Abstract
Formation of the σ phase has been observed in quite a few high-entropy alloys (HEAs) recently. The σ phase significantly enhances the hardness of the alloys but reduces their ductility. Thus, controlling the formation of σ phase through proper design is critical for HEAs. However, theories to predict the σ phase formation based on HEA composition are still not available. Here, we demonstrate that the σ phase formation is directly related to the valence electron concentration (VEC) of the alloy. The σ-phase-forming VEC range was systematically studied and revealed. Such finding is of crucial importance to the future design of HEAs.
High-entropy alloy (HEA) is a new class of metallic material featuring more than five principal elements. [Citation1] Studies have shown that this kind of alloy can have high strength/hardness,[Citation2–4] superior resistance to thermal softening,[Citation5,Citation6] outstanding resistance to wear and corrosion,[Citation7] and good thermal stability.[Citation8] Thus, these alloys have received significant attention recently. Due to their equimolar or near-equimolar composition, HEAs have high configuration entropy that enhances the formation of solid solution phases. Thus, the majority of as-cast HEAs form solid solution phases that have simple structures such as body centered cubic (BCC) and face centered cubic (FCC).[Citation9,Citation10] However, recent studies show that the as-cast solution phases may transform to intermetallic phases when the alloys are aged at intermediate temperatures.[Citation4,Citation7,Citation11–14] Since high-temperature structural component is an important potential application of HEAs, the formation of intermetallic phases at intermediate temperatures and its effect to the properties of HEAs becomes a critical issue. In particular, the formation of sigma phase is frequently observed in aged HEAs.[Citation4,Citation11–13,Citation15,Citation16] Sigma phase is a very hard but brittle phase, so its formation leads to significant changes in mechanical properties. For example, the formation of a sigma phase matrix in Al5Cr32Fe35Ni22Ti6 hardens the alloy to HV 950 but reduces the KIC to ∼5.[Citation14] Consequently, controlling the formation and amount of sigma phase according to the desired application is vitally important to the design of HEAs.
Sigma phase forms between so-called A and B elements, where A is usually a group VB or VIB element and B is usually a VIIB or VIIIB element.[Citation17] σ is known as an electron compound, which means its formation is closely related to the electron concentration of the outer shell. This characteristic provides the foundation for the theories to predict the formation of σ phase. For example, PHACOMP [Citation18,Citation19] and new PHACOMP [Citation20] were proposed to predict the σ phase formation in superalloys. The PHACOMP method evaluates the average number of holes (Nh) based on the composition of the gamma matrix. When Nh is higher than a critical value, the σ phase is likely to form. In contrast, the new PHACOMP approach calculates the average energy level of the d-orbital (Md) of Ni3Al. The σ phase formation is expected if alloying leads to a Md value higher than the threshold value. These predictive methods provide valuable guidelines in the development of superalloys. However, they cannot be used to predict the σ phase formation in HEAs. This is because they are based on the premise that the σ phase precipitates from an FCC matrix with a known composition, which is apparently not the case for HEAs. For example, in the Al0.3CrFe1.5MnNi0.5 alloy, the dendrite region is composed of B2 precipitates embedded in a BCC matrix.[Citation13] During annealing at 700°C, the BCC matrix transforms into the σ phase directly without changing its composition, just like the case in the Fe–Cr binary system. Therefore, in HEAs, the σ phase formation can be totally different from the above premise: they can form from BCC phase without precipitation.
Here, we propose a simple parameter, the average valence electron concentration (VEC), as a predictive index for σ phase formation in HEAs. We start from the Al–Cr–Fe–Mn–Ni alloy system, which is known to form a large amount of σ upon aging between 600°C and 900°C.[Citation4] The VEC in Al–Cr–Fe–Mn–Ni was changed systematically to observe the effect of VEC on the formation of σ phase. Then, a series of test alloys with VEC values spanning from 6.27 to 8.44 was designed to determine the σ-phase-forming VEC range. Finally, the high-temperature phase-stability information of other HEAs available in the literature was analyzed to test the efficacy of the VEC theory. The targets of our analysis include HEAs containing Cr and/or V as A elements. The other known A element in HEAs, Mo, is not considered in the present research because the number of Mo-containing alloys available is not sufficient for a reliable analysis yet.
