ABSTRACT
Biosimilars play an important role in reducing the burden on patients and increasing the market competition. Biosimilar monoclonal antibodies are currently one of the hotspots of research and development in China with policies support. With the continuous improvement of policies, the enthusiasm for the research and development of biosimilars has increased year by year. The policy requirements in different periods have different degrees of impact on the patent applications of pharmaceutical companies. This review introduces the biosimilar monoclonal antibodies market status and approval process in China, analyzes the patents in this field, and helps pharmaceutical companies protect their intellectual property rights.
Graphical Abstract
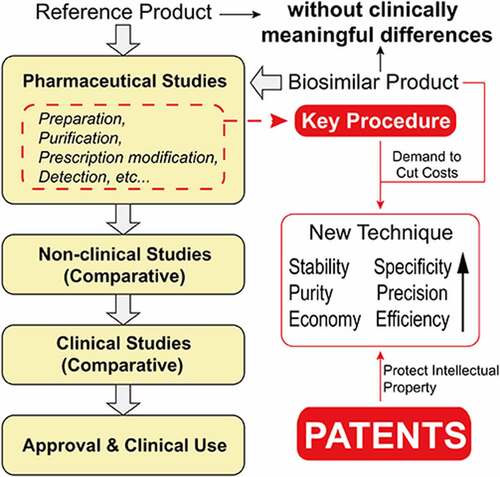
Highlights
A summary of the patents related to biosimilar mAbs in China and their advantages.
An overview of Chinese biosimilar monoclonal antibody market and development.
Patents that improve the prescription of preparations account for the majority.
Introduction
Monoclonal antibodies (mAb), first commercialized in 1986, have already developed into the vital therapeutics of diseases, especially tumor and autoimmune diseases [Citation1,Citation2]. Up to 2021, there are over 40 mAbs launched in China, most of which are imported. Compared with chemotherapeutic drugs, mAbs provide more efficacy, more specificity, but cost more. The extravagant medicinal cost becomes a burden to patients and society, which impedes the development of the mAbs market in China. In 2018, the world’s best sell drug was adalimumab (Humira®), while no mAbs ranked in the top 10 in China[Citation3]. Meanwhile, there are huge gaps in technique and equipment between home and abroad. The market of mAbs in China still has room for growth.
Because of rising competition from biosimilars, the price of mAbs decreases and access increases. For instance, in China, the price of trastuzumab (Herceptin®) in 2016 was 24,500 and then decreased to 7600 after involved in the medical insurance list. In 2020, Zercepac®, a biosimilar of Herceptin®, was listed at 1688 in China, helping patients receive efficacious and economical treatment. The 13th Five-Year Plan also pointed out the significance of developing biosimilars and regarded biosimilars as an important part of novel biomedical system. Different from generic drugs, biosimilars cannot be the same as original drugs due to their large molecular mass, complex structure, and undisclosed manufacturing process[Citation4].
Since the first biosimilar, Rituximab injection copied by Henlius, was launched in 2019, 142 biosimilar mAbs involved 16 targets have been researched and developed in China, and ten of them have been launched (). China has over 60 pharmaceutical companies in this field. Representative companies include Henlius, Hisun, Mabpharm, Qilu Pharmaceutical, Hualanbio, Biotech, Chiatai Tianqing, and Innoventbio. shows the outline of biosimilar mAbs of these companies and their trial progress.
Figure 1. Numbers of the research and development of different biosimilars in China. Adalimumab and bevacizumab are the hotspots in the Chinese biosimilar market, while mAbs with new targets show less competitions.
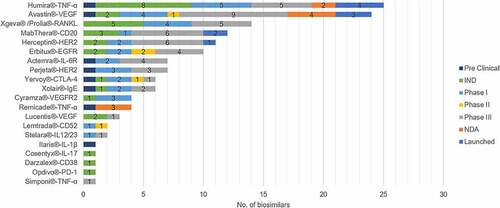
Table 1. Representative companies and the R&D progress of their biosimilar products.
In the United State of America and the European Union, the development of biosimilar products is equally rapid. Filgrastim-sndz (Zarxio®) is the first biosimilar product approved by Food and Drug Administration (FDA) in March 2015. Up to April 2022, FDA approved 35 biosimilar products and 28.6% (10/35) were approved in 2019. [Citation5]. Somatropin (Omnitrope®) is the first biosimilar product in the world and was approved by European Medicines Agency (EMA) on April 12th, 2006. Since then, 68 biosimilar products were approved by EMA, and 17 were refused and withdrawn[Citation6]. As for biosimilar mAbs, 18 and 32 biosimilar products were approved respectively by FDA and EMA. Both of these biosimilar mAbs showed similar effects and safety to their reference drugs. [Citation7–14] The markets of the USA and EU are more competitive and energetic than that of China. Interestingly, Zercepac® (trastuzumab) from China was authorized by EMA in July 2020. [Citation6]. The manufacturer of Zercepac® Henlius has reached partnerships with Eurofarma Laboratórios S.A., Accord Healthcare, Cipla, Mabxience, and other pharmaceutical companies to actively explore overseas markets[Citation15].
The booming market of biosimilars requires powerful patent protections. Patent application is an important approach to protect their rights and interests. We searched and selected the patents from the web of China National Intellectual Property Administration (http://www.cnipa.gov.cn).
This review aims to introduce the outline of biosimilar approval process, summarize the patents related to biosimilar mAbs in China and their advantages, produce an overview of Chinese biosimilar mAb market, and help pharmaceutical companies protect their patents.
Biosimilar development, review, and approval
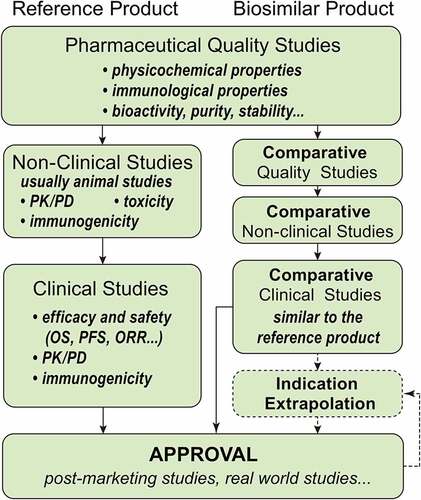
In 2015, the concept of biosimilar was first defined by National Medical Products Administration (NMPA) in China, with the announcement of Technical Guidelines for the Development and Evaluation of Biosimilars (Trial)[Citation16]. Since then, the relevant systems have been continuously improved, forming a complete approval system for biosimilars. shows the development process of the reference and biosimilar product.
