ABSTRACT
The level of miR‑135b-5p is lower in patients with preeclampsia (PE) superimposed on chronic hypertension than in healthy controls. However, the function of miR‑135b-5p in PE progression remains unknown. In the present study, we investigated the role of miR‑135b-5p in PE development and its possible mechanism for the first time. HTR8/SVneo cells (trophoblast cell line) were exposed to hypoxia/reoxygenation (H/R) to mimic PE in vitro. Hypoxia-inducible factor-1α (HIF-1α), forkhead box O3A (FOXO3a), and miR-135b-5p levels were measured using Real-time PCR. Cell proliferation, apoptosis and migration/invasion were evaluated using the Cell Counting Kit-8 (CCK-8), flow cytometry and transwell assays, respectively. Real-time PCR and Western blotting were performed to determine the levels of several pro- and anti-angiogenic factors. The binding of miR-135b-5p to the PIK3R2-3’ untranslated region (3ʹUTR) was confirmed by bioinformatics analysis and a dual-luciferase reporter assay. H/R exposure greatly upregulated HIF-1α, FOXO3a, and PIK3R2 levels, while downregulating miR-135b-5p levels in HTR8/SVneo cells. H/R exposure resulted in the inhibition of proliferation, migration, invasion, angiogenesis, and the induction of apoptosis. MiR-135b-5p overexpression reversed the effects of H/R on trophoblast cell function, while miR-135b-5p knockdown enhanced the effects. PIK3R2 knockdown had similar effects as miR-135b-5p overexpression on proliferation, apoptosis and angiogenesis. The effect of miR-135b-5p overexpression on H/R-exposed cells was enhanced by PIK3R2 knockdown. MiR-135b-5p downregulated PIK3R2 expression by pairing with its 3ʹUTR. Therefore, miR-135b-5p may regulate trophoblast function by targeting PIK3R2 in PE and could serve as a novel therapeutic target for PE.
Graphical abstract
Highlights
H/R treatment reduces miR-135b-5p while elevates PIK3R2 in trophoblast cells.
MiR-135b-5p regulates proliferation and metastasis of H/R-exposed trophoblast cells.
MiR-135b-5p modulates angiogenesis in H/R-exposed trophoblast cells.
MiR-135b-5p exerts its effects by targeting PIK3R2.
Introduction
Preeclampsia (PE) is a principal cause of maternal and perinatal deaths that affects 2–8% of pregnancies worldwide [Citation1,Citation2]. Several risk factors for PE have been identified, including obesity, hypertension, diabetes, multiple pregnancy, history of preeclampsia, nulliparity, and advanced maternal age [Citation3,Citation4]. During pregnancy, decreased trophoblast invasion, defective remodeling of uterine spiral arteries, and subsequent placental ischemia/hypoxia are associated with the initiation of PE [Citation2,Citation5]. Women suffering from PE often present with new-onset hypertension and proteinuria, which can lead to multi-organ dysfunction and even death of mothers and fetuses [Citation6]. Currently, delivery of the fetus and placenta is the only cure for PE [Citation7]. Most patient symptoms can be resolved, but postpartum hypertension may persist after delivery [Citation8]. Therefore, exploring the molecular mechanisms of PE is urgently needed to develop effective therapeutic strategies for PE.
MicroRNAs (miRNAs) are small noncoding RNAs that are approximately 19–24 nucleotides in length. They affect multiple biological processes [Citation9]. Aberrantly expressed miRNAs have been implicated in numerous human diseases, including PE [Citation10–12]. A previous study has revealed that miR-135b-5p protects against neuronal injury induced by oxygen-glucose deprivation and reoxygenation by targeting GSK-3β and activating the Nrf2/ARE signaling pathway [Citation13]. Chim et. al also demonstrated that miR‑135b-5p levels in maternal plasma were higher during pregnancy than those 24 h after delivery [Citation14]. Another study by Vashukova et. al found that miR‑135b-5p was downregulated in the placenta of patients with PE superimposed on chronic hypertension, compared to that in healthy controls [Citation15]. However, the function of miR‑135b-5p in PE progression remains unknown.
Phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2), which encodes the PI3K regulatory subunit p85β, is associated with cell proliferation, apoptosis and angiogenesis [Citation16–18]. PIK3R2 can be targeted by miR-126-3p, and in turn regulates endothelial progenitor cell function, vasculogenesis, and placenta/fetus weights in rats with PE [Citation19,Citation20]. According to a study by Wang et al., the miR-126-3p/PIK3R2 axis participated in protection against ischemia/reperfusion-induced pain hypersensitivity in rats [Citation21]. However, the correlation between miR‑135b-5p and PIK3R2 in PE has not yet been elucidated.
In the present study, HTR8/SVneo (a human trophoblast cell line) cells were subjected to hypoxia/reoxygenation (H/R) to mimic PE in vitro. We evaluated, for the first time, the function of the miR‑135b-5p/PIK3R2 axis in an in vitro model of PE. These findings will expand our understanding of the molecular mechanisms underlying PE and could guide future research toward effective new treatment strategies.
Materials and methods
Cell culture
HTR8/SVneo (human trophoblast cell line) cells were obtained from BLUEFBIO (Shanghai, China) and grown in HyClone Roswell Park Memorial Institute-1640 (Waltham, MA, USA) and Gibco fetal bovine serum (FBS; 10%; Grand Island, NY, USA) with 5% CO2 [Citation22].