The HEAs studied in the present research were prepared by vacuum arc-melting. Raw materials with purities higher than 99.5% were melted in Ar for at least four times to ensure the materials were well-mixed in their liquid state. The AlxCrFe1.5MnNi0.5 alloys were aged at 700°C for 1, 2, 5, 10, 20, and 50 h in air, and subsequently air-cooled. Other alloys were aged at 700°C for 20 h to allow the σ phase formation. Crystal structures were identified using an X-ray diffractometer (XRD) (Rigaku ME510-FM2) in the configuration. A Cu Kα radiation operated at 30 kV, 20 mA was used, and the scanning speed was 4 min−1. Hardness was measured using a MATSUZAWA SEIKI MV-1 hardness tester with a load of 5 kg. The VEC of an alloy is the weighted average VEC value of the constituent elements:
, where n is the number of components in the alloy, ci and (VEC)i are the atomic concentration, and the VEC of component i.
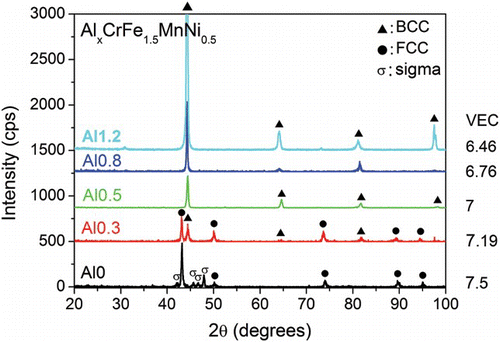
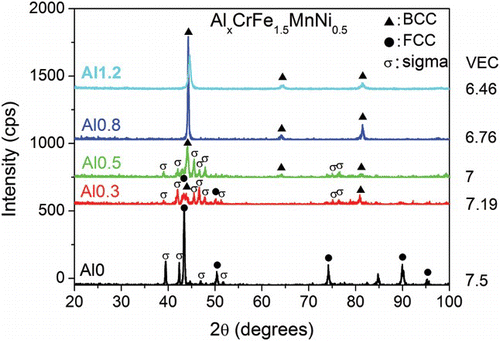
shows the XRD patterns of the AlxCrFe1.5MnNi0.5 alloys. Alloys with x=0, 0.3, 0.5, 0.8, 1.2 will henceforth be referred to as Al0, Al0.3, Al0.5, Al0.8, and Al1.2, respectively. Since Al has a small VEC value (VEC=3), Al is the most effective choice to modify the VEC of the alloy. It is seen that the main structure of the alloys changes from FCC (Al0) to (Al0.3), and eventually to solely BCC (Al0.5, Al0.8, and Al1.2). This trend agrees well with the general understanding that Al is a very effective BCC former.[Citation9,Citation21] Moreover, the trend also agrees with the conclusion of Guo et al., which states that VEC plays a decisive role in the phase formation of as-cast HEAs.[Citation22] Guo et al. suggested that when the VEC of the alloy is larger than 8, FCC phases are stable; when VEC is smaller than 6.87, BCC phases are stable. When the VEC is between these two values, BCC and FCC phases co-exist.[Citation22] The VEC of Al0.8 and Al1.2 falls below 6.87 and thus BCC phase is expected. Although the main phase in Al0 alloy is FCC, it is noted that the σ phase already exists in as-cast state. As for Al0.3 and Al0.5, XRD peaks corresponding to the σ phase appear after aging at 700°C for 20 h (). In fact, σ phase peaks are already detected after a short aging time of 2 h (XRD pattern not shown here). In contrast, the patterns of Al0.8 and Al1.2 remain almost unchanged after aging for 20 h. The σ phase was not observed even after a long aging time of 50 h (XRD pattern not shown here). This shows that for these two alloys, aging does not lead to the σ phase formation. The above results demonstrate the substantial influence of Al content on the σ phase formation. The σ phase is suppressed by the increase in Al in AlxCrFe1.5MnNi0.5 alloys. The VEC of these alloys are listed in and . It is seen that the σ phase is suppressed when VEC is smaller than a critical value.
