ABSTRACT
Cataract is a global ophthalmic disease that blinds the eye, and oxidative stress is one of its primary causes. Apoptosis of lens epithelial cells (LECs) is considered the major cytological basis of many cataracts except congenital cataracts. The purpose of this study was to investigate whether diosmetin could reduce oxidative stress-induced damage to LECs, and explore its regulatory pathway. Lens epithelial cell line SRA01/04 was used as the object of study. Using ultraviolet B (UVB) and hydrogen peroxide (H2O2) as sources of oxidative stress, the protective effects of diosmetin at different concentrations on cells were investigated, including inhibition of proliferation, apoptosis, and oxidative stress. Molecular docking was then used to predict the target proteins and validation was performed at the cellular and protein levels. The oxidative stress of SRA01/04 was induced by UVB and H2O2, and inhibition of proliferation and apoptosis were observed. Here, diosmetin has a dose-dependent cell-protecting effect. This effect is achieved by targeting the MEK2 protein and inhibiting the MAPK signaling. In conclusion, diosmetin reduces H2O2- and UVB-induced inhibition of SRA01/04 proliferation and apoptosis by reducing oxidative stress-induced activation of the MAPK pathway.
Graphical Abstract
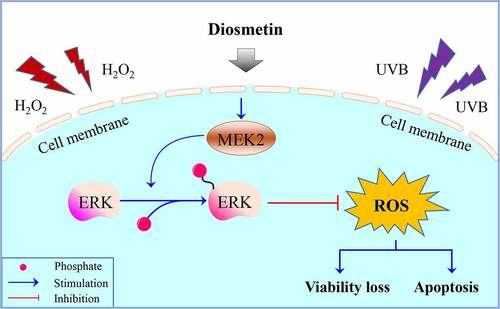
Highlights
Diosmetin promotes SRA01/04 cell proliferation;
Diosmetin inhibits H2O2- and UVB-induced apoptosis of SRA01/04 cells;
Diosmetin reduces oxidative stress induced by H2O2 and UVB;
Diosmetin attenuates H2O2- and UVB-induced cell damage via regulating MEK2.
Introduction
Cataract is the clouding of the lens caused by many reasons. It is a common senile disease and the world’s first eye disease. Surgery is still the most effective method of treating cataracts. Existing studies show many causes of cataracts. Hydrogen peroxide (H2O2) was significantly increased in the anterior chamber of most cataract patients [Citation1]. Spector A et al. reported that oxidative stress occurs in the early stage of cataract development and the H2O2 level of cataract patients is 2 to 7 times the normal range [Citation2]. The ROS concentrations in the anterior chamber and lens of cataract patients were significantly higher than in normal controls [Citation3]. H2O2 induces apoptosis of lens epithelial cells (LECs) equivalent to the pathological changes in the eyes of cataract patients [Citation4]. In the cataract process, oxidative stress first attacks the LECs.
LECs are the most metabolically active part of the lens. They can divide, proliferate, elongate at the equator, produce lens fibers, and maintain the metabolism of the entire lens. They play a crucial role in maintaining lens transparency [Citation5]. Because of the importance of their anatomical location and physiological function, LECs are the primary target of oxidative stress and other injury factors. Li et al. found that oxidative damage can cause apoptosis of LECs, and cataracts appeared several hours after apoptosis, which gradually worsened over time, eventually leading to complete opacification. Apoptosis of LECs is considered the common cytological basis of all types of cataracts, except congenital cataracts [Citation6]. Absence of epithelial cells in lenses from apoptosis-related death leads to cortical swelling, vacuolation, and subcapsular opacification, which eventually forms cataracts first in the equatorial region and then spreads to the entire lens [Citation7]. So, protecting LECs from oxidative stress is the current idea of developing drugs to treat cataracts.
In traditional Chinese medicine (TCM), chrysanthemum purifies the liver, brightens the eyes, and is a key ingredient in TCM prescriptions for cataract treatment. Recent studies have shown that chrysanthemum extracts have anti-inflammatory, antioxidant, and anti-apoptotic abilities [Citation8,Citation9]. As an essential active ingredient in chrysanthemum, and diosmetin is a flavonoid compound that can regulate so many physiological activities. Its antioxidant and anti-apoptotic properties can protect tissues under various pathological conditions. For example, it was recently reported that diosmetin plays a cardioprotective role in myocardial ischemia injury in newborn rats by reducing oxidative stress and myocardial cell apoptosis [Citation10]. Diosmetin has antioxidant, anti-inflammatory, and anti-apoptotic effects and can prevent endotoxin-induced acute liver failure in mice [Citation11].