Figure 2. The developing process of the reference and biosimilar products. The elements of their processes are similar, both containing pharmaceutical studies, non-clinical studies (animal studies), and clinical studies. However, biosimilar products pay more attention on comparative studies and their indications can be extrapolated.
Definitions and scope of application
According to the definition, the biosimilar is a therapeutic biologic that is similar in quality, safety and efficacy to the reference product, usually the original product, that has been approved for registration. The guideline emphasized that for modified products such as polyethylene glycol and antibody-coupled drug products, careful consideration should be given when developing biosimilars[Citation16].
Same as FDA, the similarity of biosimilars means they have the same amino acid sequence, structure, purity, chemical identity, bioactivity, and other product characteristics. Some minor differences are acceptable, such as host cells, prescriptions, and acceptable within-product differences. The differences between the biosimilar and reference products should be no meaning in safety and effectiveness [Citation16,Citation17]. Different with FDA, the interchangeable product does not exist in the guideline. The patients need a prescription from prescribers written specifically for biosimilar to receive the biosimilar product[Citation18].
Development and review
The requirements of FDA and NMPA in biosimilar development are similar. The manufacturer of a proposed biosimilar should provide the comparative data evaluated from a systematic process consisting of pharmaceutical studies, non-clinical studies (animal studies), and clinical studies [Citation16,Citation19].
Pharmaceutical studies include physicochemical properties, bioactivity, purity (impurities), immunological properties, and stability, demonstrating that the biological product is highly similar to the reference product[Citation20].
The design of non-clinical studies depends on the results of pharmaceutical studies, usually containing pharmacokinetics, pharmacodynamics, immunogenicity, and toxicity. Clinical studies also include assessing immunogenicity, pharmacokinetics, pharmacodynamics and comparative clinical studies in one or more of the indications for which the reference product is licensed[Citation21].
Indication extrapolation
Both FDA and NMPA agree that a biosimilar can be approved for an indication without direct studies of the biosimilar in that indication. However, the extrapolation is not automatic [Citation19,Citation20].
NMPA reckons that the indication extrapolation requires the following conditions to be met at the same time: 1) The completed comparison studies have used sensitive clinical trial models and no clinical differences have been detected; 2) The clinically relevant mechanisms and/or associated receptors of indications are the same; 3) The safety and immunogenicity of the biosimilar have been fully evaluated, and there are no special or additional safety issues for the proposed extrapolated indications[Citation20].
FDA evaluates all of the biosimilar product data from studies mentioned above to assess whether the differences between the biosimilar and the reference product may affect the indications or populations not studied by the biosimilar manufacturer. If no such differences are detected, extrapolation of these indications is generally supported[Citation19].
Patent review of biosimilar monoclonal antibodies
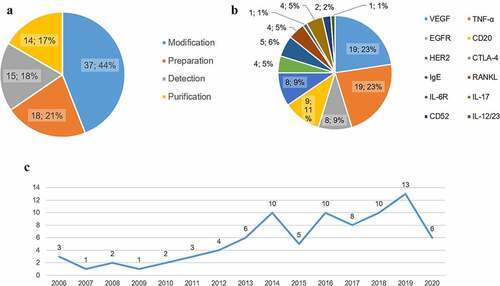
We found 84 patents for biosimilar mAbs, among 63 of which were in the protection or examination period. As shown in , most of patents are about improvement of formulations. The patents authorized from 2015 to 2020 are approximate twice the number that before 2015 (52 versus 32). The main targets we focused on are tumor necrosis factor alpha (TNF-α), vascular endothelial-derived growth factor (VEGF), receptor activator of nuclear factor-kappa B ligand (RANKL), CD20, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor (EGFR). In addition, we summarized patents of other hot spots and novel targets.
Figure 3. Outline of biosimilar patents in China. a The classification of biosimilar patents and the distribution. b Different targets of biosimilars and the patents of each target. c Trend analysis for Chinese patents on mAb biosimilars.
TNF-α-targeted antibodies
TNF-α is a cytokine produced by macrophages, lymphocytes, and natural killer cells and has effects on induction of systemic inflammation, regarded as a therapeutic target of autoimmune diseases [Citation22–24]. Adalimumab, golimumab, and infliximab are available in China and have 25, 4, and 1 biosimilar respectively. Infliximab is a chimeric IgG1 antibody, approved for using in rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, psoriasis, and ulcerative colitis since listed in China in 2007. Adalimumab and golimumab have lower immunogenicity due to human IgG1 antibodies, also approved to be used in rheumatoid arthritis and ankylosing spondylitis. By May 2021, adalimumab biosimilars of Biotech, Hisun, Innoventbio, and Henlius have been listed. BAT1406 from Biotech was the first adalimumab biosimilar in China, bringing 200 million in sales. Listed in 2019, HS016 (Hisun) has similar safety, efficacy, and immunogenicity according to clinical trials (CTR20160450, CTR20160398, http://www.cde.org.cn) and is the first biosimilar approved for all indications of Humira® [Citation25–27]. The clinical trial reports of IBI303 (Innoventbio, CTR20160219, CTR20160628, CTR20160687, http://www.cde.org.cn)[Citation28] and HLX03 (Henlius, CTR20160930, CTR20171123, http://www.cde.org.cn)[Citation29] supported the clinical development of two biosimilars.
There are 19 patents related to anti-TNF-α mAb biosimilar, including 9 (47.4%) patents about modification of prescriptions, 5 (26.3%) of preparation technique, 4 (21.0%) of purification, and 1 (5.3%) about detection method (). Lin et al. changed the prescription to enhance the stability of the antibody and allow intravenous injection. However, the concentration of BAT1406 cannot be over 80 mg/mL[Citation30]. In 2019, Wu et al. improved the prescription in CN201310093009.7, the patent mentioned above, adding acetic acid, histidine-HCl, arginine-HCl, methionine, and Tween 80 to increase the upper limit of concentration by 50%[Citation31].
Table 2. The patents of anti-TNF-α antibody biosimilar.
The innovation in industrial manufacture process also needs to protect. Leng et al. invented a combined medium for expressing adalimumab to increase quantity and quality[Citation32]. Xie et al. disclosed a production procedure of anti-TNF-α mAb improving purity and recovery rate[Citation33]. Zhang et al. enhanced the specificity of total organic carbon (TOC) detection by adopting gas chromatographic (GC) to detect ethanol residue[Citation34].