Although the effect of VEC is clearly observed in the AlxCrFe1.5MnNi0.5 alloys, the VEC range attainable in these alloys is not sufficiently wide. This is because except Al, the VEC values of composing elements in Al–Cr–Fe–Mn–Ni are similar. Therefore, within the composition range of HEAs (i.e. concentration of any major element should be between 5 and 35 at.%), variation of VEC in Al–Cr–Fe–Mn–Ni alloy is limited. In order to probe the effect of VEC on the σ phase formation over a wider spectrum of VEC, a series of test alloys with different composing elements and VEC values spanning from 6.27 to 8.44 was designed. As-cast test alloys were aged at 700°C for 20 h to allow the σ phase formation. Seven hundred degree Celsius was selected as the aging temperature because virtually all Cr- and V-containing binary σ phases are stable at this temperature.[Citation23] Moreover, it also offers rapid reaction kinetics so that 20 h is more than sufficient for σ phase to form. After aging, the phases were identified by XRD. The composition, VEC, and phase (after aging treatment) of the test alloys are listed in . The presence of σ phase in aged test alloys and AlxCrFe1.5MnNi0.5 alloys is plotted against the VEC of that alloy in , where σ-forming alloys are colored red, while σ-free alloys are colored green. clearly demonstrates the strong dependence of σ phase formation on the VEC of the alloy: there is a σ-forming VEC range within which σ phase develops upon aging at 700°C. This VEC range can be determined from . The lower critical VEC value is around 6.88 and the higher critical VEC (HCVEC) value is around 7.84. The σ phase did not develop in aged Cr- and/or V-containing HEAs with VEC values outside of this range. It is also noted that VEC not only controls the presence of σ phase, but also affects the phase formation of the alloys. Aged alloys with high VEC have a single FCC structure (T9–T13). With the decrease in VEC, the σ phase appears. Further decrease in VEC leads to the formation of single BCC structure (T2–T3, Al1.2, and Al0.8). This trend is basically similar to that observed by Guo et al. in as-cast HEAs.[Citation22]
Figure 3. Relationship between the VEC and the presence of σ phase after aging for a number of HEAs. Green and red icons indicate the absence and presence of σ phase after aging, respectively. The aging condition for the AlxCrFe1.5MnNi0.5 alloys and the test alloys is 700°C 20 h, while those for alloys collected from the literature are listed in . Note the similarity between the σ-phase-forming VEC range and the BCC+FCC VEC range proposed by Guo et al. (colour online only).
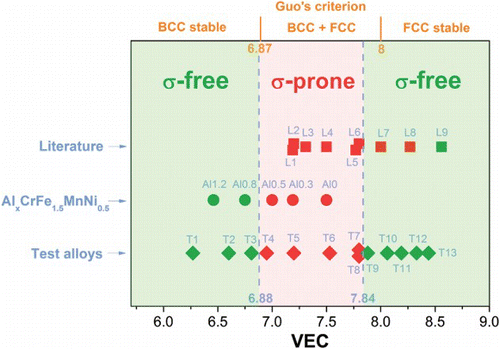
Table 1. Composition and VEC of the test alloys designed in this study.
