Abstract
Nanolamellar MAX phase compounds (Cr0.5V0.5)n+1AlCn are formed with n = 1, 2 and 3, and their 300 K structure is studied in detail by high-resolution neutron diffraction. While the n = 1 compound is found to have complete disordering of vanadium and chromium in the metallic layers, the n = 2 and 3 compounds show strong tendency for these elements' ordering, with the layer in the 2a(0,0,0) site of (Cr0.5V0.5)3AlC2 fully occupied by vanadium. The thermal expansion dependency of temperature is also studied by neutron diffraction for 2 < T < 550 K, revealing a negligible thermal expansion below 100 K for all of the compounds.
Introduction
In the field of nanolaminated compounds, Mn+1AXn phases (n = 1, 2 or 3) have attracted much interest in the last 15 years. These hexagonal ternary carbides or nitrides, also called MAX phases, for which M is an early transition metal, A is an A-group element and X is carbon and/or nitrogen,[Citation1] have an inherent nanolayered structure, Mn+1Xn layers. These layers are characterized by strong covalent M–X bonds, being interleaved with A layers through weaker M–A bonds. This nanolamellar structure gives a unique combination of metal-like and ceramic-like properties with several potential applications.[Citation2] Up to now, more than 60 MAX phases have been synthesized by using three different elements M, A and X, and new compounds, as the recently discovered Nb2GeC [Citation3] or Mn2GaC,[Citation4] will certainly be proposed in the future. Yet, probably the most promising way to fine-tune or optimize desired properties, as well as opening new routes for developing new characteristics in the MAX phases, such as magnetism, can be found by exploring solid solutions.
Isostructural solid solutions can be obtained in the M, A or X sites, thus forming either quaternary carbides or nitrides, such as (M′xM″1−x)n+1AXn and Mn+1(A′xA″1−x)Xn, or carbonitrides in the case of X-site solid solution. Recently, their potential use in tailoring desired property was demonstrated in the fine-tuning of thermal expansion coefficient (TEC) in the Cr2(AlxGe1−x)C system.[Citation5] Furthermore, solid solutions allow one to obtain new MAX phases. For example, it is possible to synthesize (CrxMn1−x)2AlC [Citation6] or (CrxMn1−x)2GeC [Citation7] compounds even when Mn2AlC and Mn2GeC are yet to be experimentally realized. Similarly, the formation of (Cr0.5V0.5)4AlC3 and (Cr0.5V0.5)3AlC2 was evidenced albeit neither bulk V3AlC2 nor Cr3AlC2 and Cr4AlC3 exist.[Citation8]
The possibility to synthesize nanolamellar metallic-based materials, especially with desired electronic and magnetic properties such as Mn-containing MAX phases, is of interest not only for basic research but also for applications. The latter may be realized through the MAX phase's 2D derivatives, the MXenes (Mn+1Xn).[Citation9] Already recognized as a promising new 2D family of compounds, there is large motivation for new MXene (M′,M″)n+1Xn compounds, preferably formed as ordered metallic layers with high n value. Order is sought for to help achieving enhanced magnetic properties such as giant magnetoresistance [Citation10,Citation11] effects. Large n is needed for higher structural stability, production yield and larger electron/spin density.[Citation9] However, up to now, no ordering was experimentally observed in the n = 1 MAX phases' solid solutions, even though it was theoretically proposed.[Citation7,Citation12,Citation13] A possible reason for the lack of observed ordering in the (M,M′) site is that in the n = 1 phase only one metallic site exists, and its ordering necessitates the formation of superlattice, and the decrease in configurational entropy.[Citation7] However, it can be postulated that it may be easier to achieve ordering of metallic layers by the formation of solid solutions with n = 2 and 3, where more than one M crystallographic site is available, and preferred occupancy may be realized.[Citation14] Indeed, very recently, Ti/Cr ordering was clearly observed in (Cr2/3Ti1/3)3AlC2, supporting this postulation.[Citation15]
The present contribution focuses on (CrxV1−x)n+1AlCn (n = 1, 2 and 3) solid-solution MAX phases whose formation was already experimentally evidenced.[Citation8] One of the main interest of this Cr,V,Al,C system consists in the possibility of studying the evolution of the structural parameters and physical properties as a function of n since it is the only other example known to date, together with Tan+1AlCn,[Citation16] of a system in which phases with n = 1, 2 and 3 can be obtained. Moreover, recently an Invar-like behavior of Cr2AlC was claimed to be observed below 80 K.[Citation17] A search for similar TEC behavior in higher order MAX phases is of interest. Finally, (Cr0.5V0.5)3AlC2 is one of only two solid-solution MXene reported to exist,[Citation9] and its detailed study is important.
