα, foxO1 was a restriction in this study, so we will further exploit and elaborate the interaction of miR-378b and these factors, and construct the network of miRNA-RNA-pathway in the future. On the other hand, the regulation of lipid metabolism by CADE and miR-378b is currently limited to animal experiments, but has not been verified in human tissues. Thus, if there is a chance, it must be a great advantage for us to perceive the expressions of miR-378b and CaMKK2 cascade in clinical specimens.
5. Conclusions
Our results suggest that CADE ameliorates alcohol-induced hepatic deposition by down-regulating miR-378b. Establishing the protective effect and mechanism of CADE in ethanol-caused fatty degeneration could possibly lay the foundation for new orientation and goals for the mechanism explore and drug exploitation of ALD.
Data availability of statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethical Committee of Guilin Medical University (protocol code GLMC201800007).
Author contributions
Jun Lu, Yan Zhang: Data curation, Writing-Original draft preparation; Ying-zhao Wang, Rui Wang: Conceptualization, Methodology; Yu-juan Zhong, Li Chen: Supervision; Meng-wei Song, Lin Shi, Yuan-yuan Li: Software, Validation; Yong-wen Li, Li Li: Project administration, Funding acquisition.
Supplemental Material
Download Zip (16.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Fuster D, Samet JH, Longo DL. Alcohol use in patients with chronic liver disease. N Engl J Med. 2018;379:1251–1261.
- Baghy K, Iozzo RV, Kovalszky I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem. 2012;60:262–268.
- Jiang Z, Zhou J, Zhou D, et al. The adiponectin-SIRT1-AMPK pathway in alcoholic fatty liver disease in the rat. Alcohol Clin Exp Res. 2015;39:424–433.
- Singal AK, Bataller R, Ahn J, et al. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113:175–194.
- Aday AW, Mitchell MC, Casey LC. Alcoholic hepatitis: current trends in management. Curr Opin Gastroenterol. 2017;33:142–148.
- Liao R, Qi Z, Tang R, et al. Methyl ferulic acid attenuates human cardiac fibroblasts differentiation and myocardial fibrosis by suppressing pRB-E2F1/CCNE2 and RhoA/ROCK2 pathway. Front Pharmacol. 2021;12:714390.
- Tao MQ, Ji CL, Wu YJ, et al. 1,7-dihydroxy-3,4-dimethoxyxanthone inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppressing TLR4/NF-κB signaling cascades. Inflammation. 2020;43:1821–1831.
- Zuo J, Dou DY, Wang HF, et al. Reactive oxygen species mediated NF-κB/p38 feedback loop implicated in proliferation inhibition of HFLS-RA cells induced by 1,7-dihydroxy-3,4-dimethoxyxanthone. Biomed Pharmacothe. 2017;94:1002–1009.
- Wang DD, Li Y, Wu YJ, et al. Xanthones from securidaca inappendiculata antagonized the antirheumatic effects of methotrexate in vivo by promoting its secretion into urine. Expert Opin Drug Metab Toxicol. 2021;17:241–250.
- Cheng Q, Li C, Yang CF, et al. Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-β1/Smad and NOX4/ROS pathways. Chem Biol Interact. 2019;299:131–139.
- Li C, Li L, Yang CF, et al. Hepatoprotective effects of methyl ferulic acid on alcohol-induced liver oxidative injury in mice by inhibiting the NOX4/ROS-MAPK pathway. Biochem Biophys Res Commun. 2017;493:277–285.
- Cheng Q, Li YW, Yang CF, et al. Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and foxo1 pathways in rats and L-02 cells. Chem Biol Interact. 2018;291:180–189.
- Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205.
- Racioppi L, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem. 2012;287:31658–31665.
- Chauhan AS, Liu X, Jing J, et al. STIM2 interacts with AMPK and regulates calcium-induced AMPK activation. FASEB J. 2019;33:2957–2970.
- Kan J, Zhao C, Lu S, et al. S100A16, a novel lipogenesis promoting factor in livers of mice and hepatocytes in vitro. J Cell Physiol. 2019;234:21395–21406.
- Kusu H, Yoshida H, Kudo M, et al. Tomatidine reduces palmitate-induced lipid accumulation by activating AMPK via vitamin D receptor-mediated signaling in human hepG2 hepatocytes. Mol Nutr Food Res. 2019;63:e1801377.
- Lee YJ, Shu MS, Kim JY, et al. Cilostazol protects hepatocytes against alcohol-induced apoptosis via activation of AMPK pathway. PLoS One. 2019;14:e0211415.