Based on the above research background, we speculated diosmetin might decrease the oxidative stress-induced damage of LECs. To test this hypothesis, we planned to use the human lens epithelial cell line SRA01/04 as a study object and induce oxidative stress of the cells by UVB radiation and H2O2 treatment. The protective effects of diosmetin on LECs were confirmed in vitro. The target protein was predicted and verified by molecular docking. Through the above experiments, we expected to confirm that diosmetin eases the damage of LECs caused by oxidative stress and explore its mechanism, providing theoretical support for a new drug therapy for cataracts.
Materials and methods
Cell culturing and treatments
Human LECs (SRA01/04; RCB1591, Riken BRC, Tsukuba, Japan) were cultured in DMEM/F-12 medium (Cat. No. 31331093, Gibco, USA) containing 10% fetal bovine serum (FBS; Cat. No. 10099141, Gibco, USA) and 1% penicillin/streptomycin (Cat. No. P1400, Solarbio, China) at 37°C with 5% CO2. When 60% confluence was reached, cells were treated with 200 μM H2O2 for 12 h or irradiated with UVB (2 W/m2) for 60 min and incubated for 12 h before the following experiments [Citation12,Citation13]. For UVB irradiation, cells in phosphate buffer saline (PBS) were irradiated from top to bottom with a UVB lamp (Photoelectric Instrument Factory, Beijing Normal University, Beijing, China) at a distance of 5 cm. The UVB light had a wavelength spectral distribution of 275–400 nm, with a peak at 310 nm. Diosmetin (Cat. No. A0927, Chengdu Must Biotechnology, China) was dissolved in dimethyl sulfoxide (DMSO) and further diluted in medium to different concentration levels (10, 20, and 40 μM).
MTT assays
Cell proliferation was determined by MTT assays. The cells were seeded in a 96-well plate with 5 × 103 cells per well. After 24 hours the cells were treated with H2O2 or UVB, and then 20 µL MTT (5 mg/mL; Cat. No. M1020, Solarbio, China) was added. Four hours later, the mixed medium was replaced with 150 µL DMSO (Cat. No. D8370, Solarbio, China). After the plate was agitated for 15 minutes, the optical density (OD) value of each well was measured using a fluorescence microplate reader at a wavelength of 490 nm.
Cell apoptosis assays
Cell was assayed with the Annexin V-FITC Apoptosis Detection Kit (Cat. No. CA1020, Solarbio, China). Briefly, SRA01/04 cells at a concentration of 5 × 105 cells/mL were collected and washed twice by PBS at 4°C, and then centrifuged at 300 g for 5 min at 4°C. The cells were then resuspended in 50 μL of binding buffer and incubated with the Annexin V-FITC and PI staining solution at room temperature for 10–15 min. Afterward, 250 μL of binding buffer was added to the mixture and cell apoptosis was measured by flow cytometry.
Western blot assays
Western blot assay was used to detect protein expression levels in SRA01/04 cells. Proteins were extracted using RIPA total protein lysate (Cat. No. P0013C, Beyotime, China) and separated by electrophoresis on 10% SDS-PAGE, then blotted onto PVDF membranes (Cat. No. FFP39, Beyotime, China). After incubation with blocking solution (Cat. No. P0252, Beyotime, China), PVDF membranes were probed with anti-p53, anti-Bax, anti-Bcl-2, anti-p-p44/42, anti-p44/42, anti-p-JNK, anti-JNK, anti-p-p38, anti-p38, and anti-GAPDH antibodies (Cat. No. ab32389, ab32503, ab32124, ab278538, ab184699, ab124956, ab179461, ab178867, ab170099, ab9485, Abcam, USA, 1:1000) at 4°C overnight. After washing, the blots were incubated with an HRP-conjugated Goat Anti-Rabbit IgG H&L secondary antibody (Cat. No. ab6721, Abcam, USA, 1:2000) for 1 h and treated with the ECL Western Blot Reagent (Cat. No. P0018S, Beyotime, China) with a chemiluminescent imaging system (ChemiDoc MP, Bio-rad, USA).