Naturally, most probable solid solutions can be achieved by mixing neighboring elements in the periodic table, due to similar atom size and electronic structure. Therefore, the study of possible structural ordering of such solid solution is greatly hindered by the inability of X-ray diffraction to distinguish neighboring elements. In contrast, neutron scattering from the nuclei can usually distinguish extremely well between neighboring atoms, and even between isotopes.[Citation18] This is especially true for the (Cr,V) system, where vanadium has negligible neutron coherent scattering compared to that of chromium (∼100 times smaller).[Citation18] This is the last, but not least, reason for our current concentration on the detailed temperature-dependent study of the (Cr,V)n+1AlCn system using neutron diffraction (ND).
2. Experimental Details
(Cr0.5V0.5)n+1AlCn compounds were synthesized using conventional powder metallurgy techniques. Chromium (−200 mesh, 99.95%, Alfa Aesar GmbH & Co. KG, Karlsruhe, Germany), vanadium (−325 mesh, 99.8%, Alfa Aesar GmbH & Co. KG, Karlsruhe, Germany), aluminum (40–325 mesh, 99.8%, Alfa Aesar GmbH & Co. KG, Karlsruhe, Germany) and carbon graphite (< 40 µm, Chemwatch) powders were weighted to obtain the nominal compositions: 0.5(n + 1)Cr:0.5(n + 1)V:1.1Al:nC. An excess of 10% Al was chosen for aluminum loss compensation by evaporation during the sintering process. Following 3 h of mixing (Turbula™ shaker), cylindrical compacts, 12 mm in diameter and 3 or 4 g in weight, were obtained using a uniaxial pressure of 250 MPa. Pressure-less reactive sintering of samples was achieved in a furnace (Nabertherm™) under Ar flow. Annealing temperature and holding time were optimized to minimize possible impurity formation (1,400°C for 1 h, 1,500°C for 1 h and 1,400°C for 3 h for n = 1, 2 and 3, respectively). Four or five cylindrical samples of each compound were synthesized in the same batch. The obtained samples have a large open porosity and poor mechanical strength so that they were easily mechanically polished to remove possible secondary phases which exist close to the surface (mainly oxides) and to adjust their diameter to the vanadium containers used for the ND experiments (8 mm in diameter).
ND measurements were conducted on both G4.1 [Citation17] and 3T2 [Citation19] neutron diffractometers at the Orphée research reactor in the Laboratoire Léon Brillouin, Saclay, France. G4.1 is a two-axis powder diffractometer, equipped with a vertical focusing graphite monochromator (wavelength: λ = 2.426(1) Å), and linear BF3 multidetector. In this study, we used an angular step of Δ2θ = 0.1° and an angular coverage of 4–83.9° (momentum-transfer coverage of Q≈0.18–3.46 Å−1). 3T2 is a high-resolution two-axis powder diffractometer, equipped with a vertical focusing Ge monochromator (λ = 1.225193(1) Å), and a banana-type 3He multidetector. In this study, we used an angular step of Δ2θ = 0.05° and an angular coverage of 4.5–118.4° (Q≈0.4–8.6 Å−1). Due to its higher resolution, and significantly larger Q-range coverage, 3T2 was used for detailed structural studies, including cell parameters, atomic positions, atomic occupancies and anisotropic atomic displacement parameters (ADPs). Due to its higher flux, G4.1 was used for a detailed study of the TEC. Four or five cylindrical samples (∼5 g in total) were put in a vanadium container, inside a helium cryofurnace. On G4.1, measurements were performed for 23–31 different temperature points in the range 1.5–340 K, whereas 5–7 different temperature points were studied on 3T2 in the range 2–500 K. Neutron counting time ranged from 20 min (for n = 1, 2) to 45 min (3) on G4.1, and between 6 and 14 h on 3T2. Results were analyzed by the Rietveld refinement method using FULLPROF (see Supplementary Material).[Citation20]
3. Results and Discussion
The positions of observed {hkil} reflections in the ND diffractograms of all three samples in all measured temperatures were consistent with the hexagonal structure (space group P63/mmc) generally used for these compounds and with lattice parameters similar to those previously found ().[Citation8] In all samples, the intended MAX phase was the major phase. The Rietveld analysis of the 3T2 data at 300 K () revealed 1.7(1) wt% of Cr2Al and 2.2(2) wt% Al2O3 as impurity phases in the n = 1 sample; 2.0(1) wt% Cr2Al, 0.4(1) wt% (Cr0.5V0.5)C and 2.6(3) wt% (Cr0.5V0.5)2AlC in n = 2; 5(1) wt% (Cr0.3V0.7)2Al, 1.1(2) wt% Al2O3, 6.7(3) wt% (Cr0.3V0.7)C, 3.1(2) wt% Cr2AlC and 2.9(2) wt% (Cr0.66V0.33)3AlC2 in n = 3.