Generation of PIK3R2 knockdown plasmids
Short hairpin RNAs (shRNAs) targeting PIK3R2 (PIK3R2 shRNA-1, 5′-ACCGGGGGTTCTCTCACCCTCTTTCTCTCGAGAGAAAGAGGGTGAGAGAACCCTTTTTGAATTC-3′; PIK3R2 shRNA-2, 5′- ACCGGGTCTCTCACCCTCTTTCTCTTTCCTTCTCGAGAAGGAAAGAGAAAGAGGGTGAGAGACTTTTTGAATTC-3′; PIK3R2 shRNA-3, 5′-ACCGGGCCATTCTCCAGATCTCCCTCTGTCTCTCGAGAGACAGAGGGAGATCTGGAGAATGGCTTTTTGAATTC-3’) were inserted into pLKO.1-EGFP (empty vector) to knockdown PIK3R2 expression, as described previously [Citation23].
Hypoxia/reoxygenation (H/R) treatment and cell transfection
HTR8/SVneo cells cultured under normoxic conditions (20% O2) were used as controls. To mimic PE in vitro, HTR8/SVneo cells were subjected to hypoxia (2% O2 for 8 h) and reoxygenation (20% O2 for 16 h) at 37°C, as described previously [Citation22]. After H/R treatment, the cells were transfected with 100 pmol miR‑135b-5p mimic/inhibitor or 4 µg PIK3R2 shRNA, or they were co-transfected with 100 pmol miR‑135b-5p mimic and 4 µg PIK3R2 shRNA using a liposome reagent (Lipofectamine 2000, Invitrogen).
Real-time PCR
HTR8/SVneo cells were harvested to extract total RNAs using the TRIzol reagent. RNA was then reverse transcribed to cDNA using the miRNA-RT primer or Oligo (dT)18. The KAPA qPCR Master Mix and Bio-Rad CFX Connect Detection System (Hercules, CA, USA) were used for PCR amplification. Relative levels of HIF-1α, FOXO3a, PIK3R2, vascular endothelial growth factor (VEGF), placental growth factor (PIGF), soluble fms-like tyrosine kinase-1 (sFlt-1), and miR-135b-5p were normalized to those of GAPDH or U6 and quantified using the 2−ΔΔCt method [Citation24]. The primer sequences are shown in .
Table 1. The primer sequences for real-time PCR
Western blotting
HTR8/SVneo cells were harvested to extract total protein using a radioimmunoprecipitation assay lysis buffer (Solarbio, Beijing, China). Protein concentrations were determined using the bicinchoninic acid method (Solarbio), and western blotting was performed as described previously [Citation25]. Briefly, the proteins (20 µg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. After blocking with nonfat milk, the membranes were treated with primary antibodies against VEGF, PIGF, sFlt-1, PIK3R2, or GAPDH (1:1000) and secondary antibodies (1:10000). The protein bands were reacted with western horseradish peroxidase substrate, and their intensities were analyzed using TANON GIS software (Shanghai, China). Primary antibodies against VEGF, PIGF, sFlt-1, PIK3R2, or GAPDH, as well as secondary antibodies, were purchased from Bioswamp (Wuhan, China).
Cell Counting Kit-8 (CCK-8) assay
After H/R treatment and transient transfection, HTR8/SVneo cells were treated with 10 µL CCK-8 (Solarbio) for an additional 4 h. An AMR-100 microplate reader (ALLSHENG Instruments Co., Ltd., Hangzhou, China) was used to examine the optical density of the cells at 450 nm [Citation26].
Transwell assays
Transwell assays were performed as previously described [Citation27]. Briefly, the Transwell chambers were uncoated for evaluation of cell migration capabilities, or pre-coated with 80 μL Matrigel (BD, USA) for 30 min for evaluation of cell invasion capabilities. Afterward, the upper and lower chambers were filled with the cell suspension (5 × 104 cells in 1% FBS) and 750 μL of culture medium (10% FBS), respectively. After culturing for 48 h at 37°C, the cells were fixed and stained with 0.5% crystal violet. Migrating and invading cells were photographed and their numbers were counted using a Leica DMIL LED microscope.
Cell apoptosis evaluation by flow cytometry
Apoptosis was evaluated as previously described [Citation28]. Briefly, after washing with PBS, cell suspensions (1 × 106 cells) were treated with Annexin V-phycoerythrin (5 μL; BD Biosciences) and 7-amino-actinomycin D (5 μL; BD Biosciences) for 30 min at 4°C. Apoptotic cells were counted using a NovoCyteTM flow cytometer (ACEA Biosciences).
Dual-luciferase reporter assay
As previously described [Citation29], PIK3R2-3ʹUTRs (wild-type and mutant) were inserted into the pmirGLO vector and verified by DNA sequencing. Then, the cells were co-transfected with plasmids carrying PIK3R2-3ʹUTR (wild-type or mutant; 0.2 μg) and miR-135b-5p mimic (5 pmol) via a liposome reagent (Lipofectamine RNAiMAX; Invitrogen, USA). A dual-luciferase reporter gene assay kit (Beyotime, Haimen, China) was used to determine firefly and Renilla luciferase activities.
Statistical analysis
Results are presented as the mean ± standard deviation. A Student’s t-test or one-way analysis of variance followed by Tukey’s test was used to analyze statistical differences between groups. A P < 0.05 was considered as statistically significant.
Results
We speculated that miR-135b-5p may participate in PE development by regulating its downstream targets. To test this hypothesis, the trophoblast cell line HTR8/SVneo was subjected to H/R treatment to mimic PE in vitro. The effects of the miR-135b-5p/PIK3R2 axis on trophoblast cell function were evaluated for the first time. The binding of miR-135b-5p to the PIK3R2-3ʹUTR was confirmed by bioinformatics analysis and a dual-luciferase reporter assay. MiR-135b-5p regulated proliferation, apoptosis, migration, invasion, and angiogenesis in H/R-exposed trophoblasts by targeting PIK3R2. The miR-135b-5p/PIK3R2 axis may lead to PE development through regulation of trophoblast cell function.