ROS detection
Reactive oxygen species (ROS) detection was performed using a ROS detection kit (Cat. No. CA1410, Solarbio, China). Briefly, the supernatant was discarded, and DCFH-DA (10 μM) diluted with DMEM/F-12 was added to the plate. The cells were then further cultured for 20 minutes, washed three times to remove DCFH-DA from the plate. The ROS content was determined by flow cytometry (BD Accuri™ C6 Plus, BD Biosciences, USA).
Enzyme-linked immunosorbent assay (ELISA)
Superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) levels were measured in SRA01/04 cells using ELISA kits (Cat. No. BC0170, BC0205, and BC1175, Solarbio, China) according to the manufacturer’s instructions. OD values were measured in each well using a microplate reader (Multiskan FC, Thermo Scientific, USA).
Target protein prediction and docking
The target protein of diosmetin was predicted using PharmMapper (http://www.lilab-ecust.cn/pharmmapper/) [Citation14,Citation15]. AutoDock Vina was then used to dock diosmetin to the predicted target protein to get minimal free energy [Citation16,Citation17], and Discovery Studio software was used for molecular interaction analysis.
Statistical analysis
The researchers used the GraphPad Prism software (version 8.0) for mapping and statistical analysis. To analyze differences between groups, a one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test was used. At p < 0.05, the data were considered significantly different. Image analysis was performed using the Fiji version of ImageJ [Citation18,Citation19].
Results
Diosmetin alleviates inhibition of SRA01/04 proliferation induced by H2O2 or UVB
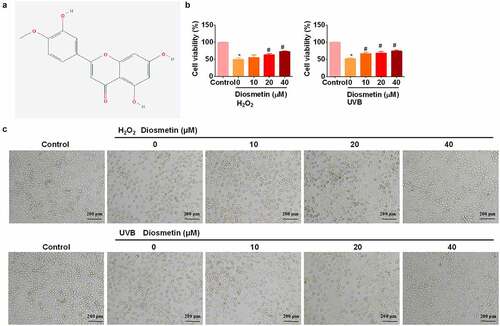
First, the effects of diosmetin on H2O2- and UVB-induced loss of viability of LECs were examined. The chemical structures of diosmetin are shown in . Based on the results of previous studies [Citation12] we treated cells with H2O2 or UVB light. Cells were treated with 200 μM H2O2 for 12 h or UVB (2 W/m2) irradiation for 60 min and an MTT assay was performed. As shown in , proliferation of SRA01/04 cells was significantly inhibited (p < 0.05) by H2O2 or UVB. Adding different doses of diosmetin to H2O2- or UVB-treated cells effectively alleviated the inhibition of proliferation in a dose-dependent manner (p< 0.05). Cell morphological changes in SRA01/04 cells were monitored by microscopy. Compared to the untreated control group, the cells exposed to H2O2 and UVB appeared to be round, shrank and many floating cells were observed (). However, the H2O2- and UVB-induced morphology changes of SRA01/04 cells were restored by diosmetin treatment in a dose-dependent manner.
Figure 1. Diosmetin alleviates inhibition of SRA01/04 proliferation induced by H2O2 or UVB. (a) The 2D structure of diosmetin; (b) MTT test results of SRA01/04 cells under different conditions; (c) Images captured by the bright filed microscope to show the morphological changes. *P < 0.05 compared with control group, #P < 0.05 compared with 0 μM diosmetin group.
Diosmetin reduced H2O2- and UVB-induced apoptosis of SRA01/04 cells
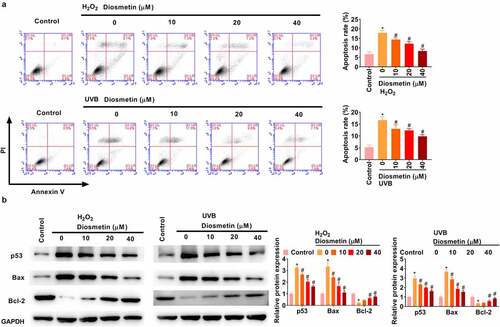
The effects of diosmetin on H2O2- and UVB-induced apoptosis were then examined. As shown in , H2O2 and UVB light could induce apoptosis of SRA01/04 cells, while diosmetin could effectively alleviate apoptosis, and its cell protective effect was dose dependent (p< 0.05). Subsequently, changes in the expression levels of the apoptosis-related proteins p53, Bax, and Bcl-2 were detected under different conditions. As shown in , H2O2 or UVB light significantly enhanced the expression levels of p53 and Bax and significantly down-regulated the expression levels of Bcl-2, while diosmetin at different doses could effectively ameliorate the changes in protein expression levels induced by H2O2 or UVB dose-dependently (p< 0.05).