Table 1. Refined crystallographic parameters of (Cr0.5V0.5)n+1AlCn with n = 1, 2 or 3, at 300 K, as determined by the Rietveld analysis of neutron diffraction data.
Figure 1. Rietveld refinement of the structural models described in (solid red lines) to the 300 K ND data collected on the 3T2 diffractometer at λ = 1.225193(1) Å (open black circles), for the (Cr0.5V0.5)n+1AlCn samples with (a) n = 1, (b) n = 2 and (c) n = 3. The difference between model and experiment is presented by the dashed green line at the bottom of each diffractogram. The blue tags represent the reflections' positions of the phases used in the model, with the first line of tags belonging to the major MAX phase.
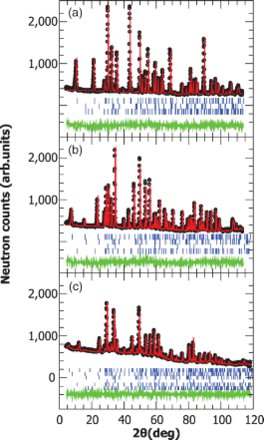
The reflection intensities in the ND diffractograms of the n = 1 sample for all measured temperatures were consistent with a complete disorder of the atoms in the (Cr,V) site ((a)). The Rietveld refinement of the Cr:V occupancy ratio resulted in with the 1:1 ratio with negligible refinement uncertainty. No additional reflections corresponding to ordered superlattice or long-range magnetic ordering were observed. Rietveld refined parameters obtained using the 300 K 3T2 data are summarized in . The resulted crystallographic structure is given in .
Figure 2. Crystal structure of the (Cr0.5V0.5)n+1AlCn compounds with n = 1 (left), n = 2 (middle) and n = 3 (right), visualized as a result of the Rietveld analysis of the 300 K ND data taken on 3T2. Refined layers’ distances are also given. For n = 2 and n = 3, a strong preferred occupation on the two (Cr,V) sites is observed. Reported (Cr,V) occupancies match the occupancies determined from the Rietveld analysis of the ND data (see also ).
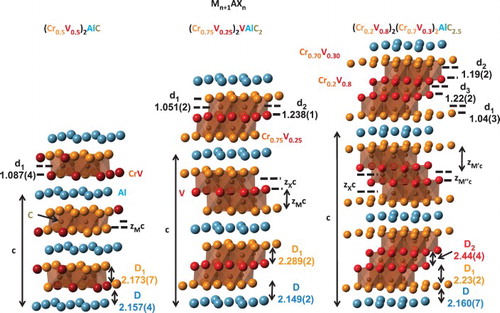
The reflection intensities in the ND diffractograms of the n = 2 and 3 samples for all measured temperatures in both diffractometers were inconsistent with a complete disorder of the atoms in the two (Cr,V) sites of each structure ((b) and 1(c)). The best fit to the data was achieved when large preferred occupancy was allowed in the (Cr,V) layers (). For the n = 2 sample, refinement resulted in 100% and 27(1)% occupancy of vanadium in the 2a(0,0,0) and 4f(1/3,2/3,0.1290(1)) layers, respectively. For n = 3, it resulted in with 32(2)% and 84(1)% occupancy of vanadium in the 4e(0,0,zM' = 0.1539(3)) and 4f(1/3,2/3,zM′′ = 0.054(1)) layers, respectively. In both cases, significant carbon vacancies were also found. Rietveld refined parameters of the ordered compounds, obtained using the 300 K 3T2 data, are summarized in . The resulted crystallographic structures are given in .