To understand the efficacy of the VEC criterion, high-temperature phase-stability information of other HEAs is also collected from literatures () [Citation11–15,Citation24–27] and summarized in (data points L1–L9). Alloys that already contain σ phase in their as-cast state are also included (L2, L4, L5, and L6). Although the data available are very limited, it does support the existence of a σ-free zone at the high-VEC end (point L9). However, apparent contradictions to the σ-forming VEC range proposed above are observed: L7 and L8 alloys have VEC values higher than the HCVEC, but they are actually σ-prone. To understand this contradiction, we should take a closer look at these alloys. The composition of L7 is Cr2CuFe2MnNi and thus it has a VEC of 8. Structure analysis indicates that the σ phase formed during aging treatment at 600–950°C.[Citation11] Careful examination of its microstructure reveals that L7 contains a Cu-rich phase which has 43.8–86.9 at.% of Cu, depending on the aging temperature. This means that most of the Cu in this alloy separates from other elements to form its own phase. Therefore, although the overall concentration of Cu in the alloy is 14.29 at.%, much less Cu actually participates in the phase formation of the rest of the alloy. Indeed, Cu concentration in the dendrite area is only 1.8–5.5 at.%.[Citation11] This factor is not considered in the calculation of VEC, so the effect of Cu is over-estimated. Unfortunately, Cu has a very high VEC of 11. Therefore, the over-estimation of Cu leads to an apparent shift of VEC to the right side of . This explains why σ phase was observed in L7 and L8, because their ‘effective’ VEC values are probably below 7.84. For example, assume only 1/5 of Cu participates in the phase formation of the non-Cu-rich phases in L7, the ‘effective’ VEC will be 7.61 only. This finding thus suggests that one should be particularly careful when using the VEC criterion on alloys containing elements that separate from other components to form their own phases, because the effective VEC of these alloys may be evidently different from their apparent VEC.
As mentioned earlier, Guo et al.[Citation22] showed that VEC strongly affects the phase formation in as-cast HEAs. They concluded that at , the stable phase is BCC; at VEC>8, the stable phase is FCC. When VEC is between the two values, BCC and FCC phases co-exist. Interestingly, the σ-prone VEC range in the present study (
) is very close to the
VEC range (
) proposed by Guo et al. (i.e. alloys whose as-cast states have
phases are prone to the σ phase formation when aged at suitable temperature). This is actually logical. Guo's conclusion suggests that if an HEA is composed mostly of BCC elements, the cast alloy will have a BCC structure (because most BCC elements have a VEC of 5 or 6, and will easily result in a VEC smaller than 6.87). Similarly, if an HEA is composed mostly of FCC/HCP elements (with the exception of Al, which is FCC but leads to the formation of BCC phase due to its low VEC), the cast alloy will have an FCC structure. If an HEA contains both BCC and FCC elements, and their amounts are similar, the cast alloy will have
structures. Recall that the formation of a binary σ phase requires an A–B element pair, where A is usually a BCC element (group VB or VIB) and B is usually an FCC or HCP element (group VIIB or VIIIB). Thus, the σ phase formation requires the alloy to contain both BCC and FCC/HCP elements—which means the as-cast state of these σ-prone alloys will have
structures.
The effect of electron concentration on phase stability has been observed for a long time.[Citation28] For example, when electron concentration is expressed as average number of valence electrons per atom (e/a), there is a strong correlation between this value and the stable phase type in many Cu and Ag alloys.[Citation28,Citation29] This phenomenon is usually explained by the formation of pseudogap.[Citation30] Pseudogap is the depression in the density of state across the Fermi level, which is a result of the Fermi surface–Brillouin zone (FsBz) interaction.[Citation30] When this happens, the electronic energy is effectively lowered and the phase is stabilized. Though in the present paper, the other definition of electron concentration (VEC) is used, it is noted that VEC is closely related to another mechanism for pseudogap formation, the orbital hybridization.[Citation30] For example, pseudogap formation around the Fermi level was suggested in Al–Mn alloys.[Citation31] It is not sure whether pseudogap formation is the reason for the stabilization of σ phase in such VEC range. Clearly, more theoretical work needs to be done to clarify this issue.