VEGF-targeted antibodies
Researchers have confirmed the vital role of VEGF in cancers and blinding eye diseases[Citation35]. VEGF is a family including VEGF-A (generally referred to as VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor (PIGF), which regulates angiogenesis and lymphangiogenesis. VEGF receptors (VEGFR1, VEGFR2, and VEGFR3) were discovered in 1992 and VEGFR2 was the main receptor for VEGF[Citation35].
Overexpression of VEGF in tumors correlates with majority, metastasis, recurrence, and prognosis. VEGF can also promote immune evasion of tumors by stimulating the proliferation of myeloid-derived suppressor cells and regulatory T cells[Citation36]. As for eye diseases, neovascularization driven by VEGF can cause bleeding, retinal detachment, and fibrovascular proliferation, which turns to blindness[Citation37]. The role of VEGF in the choriocapillaris is crucial for retinal vascular diseases.
Several mAbs targeted the VEGF-VEGFR signaling pathway have been launched. Bevacizumab, a VEGF-targeted humanized IgG1 antibody, was approved for the treatment of colorectal cancer (CRC), non-small cell lung carcinoma (NSCLC), and glioblastoma in China. Since listed in 2010, the price of Avastin® has decreased by 61.4%. In China, the antibody sequence patent of bevacizumab expired in 2018. Qilu Pharmaceutical’s bevacizumab was approved for marketing in 2019, becoming the first domestically produced bevacizumab biosimilar. Biosimilars of Innoventbio and Luye Pharma has been approved for listing, which significantly reduces patients’ medical cost. All biosimilars showed comparative efficacy, safety, and quality [Citation38–42].
Ranibizumab is a humanized IgG1 Fab that is obtained from the same parental mouse antibody as bevacizumab, approved for age-related macular degeneration (AMD). Ramucirumab is a human IgG1 antibody targeted on VEGFR2 and not available in China. Biosimilars of ranibizumab and ramucirumab are still in research and development.
As shown in , there are 19 patents about VEGF-targeted biosimilar mAbs. 9 (47.4%) patents relate to prescription innovation, while patents about preparation, purification, and detection are 4 (21.0%), 3 (15.8%), and 3 (15.8%) respectively.
Table 3. The patents of anti-VEGF antibody biosimilar.
The patents of prescription focus on the stability of mAbs. Wu et al. changed buffer solution and stabilizer into a phosphate buffer solution and trehalose to enhance the stability of the antibody[Citation43]. Wang et al. provided a buffer system containing 1.0–5.0 mg/ml of sodium acetate trihydrate, reducing the physical and chemical degradation reaction rates of mAbs[Citation44]. Cheng et al. adopted a combination buffer system of a sodium phosphate buffer agent and a second buffer agent, and adopted one or two of mannitol or sodium chloride as an osmotic pressure regulator to significantly reduce polymers and degraded materials[Citation45].
In addition, Liu et al. invented the two-step purification of cation exchange-hydrophobic chromatography with a purity of more than 95%[Citation46]. Liu et al. offered a biological activity detection method meeting the requirements on the specificity, accuracy, precision, and other verifications[Citation47]. To enhance the expression of ranibizumab, Li et al. invented a high-producing strain, deleting 11 nonessential regions in an E. coli DH1 genome[Citation48].
RANKL-targeted antibodies
Receptor activator of nuclear factor-kappa B (RANK) and its ligand (RANKL) are part of the TNF superfamily, most strongly expressed in bone. The functions of RANKL signaling include remodeling bone, immunity regulation, cell growth and differentiation, and the development of other organs [Citation49,Citation50]. Tumor metastasis affects the prognosis of patients, which is a hotspot in oncology. Tan et al. revealed that tumor-infiltrating CD4+CD25+FoxP3+T cells are a major source of RANKL production and stimulate metastatic progression, which explains more aggressive behavior in advanced breast cancers[Citation51]. Osteoclasts are the main functional cells for bone resorption and play an important role in bone development, growth, repair, and reconstruction. RANKL regulates the differentiation and activation of osteoclasts. The imbalance of osteoclasts and osteoblasts may cause osteoporosis, which means RANKL can be a drug target for bone diseases[Citation52].
Denosumab, a human IgG2 antibody, is the first mAb targeted RANKL, approved for giant cell tumor of bone, postmenopausal women with osteoporosis at high risk for fracture, and multiple myeloma and bone metastasis from solid tumors in China. Several clinical trials (NCT00926380, NCT00089674, NCT00321620, NCT00089791, NCT00523341, https://clinicaltrials.gov) [Citation53–57] support the efficacy of denosumab. The market of denosumab biosimilars is budding but competitive. Nine biosimilars of Chinese pharmaceutic companies are in clinical study and 5 biosimilars are in investigational new drug (IND).
The patents are not booming as the market. There are four patents of denosumab biosimilars. Mei et al. provided a novel purification method and a redox system composed of dithiothreitol (DTT) and sodium sulfite to prepare the denosumab biosimilar[Citation58]. Three patents about biological activity detection showed advantages in specificity, accuracy, precision, and efficiency. Liu et al. disclosed an ameliorated tartrate resistant acid phosphatase assay[Citation59]. Ye et al. and Ding et al. provided different luciferase assays, the former focused on the preparation of plasmid, while the latter focused on the tool cell selection [Citation60,Citation61].
CD20-targeted antibodies
Rituximab was the first mAb approved for clinical use in oncology which is a chimeric IgG1 antibody targeted CD20, treating non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia (CLL)[Citation62]. CD20 is a B-cell surface marker regulating the development and differentiation of B cells into plasma cells. Most NHLs originate from mature B cells, presented a malignant monoclonal proliferation of lymphoid cells. Chemotherapy combined with rituximab is the typical treatment to kill malignant CD20-positive cells [Citation62–64]. Chronic lymphocytic leukemia is another malignant tumor related to lymphoid cells, which is characterized by the clonal proliferation and accumulation of B-cells. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) is the first-line treatment of CLL and shows great efficacy and safety in long-term follow-up [Citation65,Citation66].
The patent of rituximab (Mab Thera®) expired in 2015, which stimulated the market of rituximab biosimilars. In China, 2 biosimilars from Henlius and Innoventbio have been listed in 2019 and 2020 respectively with 12 biosimilars in total.