Due to the large difference between the thermal neutron scattering from Cr and V,[Citation18] large discrepancies between observed and calculated intensities were obtained when a complete disorder on the (Cr,V) sites was fixed in the model, especially on the {0002}, {0004} and reflections. For n = 2, when 50% occupancy of Cr was forced into the model in both metallic sites, especially large goodness-of-fit, χ2 > 10 [Citation20], was resulted in the refinement, compared to χ2 = 2.82 for the ordered model. In addition, some of the anisotropic ADPs diverged into non-physically large values. For n = 3, the random occupancy model resulted in with zM″ = 0.080(6), a slightly larger χ2 = 2.78 and 35(2)% carbon vacancies, compared to zM″ = 0.055(6), χ2 = 2.67 and 19(2)% carbon vacancies in the ordered model. Moreover, non-physically high values of anisotropic ADPs are obtained. A value of zM″ = 0.080(6) is ∼45% higher than the commonly found value for this parameter in n = 3 samples,[Citation2,Citation16,Citation21] and extremely unlikely to be correct. Therefore, we conclude that a strong preferred occupation in the (Cr,V) layers occur for the n = 2 and 3 samples ( and ).
It is important to note here that ordering of the (Cr,V) atoms within a layer was also considered. However, any such ordering will result in with superlattice reflections. No such superlattice reflections were observed in any of the samples' ND data.
The temperature-dependent lattice parameters for all samples are shown in detail in Figure S1(a)–(c) in the supplementary material. The resulted thermal expansions relative to 2 K are given in , and in more detail in Figure S1(d)–(f). TECs for each direction () are calculated for 150 < T < 340 K using data collected on G4.1 and the average linear TEC method.[Citation22] In this method, the local (in temperature) linear thermal expansions are calculated using discrete derivatives of the measured data. The resulting values are then averaged to give the average linear TEC. In this case, the standard uncertainty of the mean value is the reported uncertainty. For the 3T2 data at 150 < T < 550 K, TECs were determined by simple linear-fits, due to the lack of enough temperature points (). The uncertainty of the linear-fit method is underestimated, as it ignores the variations in the local linear thermal expansion with temperature (cf. ).[Citation22]
Figure 3. Thermal expansions of a (left) and c (right) lattice parameters in (Cr0.5V0.5)n+1AlCn as determined by the Rietveld analysis of temperature-dependent ND data, measured on G4.1 (open symbols) and 3T2 (filled symbols). When not being observed, uncertainties are smaller than symbols' size.
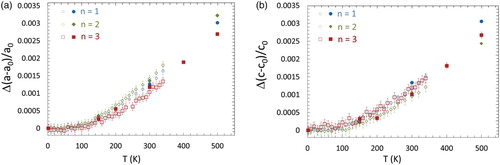
Table 2. TEC, α, in 10−6 K−1 units, along a and c lattice directions in the (Cr0.5V0.5)n+1AlCn system, as determined by temperature-dependent ND on the G4.1 [average (150–340 K)] and 3T2 [linear (150–550 K)] diffractometers at the LLB, France.