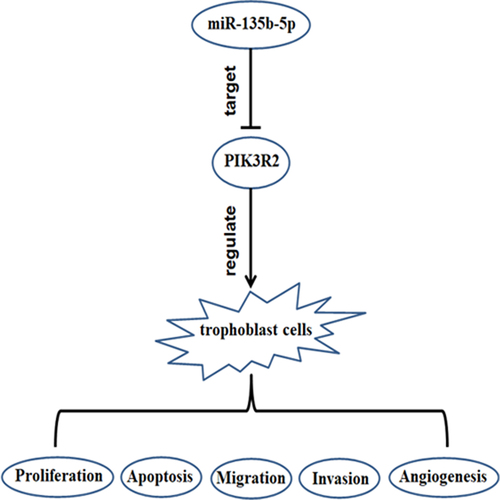
H/R exposure decreases miR-135b-5p levels and increases the levels of HIF-1α, FOXO3a, and PIK3R2 in trophoblast cells
HTR8/SVneo cells were subjected to H/R to mimic PE in vitro. HIF-1α and FOXO3a levels were determined by real-time PCR. H/R exposure significantly upregulated HIF-1α ()) and FOXO3a ()) mRNA levels in HTR8/SVneo cells compared to those in the control. Real-time PCR was also conducted to determine the miR-135b-5p and PIK3R2 levels. Following H/R exposure, miR-135b-5p ()) levels were downregulated, whereas PIK3R2 ()) levels were upregulated compared to those in the control group.
Figure 1. The effect of H/R exposure on HIF-1α, FOXO3a, miR-135b-5p, and PIK3R2 levels. (a) After exposure to hypoxia (2% O2 for 8 h) and reoxygenation (20% O2 for 16 h), HIF-1α levels in HTR8/SVneo cells were determined by real-time PCR (internal control: GAPDH). (b) Real-time PCR analysis of FOXO3a (internal control: GAPDH). (c) Real-time PCR analysis of miR-135b-5p (internal control: U6). (d) Real-time PCR analysis of PIK3R2 (internal control: GAPDH). **P < 0.01 vs. Control group.
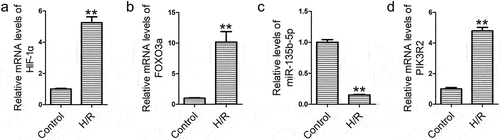
MiR-135b-5p overexpression promotes cell proliferation, migration, and invasion of H/R-exposed cells
To investigate the role of miR-135b-5p in trophoblast cell function, HTR8/SVneo cells exposed to H/R were transfected with miR-135b-5p mimic or inhibitor. MiR-135b-5p mimic transfection significantly elevated miR-135b-5p levels in HTR8/SVneo cells compared to those in the NC mimic group ()). Immunofluorescence assays showed that the NC inhibitor or miR-135b-5p inhibitor was successfully transfected into HTR8/SVneo cells ()). H/R-induced reduction in miR-135b-5p levels was reversed by miR-135b-5p mimic transfection, while enhanced by miR-135b-5p inhibitor transfection compared to those in the corresponding NC group ()). Next, cell proliferation, migration and invasion were determined. H/R treatment inhibited cell proliferation compared to that in the control. However, miR-135b-5p mimic transfection promoted proliferation of H/R-exposed cells, and miR-135b-5p inhibitor transfection suppressed this effect ()). Cell migration ()) and invasion ()) abilities were reduced by H/R exposure compared to those in the controls, which was attenuated by miR-135b-5p mimic transfection. Transfection with miR-135b-5p inhibitor further suppressed the migration and invasion of HTR8/SVneo cells exposed to H/R.
Figure 2. MiR-135b-5p overexpression enhances cell proliferation, migration, and invasion under H/R conditions. (a) HTR8/SVneo cells were transfected with miR-135b-5p mimic/NC mimic and then miR-135b-5p levels were determined by real-time PCR (internal control: U6). (b) HTR8/SVneo cells were transfected with miR-135b-5p inhibitor/NC inhibitor. Green fluorescence was observed using a fluorescence microscope. Scale bar represents 250 μm. (c) After H/R exposure and cell transfection, miR-135b-5p levels were determined by real-time PCR (internal control: U6). (d) Cell proliferation was evaluated by CCK-8 assay. (e) Cell migration was analyzed by transwell migration assay. Scale bar represents 100 μm. (f) Cell invasion was analyzed by transwell invasion assay. Scale bar represents 100 μm.**P < 0.01 vs. NC mimic group or Control group. ##P < 0.01 vs. H/R+ NC mimic group. &P < 0.05, &&P < 0.01 vs. H/R+ NC inhibitor group.