Figure 2. Diosmetin reduced H2O2- and UVB-induced apoptosis of SRA01/04 cells. (a) Cell apoptosis was detected by flow cytometry; (b) The expression of apoptosis-related proteins was detected by western blotting assay. *P < 0.05 compared with control group, #P < 0.05 compared with 0 μM diosmetin group.
Diosmetin reduces oxidative stress induced by H2O2 and UVB light
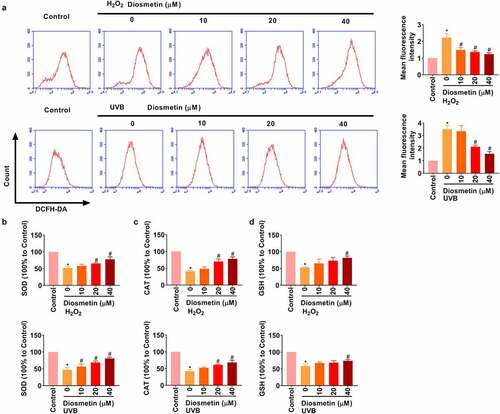
Next, the effects of diosmetin on H2O2 and UVB-induced oxidative stress were demonstrated. Under stimulation of H2O2 and UVB, intracellular ROS was detected by flow cytometry. As results in show, H2O2 or UVB light could induce the increase in ROS level in SRA01/04 cells, and diosmetin could effectively reduce ROS level in a dose-dependent manner (p< 0.05). ELISA showed that H2O2 or UVB significantly repressed SOD, CAT, and GSH levels, while different doses of diosmetin were effective in alleviating H2O2- or UVB-induced changes in protein levels in a dose-dependent manner, as shown in (p< 0.05).
Figure 3. Diosmetin reduces oxidative stress induced by H2O2 and UVB. (a) The detection of the production of ROS in cells under different conditions; The changes of (b) SOD, (c) CAT, and (d) GSH were detected by ELISA under different conditions. *P < 0.05 compared with control group, #P < 0.05 compared with 0 μM diosmetin group.
Diosmetin attenuates H2O2- and UVB-induced activation of MAPK signaling pathways
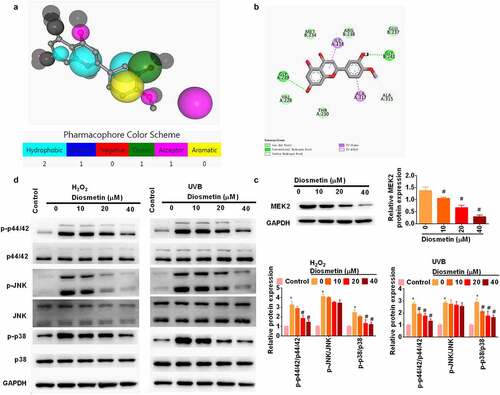
To further understand how diosmetin exerts its protective effect, PharmMapper was used to predict the target protein (http://www.lilab-ecust.cn/pharmmapper/) [Citation14,Citation15,Citation20] of diosmetin. The results showed that mitogen-activated protein kinase 2 (MEK2) may be the target protein of diosmetin. The Molecule and Pharmacophore Model are shown in . To further confirm this prediction, Autodock Vina was used for molecular docking and nine conformations were obtained, among which the lowest binding energy was −6.8 Kcal/mol. Then, discovery Studio was used for molecular interaction analysis (). MEK2 is a dual protein tyrosine/threonine kinase found in the Ras/Raf/MEK/ERK signaling pathway of mitogen-activated protein kinase (MAPK) [Citation21]. Researchers of this study further used western blotting to identify the direct regulation of diosmetin on MEK2 expression. Diosmetin significantly reduced the protein levels of MEK2 in a dose-dependent manner (p< 0.05, ). In addition, H2O2 and UVB significantly (p < 0.05, ) phosphorylated MAPK-related proteins (p44/42, JNK, and p38). Treatment with diosmetin significantly reduced phosphorylation of p44/42 and p38 (p< 0.05) rather than JNK (p> 0.05).