- Yang Z, Cappello T, Wang L. Emerging role of microRNAs in lipid metabolism. Acta Pharm Sin B. 2015;5:145–150.
- Alshehri AS, El-Kott AF, El-Kenawy AE, et al. Cadmium chloride induces non-alcoholic fatty liver disease in rats by stimulating miR-34a/SIRT1/FXR/p53 axis. Sci Total Environ. 2021;784:147182.
- Guo S, Yan K, Fang X, et al. α-lipoic acid alleviates hepatic lipid deposition by inhibiting FASN expression via miR-3548 in rats. Nutrients. 2021;14:13.
- Lyu QR, Huang N, Dong K, et al. The miR-378/PGC1β/mTOR axis as an alternative mechanism to promote autophagy during adipogenesis. Biochimica Et Biophysica Acta Mol Cell Biol Lipids. 2021;1866:158921.
- Nan K, Zhang Y, Zhang X, et al. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res Ther. 2021;12:331.
- Sun C, Liu W, Lu Z, et al. Hepatic miR-378 modulates serum cholesterol levels by regulating hepatic bile acid synthesis. Theranostics. 2021;11:4363–4380.
- Li YY, Zhong YJ, Cheng Q, et al. miR-378b regulates insulin sensitivity by targeting insulin receptor and p110α in alcohol-induced hepatic steatosis. Front Pharmacol. 2020;11:717.
- Wang YZ, Lu J, Li YY, et al. microRNA-378b regulates ethanol-induced hepatic steatosis by targeting CaMKK2 to mediate lipid metabolism. Bioengineered. 2021;12:12659–12676.
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355.
- Yang C, Li L, Ma Z, et al. Hepatoprotective effect of methyl ferulic acid against carbon tetrachloride-induced acute liver injury in rats. Exp Ther Med. 2018;15:2228–2238.
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585.
- Jadeja RN, Chu X, Wood C, et al. M3 muscarinic receptor activation reduces hepatocyte lipid accumulation via CaMKKβ/AMPK pathway. Biochem Pharmacol. 2019;169:113613.
- York B, Li F, Lin F, et al. Pharmacological inhibition of CaMKK2 with the selective antagonist STO-609 regresses NAFLD. Sci Rep. 2017;7:11793.
- Marcelo KL, Ribar T, Means CR, et al. Research resource: roles for calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in systems metabolism. Molecular endocrinology. 2016;30:557–572.
- Park SM, Kim SW, Jung EH, et al. Sipjeondaebo-tang alleviates oxidative stress-mediated liver injury through activation of the CaMKK2-AMPK signaling pathway. Evid Based Complement Alternat Med. 2018;2018:8609285.
- Xu J, Zhang L, Shu G. microRNA-16-5p promotes 3T3-L1 adipocyte differentiation through regulating EPT1.Biochem Biophys Res Commun. 2019 [Jul 5];514(4):1251–1256.
- Zhang J, Jazii FR, Haghighi MM, et al. miR-130b is a potent stimulator of hepatic very-low-density lipoprotein assembly and secretion via marked induction of microsomal triglyceride transfer protein. Am J Physiol Endocrinol Metab. 2020;318:E262–e75.
- Hyun J, Wang S, Kim J, et al. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. 2016;7:10993.
- Chen Q, Guo H, Zong Y, et al. Curcumin restrains hepatocellular carcinoma progression depending on the regulation of the circ_0078710/miR-378b/PRIM2 axis. J Recept Signal Transduct Res. 2021;1–12. DOI:10.1080/10799893.2021.1936554
- Lu YL, Jing W, Feng LS, et al. Effects of hypoxic exercise training on microRNA expression and lipid metabolism in obese rat livers. J Zhejiang Univ Sci B. 2014;15:820–829.
- Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378–390.
- Shearn CT, Smathers RL, Jiang H, et al. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J Nutr Biochem. 2013;24:1436–1445.
- Correnti JM, Gottshall L, Lin A, et al. Ethanol and C2 ceramide activate fatty acid oxidation in human hepatoma cells. Sci Rep. 2018;8:12923.
- Lieber CS, Leo MA, Wang X, et al. Alcohol alters hepatic FoxO1, p53, and mitochondrial SIRT5 deacetylation function. Biochem Biophys Res Commun. 2008;373:246–252.
- Wang Z, Yao T, Song Z. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol Clin Exp Res. 2010;34:471–478.
- Pereira IV, Stefano JT, Oliveira CP. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2011;5:245–251.
- Hussain MM, Rava P, Walsh M, et al. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond). 2012;9:14.