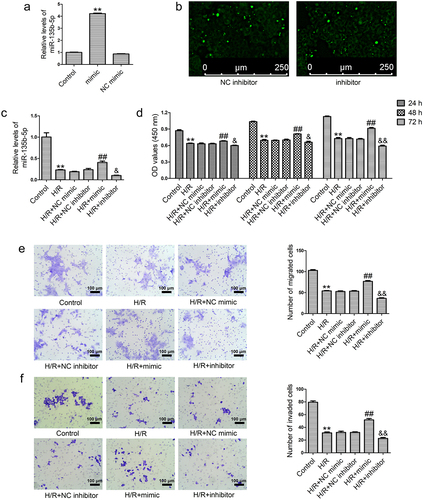
MiR-135b-5p overexpression modulates the levels of angiogenesis-associated genes in H/R-exposed cells
To investigate the role of miR-135b-5p in angiogenesis, the levels of several angiogenesis-associated genes were examined using real-time PCR. H/R exposure markedly increased PIK3R2 and sFlt-1 levels and decreased VEGF and PIGF levels in HTR8/SVneo cells compared to those in the controls, which was attenuated by the miR-135b-5p mimic ()). In addition, miR-135b-5p inhibitor transfection significantly enhanced the effects of H/R exposure on these angiogenesis-associated genes compared with NC inhibitor transfection.
Figure 3. MiR-135b-5p overexpression accelerates angiogenesis in HTR8/SVneo cells under H/R conditions. (a) HTR8/SVneo cells were transfected with miR-135b-5p mimic/NC mimic/miR-135b-5p inhibitor/NC inhibitor after H/R treatment. PIK3R2 levels were determined by real-time PCR (internal control: GAPDH). (b) VEGF, PIGF, and sFlt-1 levels were determined by real-time PCR (internal control: GAPDH). **P < 0.01 vs. Control group. ##P < 0.01 vs. H/R+ NC mimic group. &&P < 0.01 vs. H/R+ NC inhibitor group.
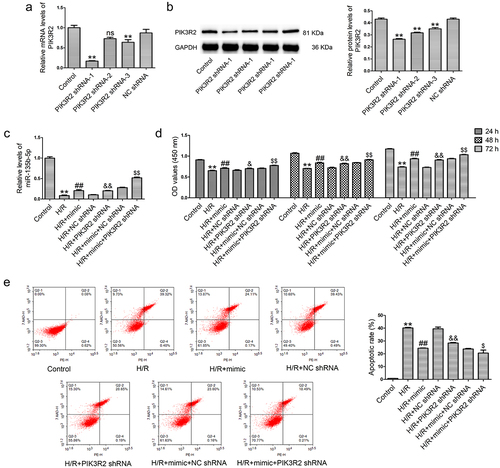
PIK3R2 knockdown enhances the pro-proliferative and anti-apoptotic effects of miR-135b-5p overexpression in H/R-exposed cells
To knockdown PIK3R2 expression, three shRNAs against PIK3R2 were synthesized and transfected into HTR8/SVneo cells. As shown in ), PIK3R2 shRNA-1 and PIK3R2 shRNA-3 significantly downregulated PIK3R2 mRNA levels compared with NC shRNA transfection. All three shRNAs against PIK3R2 markedly decreased PIK3R2 protein levels ()). PIK3R2 shRNA-1 had a stronger knockdown efficiency than the other shRNAs and was therefore used in subsequent experiments. Interestingly, PIK3R2 shRNA transfection abolished the H/R-induced decrease in miR-135b-5p levels, unlike NC shRNA transfection (). Additionally, PIK3R2 shRNA transfection enhanced the miR-135b-5p mimic-induced elevation of miR-135b-5p levels in H/R-exposed cells. We next tested whether miR-135b-5p affects trophoblast cell proliferation and apoptosis by regulating PIK3R2. PIK3R2 shRNA transfection attenuated the H/R-induced proliferation inhibition ()) and cell apoptosis ()) compared with NC shRNA transfection. PIK3R2 shRNA transfection also further enhanced the pro-proliferative and anti-apoptotic effects of miR-135b-5p mimic in H/R-exposed cells.
Figure 4. PIK3R2 knockdown enhances the effects of miR-135b-5p overexpression on proliferation and apoptosis of H/R-exposed cells. (a) HTR8/SVneo cells were transfected with PIK3R2 shRNA-1/-2/-3 or NC shRNA. PIK3R2 levels were determined by real-time PCR (internal control: GAPDH). (b) PIK3R2 levels were determined by western blotting (internal control: GAPDH). (c) HTR8/SVneo cells were transfected with miR-135b-5p mimic/PIK3R2 shRNA/NC shRNA alone, or co-transfected with miR-135b-5p mimic and PIK3R2 shRNA/NC shRNA after H/R treatment. MiR-135b-5p levels were determined by real-time PCR (internal control: U6). (d) Cell proliferation was determined by CCK-8 assay. (e) Cell apoptosis was measured by flow cytometry. **P < 0.01 vs. NC shRNA group or Control group. ##P < 0.01 vs. H/R group. &P < 0.05, &&P < 0.01 vs. H/R+ NC shRNA group. $P < 0.05, $$P < 0.01 vs. H/R+ mimic+NC shRNA group and ns indicates not significant.
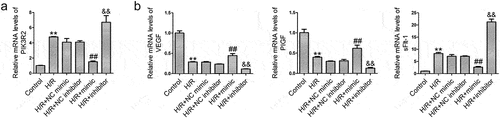
PIK3R2 knockdown enhances the pro-angiogenic effects of miR-135b-5p overexpression in H/R-exposed cells
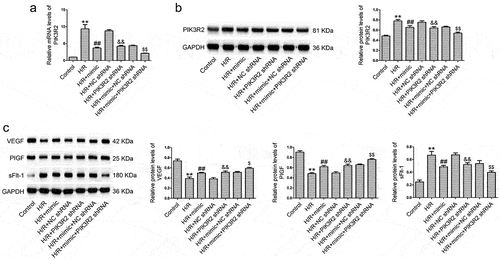
We investigated whether miR-135b-5p could regulate angiogenesis via PIK3R2 in trophoblast cells. PIK3R2 shRNA transfection reversed the H/R-induced upregulation of PIK3R2 ()) and sFlt-1 levels as well as downregulation of VEGF and PIGF levels ()) in HTR8/SVneo cells compared with NC shRNA transfection. Changes in the levels of angiogenesis-associated genes induced by the miR-135b-5p mimic were further enhanced by PIK3R2 shRNA in H/R-exposed cells.