Figure 4. Diosmetin attenuates H2O2- and UVB-induced activation of MAPK signaling pathways. (a) Molecular and pharmacophore models of diosmetin binding MEK2; (b) Molecular interaction analysis of diosmetin and MEK2; (c) Western blot was performed to detect the expression of MEK2 in diosmetin-treated cells; (d) Western blot was used to detect the phosphorylation of MAPK pathway related proteins. *P < 0.05 compared with control group, #P < 0.05 compared with 0 μM diosmetin group.
The combination of diosmetin and MEK inhibitor PD98059 reduces H2O2- and UVB-induced proliferation inhibition and apoptosis
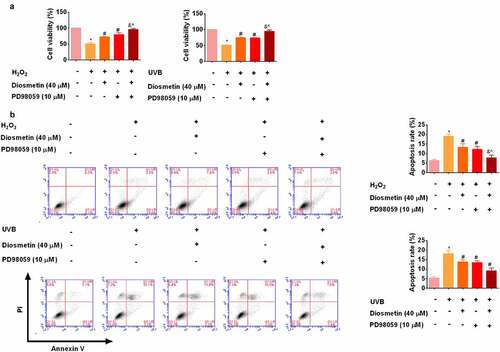
To determine whether diosmetin worked by modulating MEK2, a MEK inhibitor PD98059 was then administered to cells. As shown in , MTT results showed that diosmetin or PD98059 eased proliferation inhibition and apoptosis of cells under oxidative stress (p < 0.05). The combination of diosmetin and PD98059 enhanced the remission ability (p < 0.05). These results showed that inhibition of MAPK pathway can protect cells under oxidative stress.
Figure 5. The combination of diosmetin and MEK inhibitor PD98059 reduces H2O2- and UVB-induced proliferation inhibition and apoptosis. (a) MTT test results of SRA01/04 cells under different conditions; (b) Cell apoptosis was detected by flow cytometry. * P < 0.05 compared with control group; # P < 0.05 compared with 0 μM diosmetin, 0 μM PD98059 group; & P < 0.05 compared with 40 μM diosmetin, 0 μM PD98059 group; ^P < 0.05 compared with 0 μM diosmetin, 10 μM PD98059 group.
Discussion
This study confirmed that diosmetin reduces the damage to LECs induced by oxidative stress. The results showed diosmetin reduced H2O2- and UVB-induced proliferation inhibition and apoptosis of SRA01/04 cells by reducing oxidative stress-induced activation of the MAPK pathway. This study provides theoretical support for the new prophylactic and therapeutic methods of drug therapy for cataracts.
Currently, several bioactive compounds have been found to possess excellent antioxidant properties and protective effects against eye diseases [Citation22]. For instance, Lycium barbarum polysaccharide delivered by nano-selenium protected LECs against UVB damage [Citation23]. Epigallocatechin gallate prevented UVB-induced oxidative stress and inhibited apoptosis-related death of LECs [Citation24]. Ganoderic acid A protected LECs against UVB irradiation and delayed lens opacity [Citation25]. Paeoniflorin inhibited epithelial–mesenchymal transformation and oxidative damage to LECs and exhibited benefits in preventing diabetic cataracts [Citation26]. Diosmetin has presently been reported as a potential agent for improving eye function by restoring oxidative stress and DNA injury in the retinal pigment epithelium layer [Citation27]. We showed that diosmetin is a potential candidate to prevent cataracts. Diosmetin treatment significantly prevented oxidative stress-induced LEC damage.
After a series of verifications of the protective effects of diosmetin on SRA01/04 cells, PharmMapper was used to predict the target protein of diosmetin. PharmMapper applies Cavity to detect the potential-binding sites on the surface of a protein structure and ranks them according to the Druggability scores [Citation28]. Molecular docking by AutoDock furtherly confirmed the binding relationship and strength of diosmetin and MEK2. Several software and algorithms helped improve the accuracy of the prediction. The subsequent western blotting findings confirmed the previous experimental results.