The central finding of this work is one of the first observed bulk (M′,M″)n+1AXn phases with strong tendency for ordering of the (M′,M″) layers, and the first observed for the (Cr,V) solid solutions. It is also the first reported n = 3 phase with this tendency. ND results clearly show full ordering of the V layer between two 75%-rich Cr layers in n = 2, and two 80%-rich V layers between two 70%-rich Cr layers in n = 3 (). In both cases, the Cr-rich layers neighbor the Al layers. The possible ordering of the M layers in the MAX phase system was already previously considered for the (Cr,Mn)2GeC and (Cr,Mn)2AlC solid solutions.[Citation6,Citation7,Citation23] Calculations have shown that formation enthalpy favors ordering, when positive (negative) enthalpy was calculated for random (ordered) structure. Yet, configurational entropy destabilizes the structure at elevated temperatures. Our finding for the n = 1 phase studied here supports this idea. However, calculations have also shown that both Cr3AlC2 and Cr4AlC3 are unstable, and that the stability of their V counterparts is relatively weak, as V4AlC3 stabilizes only with a significant amount of C vacancies.[Citation24] These calculations are supported experimentally.[Citation21] Assuming similar qualitative behavior for the formation enthalpy in the n = 1, 2 and 3 solid solutions, we may presume that disordered solid solutions with n = 2,3 will, too, be unstable, and ordering of the metallic layers is needed for the stabilization of the compounds, as we observe experimentally here. More calculations on solid solutions of n > 1 MAX phases are needed to shed light on our presumption.
The above-mentioned presumption is supported experimentally by the recent finding of ordering in (Cr2/3Ti1/3)3AlC2, again, a system containing chromium.[Citation15] In this work, full ordering of Cr in the 4f(1/3,2/3,z) site and Ti in the 2a(0,0,0) site was observed using ND. This ordering is in accordance with the 2:1 ratio of the nominal Cr:Ti content. In our work, where the nominal Cr:V ratio is 1:1 for all studied samples, such full ordering is not expected in the n = 2 phase. Interestingly, however, our analysis show that the layer at the 2a(0,0,0) site is 100% occupied with V, causing a mixed, yet Cr rich, layer in the 4f(1/3,2/3,z) 2a(0,0,0) site (). Moreover, one could presume that a 1:1 Cr:V ratio is more prone to fully order in the n = 3 phase, where the two possible sites for (Cr,V) have the same multiplicity in 4e(0,0,z) and 4f(1/3,2/3,z). However, no such full occupancy is favorable in the analysis of the experimental data. Our attempts to force a Rietveld model with full-site ordering, where both (Cr,V) sites are fully occupied with either Cr or V in both n = 2 and n = 3 phases, resulted in with significantly worse fit of the model to the experimental data. Finally, as mentioned above, an n = 2 impurity phase is observed in the (Cr0.5V0.5)4AlC3 sample. It is intriguing to note that in the Rietveld analysis the (Cr,V) site occupancies of this impurity phase refine to full occupancy of Cr in the 4f(1/3,2/3,z) site and V in the 2a(0,0,0) site. The relatively small weight fraction of this phase (2.9(2)%) prevents us at this moment from claiming the more general statement, arises from the combination of our findings and those of [Citation15], that the n = 2 phase may be a true quaternary phase when the M:M′ ratio is maintained at 2:1.
Structural comparison with the Tan+1AlCn system [Citation16] may shed light on the (Cr,V)n+1AlCn system as well. In general, the interlayer distances within each compound in the present study follow similar rules as the Tan+1AlCn counterpart (compare with Figure 10 in [Citation16]). The (Cr,V)–(Cr,V) interlayer distances (Di) within each compound grow monotonically with increased distance from the Al layer, as do the (Cr,V)–C interlayer distances (di). The much shorter distances involved are attributed to the significantly shorter CrC and VC distances [Citation25,Citation26] compared to TaC.