Figure 5. PIK3R2 knockdown enhances the effects of miR-135b-5p overexpression on angiogenesis-associated genes in H/R-exposed cells. (a) PIK3R2 levels were determined by real-time PCR (internal control: GAPDH). (b) PIK3R2 levels were determined by western blotting (internal control: GAPDH). (c) VEGF, PIGF, and sFlt-1 levels were determined by western blotting (internal control: GAPDH). **P < 0.01 vs. Control group. ##P < 0.01 vs. H/R group. &&P < 0.01 vs. H/R+ NC shRNA group. $P < 0.05, $$P < 0.01 vs. H/R+ mimic+NC shRNA group.
PIK3R2 is a target of miR-135b-5p
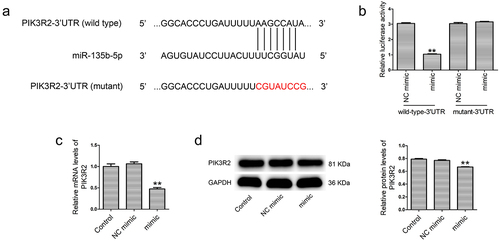
Bioinformatic analysis was conducted to predict the targets of miR-135b-5p using TargetScan and miRDB. PIK3R2-3ʹUTR contains a binding site for miR-135b-5p ()). The relationship between miR-135b-5p and PIK3R2 was confirmed by a dual-luciferase reporter assay, real-time PCR and western blotting. As shown in ), the miR-135b-5p mimic significantly reduced the luciferase activity of PIK3R2-3ʹUTR (wild-type), but had no impact on PIK3R2-3ʹUTR (mutant). In addition, miR-135b-5p mimic transfection markedly decreased the PIK3R2 mRNA ()) and protein ()) levels in HTR8/SVneo cells compared with NC mimic transfection.
Figure 6. PIK3R2 is a target of miR-135b-5p. (a) The binding relationship between miR-135b-5p and PIK3R2 was predicated using bioinformatic analysis (TargetScan and miRDB). (b) A dual-luciferase reporter assay was performed to verify binding of miR-135b-5p to the PIK3R2-3ʹUTR. (c) PIK3R2 expression was determined by real-time PCR (internal control: GAPDH). (d) PIK3R2 expression was determined by western blotting (internal control: GAPDH). **P < 0.01 vs. NC mimic+wild-type-3ʹUTR group or NC mimic group.
Discussion
Mounting evidence suggests that miRNAs are aberrantly expressed in patients with PE [Citation12,Citation30]. However, the function of miR‑135b-5p in PE progression and its underlying mechanism were unknown. In the present study, we demonstrated for the first time that miR-135b-5p is downregulated in an in vitro model of PE and may exert a protective role in this model by targeting PIK3R2.
Placental hypoxia is associated with PE [Citation31]. HIF-1α is a transcription factor that mediates the hypoxia-associated intracellular response [Citation32]. It is a key marker of tissue hypoxia and participates in cell proliferation, angiogenesis, apoptosis, and glucose metabolism [Citation33]. Upon hypoxic stimulation, HIF-1α interacts with the coactivator p300/CBP to modulate its transcriptional activity [Citation34]. FOXO3a (a member of the FOXO transcription factor family) plays a crucial role in cell proliferation, apoptosis, migration and invasion [Citation35,Citation36]. In a previous study, Zhang et al. demonstrated that HIF‑1α and FOXO3a were upregulated in PE patients and hypoxia-exposed trophoblasts [Citation37]. Moreover, elevated HIF‑1α and FOXO3a levels are correlated with trophoblast dysfunction and the occurrence of PE [Citation37,Citation38]. Therefore, we subjected HTR8/SVneo cells to H/R treatment to mimic PE in vitro and measured the levels of HIF‑1α and FOXO3a. HIF‑1α and FOXO3a mRNA levels were significantly elevated in H/R-exposed HTR8/SVneo cells compared to those in the controls, which is consistent with the findings of Zhang et al. [Citation37]. These findings indicated that we successfully established an in vitro model of PE.
MiR-135b-5p functions as an oncogene in many human cancers, including pancreatic, gastric, esophageal, and breast cancer [Citation39–43]. In addition, in a study by Vashukova et al., patients suffering from chronic hypertension with superimposed PE had lower miR‑135b-5p levels in the placenta compared to healthy controls [Citation15]. Yang et al. found that hypoxia decreased miR‑135b-5p levels in human umbilical vein endothelial cells [Citation44]. PIK3R2 is an anti-angiogenic factor that regulates cell proliferation, apoptosis, migration, invasion, and angiogenesis [Citation45–47]. PIK3R2 expression could also be elevated by hypoxia in endothelial progenitor cells [Citation48]. In the present study, we found that miR‑135b-5p was downregulated and PIK3R2 was upregulated in H/R-exposed HTR8/SVneo cells compared to those in control cells, which is consistent with previous studies [Citation44,Citation48]. Our findings indicate that hypoxia-induced aberrant expression of miR‑135b-5p and PIK3R2 may be associated with trophoblast dysfunction during PE development.