Besides inhibiting cell damage induced by oxidative stress, several studies have shown that diosmetin can also play a protective role in other ways. For example, diosmetin also has an iron-chelating ability, protecting cells by chelating excessive iron ions in cells [Citation29]. Diosmetin increases vascular tone by inhibiting dopamine and serotonin uptake [Citation30] and inhibits the activation of carcinogens by directly inhibiting cytochrome P450 1A1 (CYP1A1) [Citation31].
In the last part of the study, we explored the synergistic enhancement of diosmetin combined with the MEK inhibitor PD98059. PD98059 inhibited MEK1 and MEK2 with an IC50 value of 4 µM for MEK1 and 50 µM for MEK2 [Citation32–34]. MAPK is a phosphorylated serine/threonine protein kinase with high cytoplasmic content [Citation35]. MAPK signaling pathway is an important intracellular signal transduction pathway that regulates cell reproduction, growth, apoptosis, differentiation, and other biological activities, but can also be activated by different extracellular stimuli [Citation36–38]. The MAPK signaling pathway plays many significant roles in eye diseases. For example, studies have shown that exogenous nitric oxide (NO) can activate the MAPK pathway to regulate the expression of inflammatory cytokines on the ocular surface and enhance corneal wound healing [Citation39]. In contrast, the MAPK/ERK pathway is involved in epidermal growth factor (EGF)-mediated up-regulation of Ca (2+)-K(+)-K(+) channel in the mouse corneal alkali combustion model, and reduces EGF-induced neovascularization [Citation40]. In the current study, MEK2 was a direct target of diosmetin, and diosmetin protected LECs against oxidative stress damage, possibly by regulating MAPK signaling.
Conclusion
Taken together, by targeting MEK2 and reducing oxidative stress-induced MAPK pathway activation, diosmetin helps to protect lens epithelial cells against H2O2 and UVB-induced damage, suggesting diosmetin as a potential candidate for cataract treatment.
Ethics approval and consent to participate
No human and patients.
Authors’ contributions
Conception and design: GH G; Collection and assembly of data: GH G; Data analysis and interpretation: J D; Manuscript writing: All authors; Final approval of manuscript: All authors.
Supplemental Material
Download Zip (1.2 MB)Acknowledgements
We would like to thank TopEdit (www.topeditsci.com) for English language editing of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author (E-mail: [email protected]) on reasonable request.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254.
- Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–681.
- Yokoi N, Takehisa Y, Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am J Ophthalmol. 1996;122:818–824.
- Zatechka SD Jr., Lou MF. Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 1. The mitogenic and stress responses. Exp Eye Res. 2002;74:703–717.
- de Iongh RU, Gordon-Thomson C, Chamberlain CG, et al. Tgf beta receptor expression in lens: implications for differentiation and cataractogenesis. Exp Eye Res. 2001;72:649–659.
- Li WC, Kuszak JR, Dunn K, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169–181.
- Yan Q, Liu JP, Li DW. Apoptosis in lens development and pathology. Differentiation. 2006;74:195–211.
- Li Y, Yang P, Luo Y, et al. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019;286:8–16.
- Liang WL, Wen Y, Huang F, et al. Chrysanthemum ethanol extract induced loss of Kupffer cells via the mitochondria-dependent apoptotic pathway. Food Funct. 2020;11:8866–8877.
- Mo G, He Y, Zhang X, et al. Diosmetin exerts cardioprotective effect on myocardial ischaemia injury in neonatal rats by decreasing oxidative stress and myocardial apoptosis. Clin Exp Pharmacol Physiol. 2020;47:1713–1722.
- Yang X, Wu K, Li S, et al. MFAP5 and TNNC1: potential markers for predicting occult cervical lymphatic metastasis and prognosis in early stage tongue cancer. Oncotarget. 2017;8:2525–2535.
- Sun Y, Rong X, Li D, et al. Down-regulation of CRTAC1 attenuates UVB-induced pyroptosis in HLECs through inhibiting ROS production. Biochem Biophys Res Commun. 2020;532:159–165.
- Xiang J, Kang L, Gao H, et al. BLM can regulate cataract progression by influencing cell vitality and apoptosis. Exp Eye Res. 2019;178:99–107.