Some limitations to the present VEC criterion shall be discussed. First, the σ-forming VEC range proposed here is based on alloys composed of the following nine elements: Al, Co, Cr, Cu, Fe, Mn, Ni, Ti, and V. However, the VEC range for σ phase formation depends on the element involved. In particular, the A element appears to have a more evident effect on the σ-forming VEC range.[Citation17] Therefore, for HEAs that contain A elements other than Cr and V (e.g. Mo) or have very different composing elements (e.g. refractory HEAs), the VEC range may be different. Second, the aging temperature selected in this study is 700°C. As explained previously, this is because virtually all Cr- and V-containing binary σ phases are stable at this temperature.[Citation23] However, due to the multi-component nature of HEAs, σ phases in HEAs may contain multiple elements, too. This can lead to the shifting of their stable temperature above or below 700°C. Although unlikely, the possibility that the ‘σ-free’ alloys reported here can actually develop σ phase at other temperature cannot be completely ruled out. These two limitations clearly indicate that subsequent works involving more alloy compositions and aging temperatures are still needed to gain a more comprehensive understanding of σ phase formation in HEAs.
In summary, the first criterion to predict the existence of σ phase in HEAs is proposed. Alloys having VEC values between 6.88 and 7.84 are prone to σ phase formation either in the as-cast state or during aging at suitable temperatures. The criterion works well for Cr- and/or V-containing HEAs. Being able to predict the σ phase formation is crucial to the design of HEAs, particularly for alloys aimed for high-temperature applications. It is also found that such VEC rule needs to be applied carefully when the target alloy contains elements that do not tend to mix with other constituent elements. These elements separate from other elements and form their own phases, thus their effect to the VEC of the rest of the composition may be significantly weaker.
REFERENCES
- Yeh JW, Chen SK, Lin SJ, Gan JY, Chin TS, Shun TT, Tsau CH, Chang SY. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv Eng Mater. 2004;6:299–303. doi: 10.1002/adem.200300567
- Tong CJ, Chen MR, Chen SK, Yeh JW, Shun TT, Lin SJ, Chang SY. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall Mater Trans A. 2005;36:1263–1271. doi: 10.1007/s11661-005-0218-9
- Zhu JM, Fu HM, Zhang HF, Wang AM, Li H, Hu ZQ. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Mater Sci Eng A. 2010;527:7210–7214. doi: 10.1016/j.msea.2010.07.049
- Chen ST, Tang WY, Kuo YF, Chen SY, Tsau CH, Shun TT, Yeh JW. Microstructure and properties of age-hardenable AlxCrFe1.5MnNi0.5 alloys. Mater Sci Eng A. 2010;527:5818–5825. doi: 10.1016/j.msea.2010.05.052
- Senkov ON, Wilks GB, Scott JM, Miracle DB. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20 W20 refractory high entropy alloys. Intermetallics. 2011;19:698–706. doi: 10.1016/j.intermet.2011.01.004
- Hsu CY, Juan CC, Wang WR, Sheu TS, Yeh JW, Chen SK. On the superior hot hardness and softening resistance of AlCoCr(x)FeMo(0.5)Ni high-entropy alloys. Mater Sci Eng A. 2011;528:3581–3588. doi: 10.1016/j.msea.2011.01.072
- Chuang MH, Tsai MH, Wang WR, Lin SJ, Yeh JW. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5 Tiy high-entropy alloys. Acta Mater. 2011;59:6308–6317. doi: 10.1016/j.actamat.2011.06.041
- Tsai MH, Wang CW, Tsai CW, Shen WJ, Yeh JW, Gan JY, Wu WW. Thermal stability and performance of NbSiTaTiZr high-entropy alloy barrier for copper metallization. J Electrochem Soc. 2011;158:H1161–H1165. doi: 10.1149/2.056111jes
- Tong CJ, Chen YL, Chen SK, Yeh JW, Shun TT, Tsau CH, Lin SJ, Chang SY. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall Mater Trans A. 2005;36: 881–893. doi: 10.1007/s11661-005-0283-0
- Guo S, Liu CT. Phase stability in high entropy alloys: formation of solid-solution phase or amorphous phase. Prog Nat Sci: Mater Int. 2011;21:433–446. doi: 10.1016/S1002-0071(12)60080-X
- Ren B, Liu ZX, Cai B, Wang MX, Shi L. Aging behavior of a CuCr2Fe2NiMn high-entropy alloy. Mater Des. 2012;33:121–126. doi: 10.1016/j.matdes.2011.07.005
- Ng C, Guo S, Luan J, Shi S, Liu CT. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics. 2012;31:165–172. doi: 10.1016/j.intermet.2012.07.001
- Tsai MH, Yuan H, Cheng G, Xu W, Jian WW, Chuang MH, Juan CC, Yeh AC, Lin SJ, Zhu Y. Significant hardening due to the formation of a sigma phase matrix in a high entropy alloy. Intermetallics. 2013;33:81–86. doi: 10.1016/j.intermet.2012.09.022
- Lin CW, Tsai MH, Tsai CW, Yeh JW, Chen SK. Microstructure and aging behavior of Al5Cr32Fe35Ni22 Ti6 ternary-like high-entropy alloy. In preparation.