Both proliferation inhibition and apoptosis of trophoblast cells are correlated with PE [Citation49]. The migration and invasion of trophoblasts into the endometrium or vasculature participates in placentation in humans [Citation50]. However, inadequate trophoblast invasion impairs placentation and angiogenesis, leading to PE development [Citation51]. Previous studies have revealed that specific miRNAs participate in the regulation of proliferation and metastasis of trophoblast cells, such as miR-214-5p and miR-211-5p [Citation52,Citation53]. Zhang et al. showed that miR-135b-5p overexpression enhanced pancreatic cancer cell migration and invasion by targeting nuclear receptor subfamily 3 group C member 2 [Citation41]. Another study has reported that the overexpression of miR-135b-5p promotes proliferation and reduces cell apoptosis by targeting Krüppel-like factor 4 in gastric cancer cells [Citation54]. Conversely, miR-135b-5p knockdown suppresses proliferation and metastasis and facilitates the apoptosis of esophageal cancer cells via regulation of the thioredoxin-interacting protein (TXNIP) [Citation40]. However, the role of miR-135b-5p in trophoblast function and PE has not been fully elucidated. To investigate the function of miR-135b-5p, HTR8/SVneo cells were treated with H/R and transfected with miR-135b-5p mimic or inhibitor. MiR-135b-5p overexpression alleviated the impairment of proliferation, migration, and invasion and promoted apoptosis induced by H/R. Furthermore, we observed the opposite effects after knockdown of miR-135b-5p, which is consistent with previous findings [Citation40,Citation41,Citation54]. These findings indicate that miR-135b-5p may affect PE progression by regulating trophoblast cell function.
Impaired angiogenesis contributes to PE [Citation55]. Pro-angiogenic factors (VEGF and PIGF) and anti-angiogenic factors (sFlt-1) are often used to evaluate the angiogenic state of PE [Citation56]. VEGF and PIGF levels are downregulated, while sFlt-1 expression is upregulated, in PE patients [Citation56,Citation57]. sFlt-1 antagonizes the interaction of VEGF and PIGF with their surface receptors in endothelial cells, leading to endothelial dysfunction and PE [Citation56]. Yin et al. found that miR-135b-5p overexpression promoted angiogenesis by inhibiting TXNIP in human umbilical vein endothelial cells [Citation58]. Conversely, another study showed that miR-135b-5p knockdown impaired angiogenesis of retinal vascular endothelial cells in diabetic retinopathy in mice [Citation59]. In the present study, we found that VEGF and PIGF were downregulated, whereas sFlt-1 was upregulated, in H/R-exposed cells, indicating that an anti-angiogenic state existed in this in vitro model of PE. Overexpression of miR-135b-5p attenuated, while knockdown of miR-135b-5p enhanced, H/R-induced changes in VEGF, PIGF, and sFlt-1 levels, which is consistent with a previous study by Lin et al. [Citation59]. These findings indicate that miR-135b-5p may affect PE progression by regulating angiogenesis-related factors in trophoblasts.
PIK3R2 is an anti-angiogenic factor that is upregulated in the placental tissue of rats with PE [Citation19,Citation20]. Yan et al. determined that PIK3R2 was targeted by miR-126-3p, and its downregulation regulated endothelial progenitor cell function in vitro and placental vasculogenesis in vivo in PE [Citation20]. However, the role of PIK3R2 in trophoblast cell function was not clear. In the present study, PIK3R2 knockdown, similar to miR-135b-5p overexpression, blocked H/R-induced reduction in proliferation and angiogenesis, and promoted trophoblast cell apoptosis. Previous studies have reported that many miRNAs, such as miR-30a-5p, miR-126-3p and miR-1254-5p, can target PIK3R2 and regulate the progression of PE, non-small cell lung cancer, and breast cancer [Citation20,Citation60,Citation61]. However, whether miR-135b-5p affects PE progression through PIK3R2 regulation was previously unclear. In the present study, the binding of miR-135b-5p to PIK3R2-3ʹUTR was predicted using bioinformatics software and verified using a dual-luciferase reporter assay. PIK3R2 was reduced in miR-135b-5p overexpressing cells, but was induced after miR-135b-5p knockdown. Furthermore, PIK3R2 knockdown enhanced the pro-proliferative, anti-apoptotic, and pro-angiogenic effects of miR-135b-5p overexpression in H/R-exposed cells. These findings indicate that miR-135b-5p may affect PE progression by regulating PIK3R2 in trophoblast cells.
Conclusion
In conclusion, miR-135b-5p was downregulated, whereas PIK3R2 was upregulated, after H/R treatment. MiR-135b-5p overexpression attenuated the H/R-induced suppression of proliferation, migration, invasion, and angiogenesis, as well as the promotion of apoptosis. PIK3R2 knockdown enhanced the effects of miR-135b-5p overexpression in H/R-exposed cells. In addition, we showed that PIK3R2 is a target of miR-135b-5p. The next challenge will be to directly confirm the effects of miR-135b-5p on PE development in vivo. Studies are currently underway to verify our findings using animal models of PE. The miR-135b-5p/PIK3R2 axis may participate in PE development and may serve as a novel therapeutic target for PE.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- de Alwis N, Binder NK, Beard S, et al. Novel approaches to combat preeclampsia: from new drugs to innovative delivery. Placenta. 2020;102:10–16.
- Ives CW, Sinkey R, Rajapreyar I, et al. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(14):1690–1702.
- Phipps EA, Thadhani R, Benzing T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–289.
- Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387(10022):999–1011.
- Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24(2):131–138.
- Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112.
- Ma’ayeh M, Costantine MM. Prevention of preeclampsia. Semin Fetal Neonatal Med. 2020;25(5):101123.