9 patents were found in this field (). Liu et al. improved the prescription of anti-CD20 antibody, adding hyaluronidase to help hypodermic injection, which is the only patent about prescription improvement[Citation67]. Several scientists paid their attention on increasing production. Song et al. established a large-scale high-expression production technology for 300 L of eukaryocytes, wherein the anti-CD20 monoclonal antibody (consistent to rituximab) expression quantity is more than 1.2 g/L and the protein purification yield is improved to more than 60%[Citation68]. Shen et al. established an efficient expression system whose yield of rituximab is 1.7–2.2 g/L, which laid a foundation for large-scale industrial production[Citation69]. Zhang et al. improved the chromatographic performance by adding arginine, glycine, or mannitol in the ion-exchange chromatography process and adjusting electric conductivity with salt, having advantages in operation, cost, and safety[Citation70]. Liu et al. disclosed a detection method for anti-CD20 mAb binding activities based on flow cytometry (FCM) with good specificity, precision, linearity and range, accuracy, and durability[Citation71].
Table 4. The patents of anti-CD20 antibody biosimilar.
HER2-targeted antibodies
HER2 (also called receptor tyrosine-protein kinase ErbB-2) from ErbB family, is a product of proto-oncogene. The ErbB pathway is a complex biology signaling network that regulates the apoptosis, migration, growth, adhesion, and differentiation of cells [Citation72–74]. The HER2 overexpression leads to the occurrence and invasion of tumors and can increase the risk of metastasis, which can be observed in 25–30% of breast and ovarian cancers[Citation73]. Recent researches found HER2 overexpression in other solid tumors such as gastric cancer, biliary tract cancer, colorectal cancer, NSCLC, and bladder cancer[Citation75].
Anti-HER2 therapy is a typical treatment for HER2-positive breast cancer, such as trastuzumab and pertuzumab (anti-HER2 humanized IgG1 antibodies)[Citation76]. According to several clinical trials (NCT00567190, NCT00567190, NCT02131064, the drug combination of trastuzumab, pertuzumab, and chemotherapy showed efficacy in HER2-positive metastatic breast cancer, which benefits these patients [Citation77–80]. However, the price of these mAbs hinders patients from effective treatment.
The availability of Zercepac® changed actuality. Produced by Henlius, Zercepac® is the first biosimilar of trastuzumab and has obtained all the indications that Herceptin® (trastuzumab) has been approved in China due to the pleasure results of clinical trials (NCT02581748, NCT03084237, ; CTR20160526, [Citation81].,[Citation82] Up to 2021, 18 biosimilars of trastuzumab and pertuzumab have entered the competition of anti-HER2 biosimilar.
There are 8 patents related to the biosimilars, 6 of which were valid. Wang et al. changed α,α-Dicarboxylic trehalose into sucrose or trehalose, reducing the cost of trastuzumab preparation[Citation83]. Ma et al. provided a new pharmaceutical preparation of HLX11, the biosimilar of pertuzumab, containing sorbitol to control the budget[Citation84]. Wu et al. replaced the L-histidine by a histidine-hydrochloride buffer in the prescription of pertuzumab [Citation85] As for purification technology, Xu et al. improved and disclosed the purification method with simple steps, high recovery rate, and low potential virus risk[Citation86]. In 2016, Li et al. modified the cation-exchange chromatography packing, increasing the productivity further[Citation87]. Xie et al. simplified the procedure of purification and removed impurities effectively [Citation88,Citation89].
EGFR-targeted antibodies
EGFR is a transmembrane protein of the ErbB family, similar to HER2. The EGFR pathway regulates cancer-cell proliferation, apoptosis blocking, invasion, and metastasis, and the mutation or overexpression of EGFR exists in different human cancers, which means EGFR is an ideal target for tumor therapy[Citation89]. The activation of EGFR transduces the Ras/MAPK pathway, the most important pathway in EGFR mediation related to growth, survival, and differentiation of cells [Citation90,Citation91]. Cetuximab (Erbitux®), an anti-EGFR chimeric IgG1 antibody, was used to metastatic colon cancer and head and neck squamous cell carcinoma (HNSCC). Due to the treatment failure caused by Ras mutation, cetuximab is not suitable for Ras-mutated patients.
The patent of Erbitux® has expired in 2017; however, there are only 10 biosimilars in China and none of them complete their clinical trials. Kelun, Annpobio, and Mabpharm are pushing their phase 3 clinical trials (CTR20202451, CTR20192102, CTR20170701).
8 patents were selected from the database, while three patents were valid. He et al. donated an accurate and effective anti-EGFR monoclonal antibody biological activity detection method; the relative standard deviation using the method of the present invention was 4.2%, and the average recovery rate was 103.8%[Citation92]. Qian disclosed an improved method that using CHO cells as host cells to prepare a new anti-EGFR with different glycoforms[Citation93]. Yu et al. disclosed a screening culture method of cells capable of efficiently expressing an anti-EGFR mAb without fucosyl modification, which enhanced the antibody-dependent cell-mediated cytotoxicity (ADCC) effect [Citation94,Citation95].
Other monoclonal antibodies and their biosimilar patents
The market of newborn mAbs in China shows less liveliness than that of mAbs mentioned in the previous article, which also explains fewer patents of their biosimilars. However, biosimilars and patents cannot completely reflect the market of mAbs due to the existence of innovative drugs.
CD38 and programmed cell death 1 (PD-1) are the targets that have no biosimilar patents. CD38 is a glycoprotein located on the membrane, which can catalyze the synthesis and degradation of cyclic adenosine diphosphate ribose and has high expression in multiple myeloma (MM) cells[Citation95]. The current research on CD38 and tumors confirms that CD38 has important functions in promoting tumor cell growth and immune escape[Citation96]. A human IgG1 antibody daratumumab is the only anti-CD38 mAb approved for MM and not launched in China. The IND of Henlius’s biosimilar HLX15 has been approved by NMPA.
PD-1 is a checkpoint that has been studied deeply in recent years. It is confirmed that PD-1 plays a vital role in balancing immunity and tolerance; however, high expression of PD-1 ligand (PD-L1) on tumor cells can lead to immune escape[Citation97]. Nivolumab (Opdivo®) is a typical PD-1 mAb treating NSCLC, HNSCC, and gastric cancer (GC), having a biosimilar (LY01015) in IND. There are four innovative PD-1 mAbs in China, sintilimab (TYVYT®), camrelizumab (Airuika®), tislelizumab (Baizean®), and toripalimab (Tuoyi®), which enriches the market and provides more choices for patients.