- Wang X, Shen Y, Wang S, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45:W356–W60.
- Liu X, Ouyang S, Yu B, et al. PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010;38:W609–14.
- Li H, Leung KS, Wong MH, et al. Improving autodock vina using random forest: the growing accuracy of binding affinity prediction by the effective exploitation of larger data sets. Mol Inform. 2015;34:115–126.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461.
- Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30.
- Aragon-Sanchez J, Quintana-Marrero Y, Aragon-Hernandez C, et al. ImageJ: a free, easy, and reliable method to measure leg ulcers using digital pictures. Int J Low Extrem Wounds. 2017;16:269–273.
- Wang X, Pan C, Gong J, et al. Enhancing the enrichment of pharmacophore-based target prediction for the polypharmacological profiles of drugs. J Chem Inf Model. 2016;56:1175–1183.
- Ohren JF, Chen H, Pavlovsky A, et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11:1192–1197.
- Head KA. Natural therapies for ocular disorders, part two: cataracts and glaucoma. Alternative Med Rev J Clin Ther. 2001;6:141–166.
- Zhong JX, Jin SS, Wu KS, et al. Effect of nano-selenium loaded with lycium barbarum polysaccharide on the proliferation of lens epithelial cells after UVB damage in vitro. Int J Ophthalmol. 2022;15:9–14.
- Wu Q, Song J, Gao Y, et al. Epigallocatechin gallate enhances human lens epithelial cell survival after UVB irradiation via the mitochondrial signaling pathway. Mol Med Rep. 2022;25. DOI:10.3892/mmr.2022.12603.
- Kang LH, Zhang GW, Zhang JF, et al. Ganoderic acid A protects lens epithelial cells from UVB irradiation and delays lens opacity. Chin J Nat Med. 2020;18:934–940.
- Zeng K, Xi W, Qiao Y, et al. Paeoniflorin inhibits epithelial mesenchymal transformation and oxidative damage of lens epithelial cells in diabetic cataract via sirtuin 1 upregulation. Bioengineered. 2022;13:5903–5914.
- Shen Z, Shao J, Dai J, et al. Diosmetin protects against retinal injury via reduction of DNA damage and oxidative stress. Toxicol Rep. 2016;3:78–86.
- Yuan Y, Pei J, Lai L. LigBuilder 2: a practical de novo drug design approach. J Chem Inf Model. 2011;51:1083–1091.
- Morel I, Lescoat G, Cogrel P, et al. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Pharmacol. 1993;45:13–19.
- Sher E, Codignola A, Biancardi E, et al. Amine uptake inhibition by diosmin and diosmetin in human neuronal and neuroendocrine cell lines. Pharmacol Res. 1992;26:395–402.
- Ciolino HP, Wang TT, Yeh GC. Diosmin and diosmetin are agonists of the aryl hydrocarbon receptor that differentially affect cytochrome P450 1A1 activity. Cancer Res. 1998;58:2754–2760.
- Crews CM, Alessandrini A, Erikson RL. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480.
- Alessi DR, Saito Y, Campbell DG, et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619.
- Rosen LB, Ginty DD, Weber MJ, et al. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–1221.
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471.
- Drosten M, Barbacid M. Targeting the MAPK pathway in KRAS-driven tumors. Cancer Cell. 2020;37:543–550.
- Ye MJ, Meng N. Resveratrol acts via the mitogen-activated protein kinase (MAPK) pathway to protect retinal ganglion cells from apoptosis induced by hydrogen peroxide. Bioengineered. 2021;12:4878–4886.
- Wang Y, Yu Y, Yu W, et al. IL-35 inhibits cell pyroptosis and attenuates cell injury in TNF-α-induced bronchial epithelial cells via p38 MAPK signaling pathway. Bioengineered. 2022;13:1758–1766.
- Park JH, Kim JY, Kim DJ, et al. Effect of nitric oxide on human corneal epithelial cell viability and corneal wound healing. Sci Rep. 2017;7:8093.
- Yang H, Li X, Ma J, et al. Blockade of the intermediate-conductance Ca(2+)-activated K+ channel inhibits the angiogenesis induced by epidermal growth factor in the treatment of corneal alkali burn. Exp Eye Res. 2013;110:76–87.