- Rosser ML, Katz NT. Preeclampsia: an obstetrician’s perspective. Adv Chronic Kidney Dis. 2013;20(3):287–296.
- Low SS, Ji D, Chai WS. Recent progress in nanomaterials modified electrochemical biosensors for the detection of MicroRNA. Micromachines (Basel). 2021;13(1):12.
- Lv Y, Lu C, Ji X, et al. Roles of microRNAs in preeclampsia. J Cell Physiol. 2019;234(2):1052–1061.
- Bounds KR, Chiasson VL, Pan LJ, et al. MicroRNAs: new players in the pathobiology of preeclampsia. Front Cardiovasc Med. 2017;4:60.
- Chen Z, Zhang W, Wu M, et al. Pathogenic mechanisms of preeclampsia with severe features implied by the plasma exosomal mirna profile. Bioengineered. 2021;12(2):9140–9149.
- Duan Q, Sun W, Yuan H, et al. MicroRNA-135b-5p prevents oxygen-glucose deprivation and reoxygenation-induced neuronal injury through regulation of the GSK-3beta/Nrf2/ARE signaling pathway. Arch Med Sci. 2018;14(4):735–744.
- Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54(3):482–490.
- Vashukova ES, Glotov AS, Fedotov PV, et al. Placental microRNA expression in pregnancies complicated by superimposed preeclampsia on chronic hypertension. Mol Med Rep. 2016;14(1):22–32.
- Rao L, Mak VCY, Zhou Y, et al. p85beta regulates autophagic degradation of AXL to activate oncogenic signaling. Nat Commun. 2020;11(1):2291.
- Zhang L, Ouyang P, He G, et al. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med. 2021;25(4):2148–2162.
- Gao J, Zhou XL, Kong RN, et al. microRNA-126 targeting PIK3R2 promotes rheumatoid arthritis synovial fibro-blasts proliferation and resistance to apoptosis by regulating PI3K/AKT pathway. Exp Mol Pathol. 2016;100(1):192–198.
- Yan T, Cui K, Huang X, et al. Assessment of therapeutic efficacy of miR-126 with contrast-enhanced ultrasound in preeclampsia rats. Placenta. 2014;35(1):23–29.
- Yan T, Liu Y, Cui K, et al. MicroRNA-126 regulates EPCs function: implications for a role of miR-126 in preeclampsia. J Cell Biochem. 2013;114(9):2148–2159.
- Wang H, Chen FS, Zhang ZL, et al. MiR-126-3p-enriched extracellular vesicles from hypoxia-preconditioned VSC 4.1 neurons attenuate ischaemia-reperfusion-induced pain hypersensitivity by regulating the PIK3R2-mediated pathway. Mol Neurobiol. 2021;58(2):821–834.
- Deng Q, Yin N, Chen Y, et al. Downregulated N-acetylglucosaminyltransferase III is involved in attenuating trophoblast migration and invasion under hypoxia-reoxygenation condition. J Matern Fetal Neonatal Med. 2019;32(14):2369–2375.
- Li Z, Xue TQ, Yang C, et al. EGFL7 promotes hepatocellular carcinoma cell proliferation and inhibits cell apoptosis through increasing CKS2 expression by activating Wnt/beta-catenin signaling. J Cell Biochem. 2018;119(12):10327–10337.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408.
- Zhang Z, Zhao H, Zhou G, et al. Circ_0002623 promotes bladder cancer progression by regulating the miR-1276/SMAD2 axis. Cancer Sci. 2022;113(4):1250–1263.
- Luo N, Liu S, Li X, et al. Circular RNA circHIPK3 promotes breast cancer progression via sponging MiR-326. Cell Cycle. 2021;20(13):1320–1333.
- Zhang X, Hu F, Liu L, et al. Effect of silencing of mediator of DNA damage checkpoint protein 1 on the growth of oral squamous cell carcinoma in vitro and in vivo. Eur J Oral Sci. 2019;127(6):494–499.
- Deng H, Ma J, Liu Y, et al. Combining alpha-Hederin with cisplatin increases the apoptosis of gastric cancer in vivo and in vitro via mitochondrial related apoptosis pathway. Biomed Pharmacother. 2019;120:109477.
- Zhao CC, Jiao Y, Zhang YY, et al. Lnc SMAD5-AS1 as ceRNA inhibit proliferation of diffuse large B cell lymphoma via Wnt/beta-catenin pathway by sponging miR-135b-5p to elevate expression of APC. Cell Death Dis. 2019;10(4):252.
- Zhong Y, Zhu F, Ding Y. Differential microRNA expression profile in the plasma of preeclampsia and normal pregnancies. Exp Ther Med. 2019;18(1):826–832.
- Thompson LP, Pence L, Pinkas G, et al. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95(6):128.
- Warbrick I, Rabkin SW. Hypoxia-inducible factor 1-alpha (HIF-1alpha) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes Rev. 2019;20(5):701–712.
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469–1480.
- Lee JW, Bae SH, Jeong JW, et al. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12.
- Li J, Yang R, Dong Y, et al. Knockdown of FOXO3a induces epithelial-mesenchymal transition and promotes metastasis of pancreatic ductal adenocarcinoma by activation of the beta-catenin/TCF4 pathway through SPRY2. J Exp Clin Cancer Res. 2019;38(1):38.
- Yan F, Liao R, Farhan M, et al. Elucidating the role of the FoxO3a transcription factor in the IGF-1-induced migration and invasion of uveal melanoma cancer cells. Biomed Pharmacother. 2016;84:1538–1550.