- Li C, Li JC, Zhao M, Jiang Q. Effect of alloying elements on microstructure and properties of multiprincipal elements high-entropy alloys. J Alloys Compd. 2009;475:752–757. doi: 10.1016/j.jallcom.2008.07.124
- Tsao LC, Chen CS, Chu CP. Age hardening reaction of the Al0.3CrFe1.5MnNi0.5 high entropy alloy. Mater Des. 2012;36:854–858. doi: 10.1016/j.matdes.2011.04.067
- Joubert JM. Crystal chemistry and Calphad modeling of the sigma phase. Prog Mater Sci. 2008;53:528–583. doi: 10.1016/j.pmatsci.2007.04.001
- Boesch WJ, Slaney JS. Preventing sigma phase embrittlement in nickel-base superalloys. Met Prog. 1964;86:109–111.
- Sims CT, Stoloff NS, Hagel WC. Superalloys II. New York: John Wiley & Sons; 1987.
- Morinaga M, Yukawa N, Adachi H, Ezaki H. New PHACOMP and its applications to alloy design. In: Gell M, Kortovich CS, Bricknell RH, Kent WB, Radavich JF, editors. Superalloys 1984. Warrendale, PA: Metallurgical Society of AIME; 1984. p. 523–532.
- Tung CC, Yeh JW, Shun TT, Chen SK, Huang YS, Chen HC. On the elemental effect of AlCoCrCuFeNi high-entropy alloy system. Mater Lett. 2007;61:1–5. doi: 10.1016/j.matlet.2006.03.140
- Guo S, Ng C, Lu J, Liu CT. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J Appl Phys. 2011;109:103505. doi: 10.1063/1.3587228
- Baker H, editor. ASM handbook: alloy phase diagrams. Materials Park, OH: ASM International; 1992.
- Zhou YJ, Zhang Y, Wang YL, Chen GL. Microstructure characterization of Al-x(TiVCrMnFeCoNiCu)(100-x) high-entropy alloy system with multi-principal elements. Rare Metal Mater Eng. 2007;36: 2136–2139.
- Chen MR, Lin SJ, Yeh JW, Chen SK, Huang YS, Chuang MH. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall Mater Trans A. 2006;37:1363–1369. doi: 10.1007/s11661-006-0081-3
- Kuznetsov AV, Shaysultanov DG, Stepanov ND, Salishchev GA, Senkov ON. Tensile properties of an AlCrCuNiFeCo high-entropy alloy in as-cast and wrought conditions. Mater Sci Eng A. 2012;533:107–118. doi: 10.1016/j.msea.2011.11.045
- Lin CM, Tsai HL, Bor HY. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics. 2010;18: 1244–1250. doi: 10.1016/j.intermet.2010.03.030
- Massalski TB, Mizutani U. Electronic-structure of Hume-Rothery phases. Prog Mater Sci. 1978;22: 151–262. doi: 10.1016/0079-6425(78)90001-4
- Barrett CS, Massalski TB. Structure of metals: crystallographic methods, principles and data. Oxford: Pergamon; 1980.
- Mizutani U. Hume-Rothery rules for structurally complex alloy phases. Boca Raton, FL: CRC Press; 2010.
- Fujiwara T. Electronic-structure in the Al–Mn alloy crystalline analog of quasicrystals. Phys Rev B. 1989;40: 942–946. doi: 10.1103/PhysRevB.40.942