Cytotoxic T Lymphocyte antigen 4 (CTLA-4), also called CD152, is the other checkpoint and expressed by regulatory T cells (Treg). In normal, CTLA-4 binds CD80/CD86 to avoid conventional T cells (Tcon) stimulation, which is of significance to prevent autoimmune diseases. In the tumor microenvironment, overexpression of CTLA-4 on Treg leads to the downregulation of immune responses [Citation98,Citation99]. Anti-CTLA-4 mAb can cut off the pathway and enhance the immune responses to tumor cells, such as human IgG1 antibody ipilimumab (Yervoy®). There are six biosimilars of ipilimumab in China and Innoventbio are undergoing their phase 3 clinical trial (CTR20210080, http://www.cde.org.cn). Four patents were found in the database. Scientists from Innoventbio, DongFang Biotech, and Shanghai Celgen disclosed their prescriptions, respectively [Citation100–102].
Omalizumab is a humanized IgG1 anti-IgE mAb approved for asthma. IgE, produced by B cells in response to allergen, plays a vital role in the inflammatory of asthma. Omalizumab inhibits allergic reactions and downregulates the expression of IgE receptors to alleviate symptoms and prevent recurrence[Citation103]. Six biosimilars and three patents with validity were found. These patents ameliorated the stability and quality of the anti-IgE mAbs [Citation104–106].
CD52 is a membrane glycoprotein expressed on both B cells and T cells, but not expressed on CD34-positive lymphocytes. Alemtuzumab is a humanized IgG1 antibody against CD52, approved for CLL and multiple sclerosis (MS). However, Campath® (alemtuzumab for CLL) was not on the market in 2012 due to high toxicity. There are two biosimilars but no patent in legality.
Interleukins (ILs) are cytokines with low molecular weight produced by lymphocytes, macrophages, and monocytes, involved in the immune response and cell signaling [Citation107–109]. A variety of ILs have been widely studied and developed as new therapeutic targets and most of the mAbs against these ILs are approved for autoimmune diseases, such as ustekinumab (anti-IL-12/23), tocilizumab (anti-IL-6), and secukinumab (anti-IL-17). Tocilizumab has more biosimilars in China than the other two mAbs, and there are 6 biosimilars in clinical trials (CTR20190174, CTR20190739, CTR20201263, CTR20190002, CTR20191204, CTR20192563, http://www.cde.org.cn). Canakinumab is a human IgG1 antibody against IL-1β approved for cryopyrin-associated periodic syndromes and other spontaneous inflammatory diseases. According to the company announcement, Mabpharm has commenced the pre-clinical research of canakinumab biosimilar. Five patents of anti-IL mAbs were found and four patents were valid. Lin et al. provided a novel prescription of BAT1806, the biosimilar of tocilizumab[Citation110]. Ouyang et al. improved the technique of removing cysteinylated variant from secukinumab product[Citation111].
Discussion
The high price of most bioproductions, leading to high medical costs of related diseases, has affected the cost control of national health insurance and the sustainability of financial health expenditure. With the expiration of patents and intellectual property rights for the original drugs, more and more pharmaceutical companies are working on the development of low-cost biosimilars, which benefits patients and medical insurance payers from effective and relatively economical treatment. Biosimilars have emerged as a result.
In March 2015, NMPA in China announced the ‘Technical Guidelines for the Development and Evaluation of Biosimilars (Trial)’, which clarified the definition of biosimilars for the first time, proposed the basic principles for the development and evaluation of biosimilars, and put forward specific requirements for the contents of pharmacy, non-clinical and clinical research and evaluation of biosimilars. In July 2016, the Registration Management Measures (Amendment) further regulated the concept of biosimilars and tightened the approval criteria for biosimilar. Similar with the FDA and the EMA [Citation112], biosimilars have not been given a simple approval but adopted the same approval as innovative bioproducts, which raised the requirement of pharmaceutical companies and promoted their development invisibly. In December 2017, the National Development and Reform Commission (NDRC) in China issued the Three-Year Action Plan to Enhance the Core Competitiveness of Manufacturing (2018–2020), encouraging the development and industrialization of bioproducts with high market potential, high clinical value, and expired patents, regarding the first biosimilar drug as a high-end drug, which helps technical cooperation and the import of advanced technology.
With the policies guiding and the needs of companies’ development, the overall innovation awareness of the biopharmaceutical industry in China has increased. Since the guide of biosimilar announced in 2015, the quantity of patents of biosimilar mAbs has gradually raised.
Patents that improve the prescription of preparations account for the majority. Usually, the excipients and buffer systems used in the original biologic products are of good quality and stable but high-priced. The manufacturers of biosimilars aspire to seek the same stable and lower-cost alternative excipients and substantiate that such changes do not generate clinical differences that affect the efficacy and safety.
Meanwhile, the transformation of new technologies in the field of biochemistry is also related to the booming growth of patents. When evaluating the quality characteristics of mAb products or formulating quality evaluation standards, a detecting technique with high sensitivity, selectivity, specificity is needed, which encourages manufacturers paying more attention on technology fundamentals and refinements. For instance, enzyme-linked immunosorbent assays (ELISA) are an important technique for pharmacokinetic studies of mAbs. However, due to the interference of endogenous proteins, anti-drug antibodies (ADAs), and soluble target ligands, the application of ELISA faces limitations[Citation113]. Some of the patents about detection method provides new capture antibodies, new detecting systems, and combination methods to overcome these challenges.
With these improving techniques and scientific studies, the similarity of the marketed anti-tumor biosimilars with their reference drugs in terms of clinical efficacy, safety, and immunogenicity has been confirmed in the results of different registered clinical trials and clinical studies [Citation25–27,Citation29,Citation38–41,Citation81,Citation82,Citation114,Citation115].
However, the protection of intellectual property should be multidimensional. Compared with the USA and EU, China could pay more attention to data exclusivity and market protection. According to current laws and regulations, data exclusivity and market protection are out of implementation. In the Measures for the Administration of Drug Registration (2007 Version) released by NMPA, 6-year-data exclusivity for new chemical drugs and 5-year-market protection for new drugs were valid but are invalid in the latest version[Citation116]. Optimistically, a draft for comments was released by NMPA on May 9, 2022, emphasizing the importance of data exclusivity and market protection[Citation117]. It will be a concrete implementation of encouraging pharmaceutical innovation and a huge improvement for intellectual property protection. Due to the development of biotechnology and bioproducts markets, the market size of biosimilars in China will gradually grow with the improvement of the national guidelines.
Conclusion
In this article, we reviewed the patents of biosimilar mAbs in China. A total of 263 patents were found, among which 84 patents are related to biosimilar mAbs. In total, 63 (75.0%) of these patents are in the protection period. The main targets of biosimilar mAb patents are VEGF, TNF-α, CD20, EGFR, and HER2.