- Zhang Z, Huang C, Wang P, et al. HIF1alpha affects trophoblastic apoptosis involved in the onset of preeclampsia by regulating FOXO3a under hypoxic conditions. Mol Med Rep. 2020;21(6):2484–2492.
- Iriyama T, Wang W, Parchim NF, et al. Reciprocal upregulation of hypoxia-inducible factor-1 alpha and persistently enhanced placental adenosine signaling contribute to the pathogenesis of preeclampsia. FASEB J. 2020;34(3):4041–4054.
- Lu M, Huang Y, Sun W, et al. miR-135b-5p promotes gastric cancer progression by targeting CMTM3. Int J Oncol. 2018;52(2):589–598.
- Di Y, Jiang Y, Shen X, et al. Downregulation of miR-135b-5p suppresses progression of esophageal cancer and contributes to the effect of cisplatin. Front Oncol. 2021;11:679348.
- Zhang Z, Che X, Yang N, et al. miR-135b-5p Promotes migration, invasion and EMT of pancreatic cancer cells by targeting NR3C2. Biomed Pharmacother. 2017;96:1341–1348.
- Han X, Saiyin H, Zhao J, et al. Overexpression of miR-135b-5p promotes unfavorable clinical characteristics and poor prognosis via the repression of SFRP4 in pancreatic cancer. Oncotarget. 2017;8(37):62195–62207.
- Hua K, Jin J, Zhao J, et al. miR-135b, upregulated in breast cancer, promotes cell growth and disrupts the cell cycle by regulating LATS2. Int J Oncol. 2016;48(5):1997–2006.
- Yang S, Yin J, Hou X. Inhibition of miR-135b by SP-1 promotes hypoxia-induced vascular endothelial cell injury via HIF-1alpha. Exp Cell Res. 2018;370(1):31–38.
- Song L, Li D, Gu Y, et al. MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell proliferation, migration, and invasion by regulation of PTEN/PI3K/AKT pathway. Clin Lung Cancer. 2016;17(5):e65–e75.
- Qu Y, Zhang YP, Wu J, et al. Retracted: downregulated microRNA-135a ameliorates rheumatoid arthritis by inactivation of the phosphatidylinositol 3-kinase/AKT signaling pathway via phosphatidylinositol 3-kinase regulatory subunit 2. J Cell Physiol. 2019;234(10):17663–17676.
- Du C, Lv Z, Cao L, et al. MiR-126-3p suppresses tumor metastasis and angiogenesis of hepatocellular carcinoma by targeting LRP6 and PIK3R2. J Transl Med. 2014;12(1):259.
- Zhang Y, Xu Y, Zhou K, et al. MicroRNA-126 and VEGF enhance the function of endothelial progenitor cells in acute myocardial infarction. Exp Ther Med. 2022;23(2):142.
- Mo HQ, Tian FJ, Ma XL, et al. PDIA3 regulates trophoblast apoptosis and proliferation in preeclampsia via the MDM2/p53 pathway. Reproduction. 2020;160(2):293–305.
- Zhang Y, Cao L, Jia J, et al. CircHIPK3 is decreased in preeclampsia and affects migration, invasion, proliferation, and tube formation of human trophoblast cells. Placenta. 2019;85:1–8.
- Jiang S, Chen Q, Liu H, et al. Preeclampsia-Associated lncRNA INHBA-AS1 regulates the proliferation, invasion, and migration of placental trophoblast cells. Mol Ther Nucleic Acids. 2020;22:684–695.
- Zhang Q, Wang Z, Cheng X, et al. lncRNA DANCR promotes the migration an invasion and of trophoblast cells through microRNA-214-5p in preeclampsia. Bioengineered. 2021;12(2):9424–9434.
- Tang D, Geng L, Ma J. lncRNA PROX1-AS1 mediates the migration and invasion of placental trophoblast cells via the miR-211-5p/caspase-9 axis. Bioengineered. 2021;12(1):4100–4110.
- Chen Z, Gao Y, Gao S, et al. MiR-135b-5p promotes viability, proliferation, migration and invasion of gastric cancer cells by targeting Kruppel-like factor 4 (KLF4). Arch Med Sci. 2020;16(1):167–176.
- Pereira RD, De Long NE, Wang RC, et al. Angiogenesis in the placenta: the role of reactive oxygen species signaling. Biomed Res Int. 2015;2015:814543.
- Yelumalai S, Muniandy S, Zawiah Omar S, et al. Pregnancy-induced hypertension and preeclampsia: levels of angiogenic factors in Malaysian women. J Clin Biochem Nutr. 2010;47(3):191–197.
- Varughese B, Bhatla N, Kumar R, et al. Circulating angiogenic factors in pregnancies complicated by pre-eclampsia. Natl Med J India. 2010;23(2):77–81.
- Yin H, Yu S, Xie Y, et al. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell Signal. 2021;84:110029.
- Liu L, Xu H, Zhao H, et al. MicroRNA-135b-5p promotes endothelial cell proliferation and angiogenesis in diabetic retinopathy mice by inhibiting Von Hipp-el-Lindau and elevating hypoxia inducible factor alpha expression. J Drug Target. 2021;29(3):300–309.
- Mohamadzade Z, Hasannia Kolagar T, Nemati H, et al. Molecular and cellular evidence for hsa-miR-1254 suppressor effect against HER2 signaling in breast cancer. J Cell Biochem. 2022;123(4):746–758.
- Meng F, Wang F, Wang L, et al. MiR-30a-5p overexpression may overcome EGFR-Inhibitor resistance through regulating PI3K/AKT signaling pathway in non-small cell lung cancer cell lines. Front Genet. 2016;7:197.