[Citation27] However, between the compounds, the Tan+1AlCn-like layers distances' evolution with n being maintained for n = 2,3, but not for n = 1. Specifically, d1(n = 1) > d1(2)≈d1(3) in (Cr,V)n+1AlCn, whereas in Tan+1AlCn d1 is the smallest of the three. Clearly, this finding is consistent with the complete disorder of (Cr,V) in n = 1 and the strong tendency for ordering of the (Cr,V) sites in n = 2,3. Our results show that d1 in n = 2,3 is the distance between the Cr-rich layer and carbon (). Since the Cr–C distance is predicted to be one of the shortest in the MC family,[Citation28] it is not surprising to find the connection between the d1(2,3) < d1(1) relation and (Cr,V) ordering/disordering. Finally, one of the arguments in the study of Tan+1AlCn is that the central carbide layer is nearly undisturbed compared to the binary carbide.[Citation16] If this argument is considered general for the MAX phases it may explain the strong tendency of the central metallic layer in (Cr,V)n+1AlCn to be V rich (), since CrC is metastable.[Citation25]
In a previous paper dedicated to the MAX phase Cr2AlC, Jaouen et al. obtained very similar results as far as the evolution of the thermal expansion is concerned.[Citation17] A strong increase in the thermal expansion occurs at a temperature close to 70 K (around 100 K in the present study). They concluded on an invar-like behavior of Cr2AlC, not only on the basis of this result but also because strong modifications of the position of the Cr atoms occurred for a so-called transition temperature which would correspond to a magnetic transition. In the present case, such modifications of the atomic positions are not observed and it is impossible to propose that a magnetic transition occurs around 100 K. Furthermore, it must be pointed out that the observed behavior in the present work (a transition from very low TEC towards higher values around 100 K) is quite common for ceramic materials.[Citation29] In other words, it is impossible to conclude on an invar-like behavior for the (CrxV1−x)n+1AlCn phases studied here. Also of note is the lack in monotonic evolution of the TEC as a function of n. Our results show that the n = 1,3 TECs are isotropic while that of n = 2 is anisotropic (). Currently, no simple explanation is offered on the basis of the previous study. However, it may be that the appearance of ordering, especially the full vanadium layer in n = 2, strongly influences the interatomic bonding, and therefore, the TEC.
4. Conclusions
In conclusion, we report here on the formation of (Cr0.5V0.5)n+1AlCn compounds with n = 1, 2, 3. The n = 2,3 compounds show strong tendency for ordering in the form of rich chromium and vanadium layers. It is shown that the general structural evolution as a function of n of this system slightly deviates from that of the only other example of existing n = 1, 2 and 3 (Tan+1AlCn), in a way that supports the existence of ordering. In addition, the observed low-temperature TEC behavior of Cr2AlC [Citation17] is maintained throughout the studied system, albeit ordering. Finally, we suggest that the formation of metallic nanolaminate ordering, which may be especially desirable for magnetic elements, should be sought out in these high-level MAX phases rather than in n = 1. However, further calculations and experiment are needed to decide whether the present case is common or rare.
Supplementary Online Material.
A more detailed information on experiments is available at http://dx.doi.org/10.1080/21663831.2014.975294002014.
Supplementary material
Download MS Word (132.6 KB)Acknowledgements
This paper is partially based on experiment nos. 11689 and 11690, performed at the Orphée research reactor in the Laboratoire Léon Brillouin, CEA-CNRS, Saclay, France.
E.N.C. would like to thank the University of Poitiers for the visiting professor position received during this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Nowotny VH. Strukturchemie einiger Verbindungen der Übergangsmetalle mit den elementen C, Si, Ge, Sn. Prog Solid State Chem. 1971;5:27–70. doi: 10.1016/0079-6786(71)90016-1
- Barsoum MW. Introduction. In: MAX Phases. Weinheim: Wiley; 2013. p. 1–12.