Abbreviation
eADA: anti-drug antibody; ADCC: antibody-dependent cell-mediated cytotoxicity; AMD: age-related macular degeneration; AS: ankylosing spondylitis; BC: breast cancer; CD: Crohn’s disease; CLL: chronic lymphocytic leukemia; CRC: colorectal cancer; CTLA-4: Cytotoxic T Lymphocyte antigen 4; DLBCL: diffuse large B-cell lymphoma; DTT: dithiothreitol; EGFR: epidermal growth factor receptor; ELISA: enzyme-linked immunosorbent assay; EMA: European Medicines Agency; FCM: flow cytometry; FCR: fludarabine, cyclophosphamide, and rituximab; FDA: Food and Drug Administration; GC: gastric cancer; HCC: hepatocellular carcinoma; HER2: human epidermal growth factor receptor 2; HNSCC: head and neck squamous cell carcinoma; IL: interleukin; IND: investigational new drug; mAb: monoclonal antibodies; MM: multiple myeloma; MS: multiple sclerosis; NDA: new drug application; NDRC: National Development and Reform Commission; NHL: non-Hodgkin’s lymphoma; NMPA: National Medical Products Administration; NSCLC: non-small cell lung carcinoma; PD-1: programmed cell death 1; PLGF: placental growth factor; PMO: post-menopausal osteoporosis; PS: psoriasis; PsA: psoriatic arthritis; RA: rheumatoid arthritis; RANK: receptor activator of nuclear factor-kappa B; Tcon: conventional T cell; TNF-α: tumor necrosis factor alpha; TOC: total organic carbon; Treg: regulatory T cell; UC: ulcerative colitis; VEGF: vascular endothelial-derived growth factor
Declaration of ethical statement
No ethics approval was required for this study as it involved no human participants or animals.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ecker DM, Jones SD, Levine HL, et al. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9–14.
- Elgundi Z, Reslan M, Cruz E, et al. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev. 2017;122:2–19.
- Lu RM, Hwang Y-C, Liu I-J, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1.
- Ishii-Watabe A, Kuwabara T. Biosimilarity assessment of biosimilar therapeutic monoclonal antibodies. Drug Metab Pharmacokinet. 2019;34(1):64–70.
- FDA. FDA-approved biosimilar products. 2022.https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
- EMA Medicines . 2022May 18. https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar/ema_group_types/ema_medicine
- Yang J, Yu S, Yang Z, et al. Efficacy and safety of anti-cancer biosimilars compared to reference biologics in oncology: a systematic review and meta-analysis of randomized controlled trials. BioDrugs. 2019;33(4):357–371.
- Berg T, Jensen M-B, Jakobsen EH, et al. Neoadjuvant chemotherapy and HER2 dual blockade including biosimilar trastuzumab (SB3) for HER2-positive early breast cancer: population based real world data from the Danish Breast Cancer Group (DBCG). Breast. 2020;54:242–247.
- Egeberg A, Girolomoni G, Feldman SR, et al. Real world SB4 (Etanercept Biosimilar) use in patients with psoriasis: data from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Drugs Dermatol. 2020;19(3):316–318.
- Lee K, Ha JY, Jung AR, et al. The clinical outcomes of rituximab biosimilar CT-P10 (Truxima ®) with CHOP as first-line treatment for patients with diffuse large B-cell lymphoma: real-world experience. Leuk Lymphoma. 2020;61(7):1575–1583.
- Bae SJ, Kim JH, Ahn SG, et al. Real-world clinical outcomes of biosimilar trastuzumab (CT-P6) in HER2-positive early-stage and metastatic breast cancer. Front Oncol. 2021;11:689587.
- Melville AR, Md Yusof MY, Fitton J, et al. Real-world experience of effectiveness of non-medical switch from originator to biosimilar rituximab in rheumatoid arthritis. Rheumatology (Oxford). 2021;60(8):3679–3688.
- Rhodes W, DeClue RW, Accortt NA, et al. Real-world use of bevacizumab-awwb, a bevacizumab biosimilar, in US patients with metastatic colorectal cancer. Future Oncol. 2021;17(36):5119–5127.
- Shelbaya A. Real-world use and acceptance of biosimilar monoclonal antibodies of rituximab in oncology practice in the .Future Oncol. 2021
- Henlius. Company News. 2022.https://www.henlius.com/News.html
- NMPA. The state food and drug administration issued technical guidelines for the development and evaluation of biosimilar drugs. May 11.https://www.nmpa.gov.cn/directory/web/nmpa/yaopin/ypjgdt/20150303155101860.html
- Biosimilars FDA. 2021May 7.https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
- FDA. Biosimilar and interchangeable products. 2021.https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products
- FDA. 2021. Biosimilar Development. Review, and Approval https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval
- CDE of NMPA. Technical guidelines for the evaluation of similarities of biosimilars and the extrapolation of indications. 2021 Accessed 5 Mar 2022.https://www.cde.org.cn/main/news/viewInfoCommon/d92c6507a57bee9ccfc5baa1ee87fda9
- CDE of NMPA. Technical guidelines for clinical pharmacological research of biosimilars. 2022 Accessed 5 Mar 2022.https://www.cde.org.cn/main/news/viewInfoCommon/58b06d54af4f19d02a17e4d4d8d374f7
- Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–371.
- Torres-Acosta N, O’Keefe JH, O’Keefe EL, et al. Therapeutic potential of tnf-α inhibition for alzheimer’s disease prevention. J Alzheimers Dis. 2020;78(2):619–626.
- van Schie Ka, van Schie KA, Hart MH, et al. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis. 2015;74(1):311–314.
- Su J, Li M, He L, et al. Comparison of the efficacy and safety of adalimumab (humira) and the adalimumab biosimilar candidate (HS016) in Chinese patients with active ankylosing spondylitis: a multicenter, randomized, double-blind, parallel, phase iii clinical trial. BioDrugs. 2020;34(3):381–393.
- Su J, Li M, He L, et al. Changes in efficacy indicators for adalimumab biosimilar candidate (Hs016) for the treatment of active ankylosing spondylitis at various time points. Front Pharmacol. 2020;11:606497.
- Cao G, Yu J, Wu J, et al. A randomized, double-blind, parallel-group, phase 1 clinical trial comparing the pharmacokinetic, safety, and immunogenicity of the biosimilar hs016 and the originator adalimumab in Chinese healthy male subjects. Clin Pharmacol Drug Dev. 2021;10(3):317–325.
- Liu Y, Yang G, Zhang J, et al. Anti-TNF-α monoclonal antibody reverses psoriasis through dual inhibition of inflammation and angiogenesis. Int Immunopharmacol. 2015;28(1):731–743.
- Zhang H, Wu M, Sun J, et al. Pharmacokinetics, safety, and immunogenicity of HLX03, an adalimumab biosimilar, compared with reference biologic in healthy Chinese male volunteers: results of a randomized, double-blind, parallel-controlled, phase 1 study. Pharmacol Res Perspect. 2021;9(2):e00733.
- Lin J. BIO THERA SOLUTIONS LTD,assignee; Human antibody preparation for treating TNF (tumour necrosis factor)-alpha related diseases. China patent CN201310093009.7. 2013.
- Wu Y. BIO THERA SOLUTIONS LTD,assignee; Human antibody preparation for targeted therapy of TNF-alpha related diseases. China patent CN201910585391.0. 2019
- Leng CS. TONGHUA DONGBAO BIOLOGICAL TECH CO LTD,assignee; Combined medium for expressing Adalimumab. China patent CN201710981104.9. 2018
- Xie YL. CHIA TAI TIANQING PHARMACEUTICAL GROUP CO LTD,assignee; Production method of anti-TNF-alpha monoclonal antibody. China patent CN201911005798.8. 2020
- Zhang ZB. SUZHOU ZHONGHE BIOMEDICAL TECH CO LTD,assignee; Method for detecting ethanol residue in recombinant hominine anti-TNF-a project cleaning validation sample. China patent CN201910038495.X. 2019;
- Apte RS, Chen DS, Ferrara N, et al. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264.
- Hegde PS, Wallin JJ, Mancao C, et al. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–124.
- Sene A, Chin-Yee D, Apte RS, et al. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol Med. 2015;21(1):43–51.
- Liu YN, Huang J, Guo C, et al. A randomized, double-blind, single-dose study to evaluate the biosimilarity of QL1101 with bevacizumab in healthy male subjects. Cancer Chemother Pharmacol. 2020;85(3):555–562.
- Chu T, Lu J, Bi M, et al. Equivalent efficacy study of QL1101 and bevacizumab on untreated advanced non-squamous non-small cell lung cancer patients: a phase 3 randomized, double-blind clinical trial. Cancer Biol Med. 2021;18(3). 10.20892/j.2095-3941.2020.0212
- Zhang H. A phase I, randomized, double-blinded, single-dose study evaluating the pharmacokinetic equivalence of the biosimilar IBI305 and bevacizumab in healthy male subjects Int. J Clin Pharmacol Ther. 2019;57: 167–174.
- Yang Y, Wu B, Huang L, et al. Biosimilar candidate IBI305 plus paclitaxel/carboplatin for the treatment of non-squamous non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):989–999.
- Duan X, Zhang H, Zhou L, et al. Complete response to the combination of sintilimab and IBI305 for a patient with HBV-associated hepatocellular carcinoma with multiple lung metastasis. Dig Liver Dis. 2020;52(7):794–796.
- Wu XY. Bio thera solutions LTD,assignee; High-stability humanized antibody preparation for treating VEGF related diseases. China patent CN201410198778.8. 2015
- Wang YJ. INNOVENT BIOLOG INC,assignee; Stable anti-VEGF antibody preparation and application thereof. China patent CN201410757524.5. 2015
- Cheng YJ. Chia tai tianqing pharmaceutical group co LTD,assignee; Pharmaceutical composition of humanized antibody for vascular endothelial growth factor. China patent CN201410487742.1. 2016
- Liu N. Shandong quangang pharmaceutical co LTD,assignee; method for purifying and preparing anti-VEGF antibody fragment. China patent CN201210185573.7. 2012
- Liu PP. Shanghai Cp Guojian Pharmaceutical Co LTD,assignee; Biological activity detection method for VEGF targeted therapy drugs. China patent CN201410625267. 2016
- Li QL. UNIV ANHUI,assignee; High-producing strain with capability of efficient secretion expression of anti-VEGF-Fab antibody fragment, and construction method thereof. China patent CN201810364192.2. 2018
- Raje NS, Bhatta S, Terpos E, et al. Role of the RANK/RANKL pathway in multiple myeloma. Clin Cancer Res. 2019;25(1):12–20.
- Honma M, Ikebuchi Y, Suzuki H, et al. RANKL as a key figure in bridging between the bone and immune system: its physiological functions and potential as a pharmacological target. Pharmacol Ther. 2021;218:107682.
- Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553.
- Lorenzo J. The many ways of osteoclast activation. J Clin Invest. 2017;127(7):2530–2532.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–56.
- Smith MR, Egerdie B, Toriz NH, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822.
- Cummings SR, Martin JS, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523.
- Mei F. MABWELL SHANGHAI BIOSCIENCE CO LTD,assignee; Disulfide bond isomer of recombinant anti-RANKL antibody IgG2 type and purification method of disulfide bond isomer. China patent CN202010828720.2. 2020
- Liu PP. Sunshine Guojian Pharmaceutical Shanghai CO LTD,assignee; Method for detecting biological activity of RANKL (receptor activator for nuclear factor-kappa B ligand) targeted therapeutic drug. China patent CN201610859356.X. 2018;
- Ye CG, Xu P, Cai W-J, et al. AMPO BIOTECHNOLOGY INC,assignee; Method for detecting biological activity of RANKL (receptor activator for nuclear factor-KB ligands) targeted therapy medicines. BMC Cancer. 2018 China patent CN201810642794.X; 186: 10.1186/s12885-018-4349-y
- Ding MS. JIANGSU T MAB BIOPHARMA CO LTD,assignee; In vitro test tool and method for evaluating biological activity of RANKL target spot compound. China patent CN201810743674.9. 2018
- Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359–7368.
- Shanehbandi D, Majidi J, Kazemi T, et al. CD20-based immunotherapy of b-cell derived hematologic malignancies. Curr Cancer Drug Targets. 2017;17(5):423–444.
- Al-Naeeb A B Non-Hodgkin lymphoma. Bmj. 2018;362:k3204.
- Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215.
- Hallek M, Shanafelt TD, Eichhorst B, et al. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–1537.
- Liu YJ.2020. Shanghai Pharmaceuticals Holding Co Ltd,assignee; Medicine preparation containing anti-CD20 antibody as well as preparation method and application of medicine preparation. China patent CN201911157456.8.
- Song H. Shanghai Hankang Bio Pharmaceutical Technology CO LTD,assignee; Anti-CD20 monoclonal antibody, preparation method and application thereof. China patent CN201210356034.5. 2013
- Shen BB. ZHEJIANG TERUISI PHARMACEUTICAL INC,assignee; High-expression and high-stability CHO cell line for producing Rituximab and constructing method thereof. China patent CN201710087562.8. 2018
- Zhang GM. Lunan Pharmaceutical group corp,assignee; method for purifying anti-CD20 human-mouse chimeric monoclonal antibody. China patent CN201810135774.3. 2019
- Liu PP. GENOR BIOPHARMA CO LTD,assignee; Detection method for anti-CD20 monoclonal antibody binding activities. China patent CN201110302889.5. 2013
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137.
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487.
- Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135(1):55–62.
- Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48.
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429.
- Swain SM, Miles D, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530.
- Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734.
- Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19(1):115–126.
- Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119.
- Zhu X, Ding Y, Yu Y, et al. A Phase 1 randomized study compare the pharmacokinetics, safety and immunogenicity of HLX02 to reference CN- and EU-sourced trastuzumab in healthy subjects. Cancer Chemother Pharmacol. 2021;87(3):349–359.
- Xu B, Zhang Q, Sun T, et al. Efficacy, safety, and immunogenicity of hlx02 compared with reference trastuzumab in patients with recurrent or metastatic HER2-positive breast cancer: a randomized phase iii equivalence trial. BioDrugs. 2021;35(3):337–350.
- Wang H. SHANGHAI ZHONGJIAN BIOTECHNOLO,assignee; Stable anti-HER2 humanized antibody preparation. China patent CN200610147280.4. 2008
- Ma CJ. SHANGHAI HENLIUS BIOTECH INC,assignee; Pharmaceutical preparation containing anti-Her2 monoclonal antibody. China patent CN201811628635.0. 2020
- Wu TT. Jiangsu hengrui medicine co;shanghai hengrui pharmaceutical co ltd,assignee; HER2 antibody pharmaceutical composition and application thereof. China patent CN201910643662.3. 2020
- Xu ZH. GENOR BIOPHARMA CO LTD,assignee; Method for purifying anti-HER2 or/and anti-HER3 antibody proteins. China patent CN201110452837.6. 2012
- Li L. SHENZHEN Main Luck Pharmaceuticals Inc,assignee; Purification method of monoclonal antibodies. China patent CN201610901017.3. 2017
- Xie YL. Chia tai tianqing pharmaceutical group nanjing shunxin pharmaceutical co ltd;chia tai tianqing pharmaceutical group co ltd,assignee; Purification method of anti-HER2 monoclonal antibody. China patent CN201911191084.0. 2020
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174.
- Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017;9:52.
- Degirmenci U, Wang M, Hu J, et al. Targeting Aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells. 2020;9(1):198.
- He WH. AMPO BIOTECHNOLOGY CO LTD,assignee; Detection method for biological activity of anti-EGFR monoclonal antibodies. China patent CN201410840995.2. 2015
- Qian WZI SHANGHAI ZHANGJIANG BIOTECHNOLOGY CO LTD assignee; Method for preparing novel anti-EGFR monoclonal antibody and application thereof. China patent CN201510006233.7. 2016
- Yu HY, et al. Beijing sl pharmaceutical co ltd;beijing sl lisheng medical tech co ltd;beijing shuanglu biological tech co ltd,assignee; Screening culture method of cells capable of efficiently expressing anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibody. China patent CN202011192793.3. 2021
- van de Donk N, van de Donk NWCJ, Richardson PG, et al. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131:13–29.
- Chen L, Diao L, Yang Y, et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 2018;8(9):1156–1175.
- Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153–167.
- Rowshanravan B, Halliday N, Sansom DM, et al. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67.
- Mitsuiki N, Schwab C, Grimbacher B, et al. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol Rev. 2019;287(1):33–49.
- Xiao H. SHANGHAI HENLIUS BIOTECH CO LTD,assignee; Anti-CTLA-4 humanized antibody. China patent CN201410838544.5. 2015
- Bai Y. Beijing Dongfang Biotech Co Ltd,assignee; Injection preparation of anti-CTLA-4 monoclonal antibody. China patent CN201911022188.9. 2020
- Fang GB. Shanghai Celgen Biopharma Co LtD,assignee; Anti-CTLA-4 antibody and fusion protein preparation. China patent CN202011462549.4. 2021 Mar
- McGregor MC, Krings JG, Nair P, et al. Role of Biologics in Asthma. Am J Respir Crit Care Med. 2019;199(4):433–445.
- Chen SK. PKU UNIV V-MING (HEFEI) BIOLOGICS CO LTD,assignee; Arginine-based method for preventing aggregation of omalizumab. China patent CN201910977910.8. 2020
- Wang SX. Shanghai Mab Venture Biological Pharmaceutical CO LTD,assignee; Method for detecting hydrophobicity of Omalizumab. China patent CN201911423842.7. 2020
- Wang SX. Shanghai Mab Venture Biological Pharmaceutical Co Ltd, assignee; Separation method of charge heteroplasmon of omalizumab. China patent CN201911417917.0. 2020
- Tait Wojno ED, Hunter CA, Stumhofer JS, et al. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50(4):851–870.
- Kishimoto T. The biology of interleukin-6. Blood. 1989;74(1):1–10.
- Iwakura Y, Ishigame H, Saijo S, et al. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162.
- Lin J. Bio-thera solutions ltd assignee; Liquid preparation of humanized antibody for treating IL-6 related diseases. China patent CN202010129494.9. 2020
- Ouyang ZY. Mabwell shanghai bioscience co ltD,assignee; Method for removing cysteinylated variant from secukinumab product. China patent CN201910029571.0. 2019
- Sarpatwari A, Barenie R, Curfman G, et al. The US biosimilar market: stunted growth and possible reforms. Clin Pharmacol Ther. 2019;105(1):92–100.
- Iwamoto N. Antibody drug quantitation in coexistence with anti-drug antibodies on nSMOL bioanalysis. Anal Biochem. 2018;540-541:30–37.
- Zhang H, Li Q, Zhu X, et al. Tolerance, variability, and pharmacokinetics of bevacizumab biosimilars in Chinese healthy male subjects. Cancer Chemother Pharmacol. 2018;82(4):615–623.
- Zhang E, Xie L, Qin P, et al. Quality by design-based assessment for analytical similarity of adalimumab biosimilar HLX03 to Humira®. Aaps j. 2020;22(3):69.
- NMPA. Decree of the state food and drug administration no. 2022. http://www.gov.cn/gongbao/content/2008/content_934084.htm
- NMPA. Implementing regulations of the drug administration law of the PRC (Draft amendment for solicitation of comments). 2022.https://www.nmpa.gov.cn/xxgk/zhqyj/zhqyjyp/20220509220456183.htm