- Eklund P, Dahlqvist M, Tengstrand O, Hultman L, Lu J, Nedfors N, Jansson U, Rosen J. Discovery of the ternary nanolaminated compound Nb2GeC by a systematic theoretical–experimental approach. Phys Rev Lett. 2012;109(3):035502. doi: 10.1103/PhysRevLett.109.035502
- Ingason AS, Petruhins A, Dahlqvist M, Magnus F, Mockute A, Alling B, Hultman L, Abrikosov IA, Persson POÃ, Rosen J. A nanolaminated magnetic phase: Mn2GaC. Mater Res Lett. 2014;2(2):89–93. doi: 10.1080/21663831.2013.865105
- Cabioch T, Eklund P, Mauchamp V, Jaouen M, Barsoum, MW. Tailoring of the thermal expansion of Cr2(Alx,Ge1−x)C phases. J Eur Ceram Soc. 2013;33(4):897–904. doi: 10.1016/j.jeurceramsoc.2012.10.008
- Mockute A, Dahlqvist M, Emmerlich J, Hultman L, Schneider JM, Persson POÅ, Rosen J. Synthesis and ab initio calculations of nanolaminated (Cr,Mn)2AlC compounds. Phys Rev B. 2013;87(9):094113. doi: 10.1103/PhysRevB.87.094113
- Ingason AS, Mockute A, Dahlqvist M, Magnus F, Olafsson S, Arnalds UB, Alling B, Abrikosov IA, Hjörvarsson B, Persson POÅ, Rosen J. Magnetic self-organized atomic laminate from first principles and thin film synthesis. Phys Rev Lett. 2013;110(19):195502. doi: 10.1103/PhysRevLett.110.195502
- Zhou Y, Meng F, Zhang J. New MAX-phase compounds in the V–Cr–Al–C system. J Amer Ceram Soc. 2008;91(4):1357–1360. doi: 10.1111/j.1551-2916.2008.02279.x
- Naguib M, Mochalin VN, Barsoum MW, Gogotsi Y. 25th Anniversary article: MXenes: a new family of two-dimensional materials. Adv Mater. 2014;26:992–1005. doi: 10.1002/adma.201304138
- Binasch G, Grünberg P, Saurenbach F, Zinn W. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Phys Rev B. 1989;39(7):4828–4830. doi: 10.1103/PhysRevB.39.4828
- Baibich MN, Broto JM, Fert A, Nguyen Van Dau F, Petroff F. Giant magnetoresistance of (001)Fe/(001)Cr magnetic superlattices. Phys Rev Lett. 1988;61(21):2472–2475. doi: 10.1103/PhysRevLett.61.2472
- Cabioc'h T, Eklund P, Mauchamp V, Jaouen M. Structural investigation of substoichiometry and solid solution effects in Ti2Al(C−x,N1−x)(y) compounds. J Eur Ceram Soc. 2012;32(8):1803–1811. doi: 10.1016/j.jeurceramsoc.2011.12.011
- Arróyave R, Radovic M. Ab initio investigation of Ti2Al(C,N) solid solutions. Phys Rev B. 2011;84(13):134112. doi: 10.1103/PhysRevB.84.134112
- Liu Z, Zheng L, Sun L, Qian Y, Wang J, Li M. (Cr2/3Ti1/3)(3)AlC2 and (Cr5/8Ti3/8)(4)AlC3: new MAX-phase compounds in Ti–Cr–Al–C system. J Amer Ceram Soc. 2014;97(1):67–69. doi: 10.1111/jace.12731
- Liu Z, Wu E, Wang J, Qian Y, Xiang H, Li X, Jin Q, Sun G, Chen X, Wang J, Li M. Crystal structure and formation mechanism of (Cr2/3Ti1/3)3AlC2 MAX phase. Acta Mater. 2014;73:186–193. doi: 10.1016/j.actamat.2014.04.006
- Etzkorn J, Ade M, Hillebrecht H. Ta3AlC2 and Ta4AlC3—Single-crystal investigations of two new ternary carbides of tantalum synthesized by the molten metal technique. Inorg Chem. 2007;46(4):1410–1418. doi: 10.1021/ic062231y
- Jaouen M, Chartier P, Cabioc'h T, Mauchamp V, André G, Viret M. Invar like behavior of the Cr2AlC MAX phase at low temperature. J Amer Ceram Soc. 2013;96(12):3872–3876. doi: 10.1111/jace.12635
- Sears VF. Neutron scattering lengths and cross sections. Neutron News. 1992;3(3):26–37. doi: 10.1080/10448639208218770
- Porcher F, Rieu B, Damay F, Bourée F. News and reports. Neutron News. 2010;21(4):25–27. doi: 10.1080/10448632.2010.519640
- Rodríguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys B Condens Matter. 1993;192(1–2):55–69. doi: 10.1016/0921-4526(93)90108-I
- Hu C, Zhang H, Li F, Huang Q, Bao Y. New phases’ discovery in MAX family. Int J Refract Metals Hard Mater. 2013;36:300–312. doi: 10.1016/j.ijrmhm.2012.10.011
- Lane NJ, Vogel SC, Caspi EN, Dubois S, Gauthier-Brunet V, Bei GP, Barsoum MW. A high-temperature neutron diffraction and first-principles study of Ti3AlC2 and Ti3(Al0.8Sn0.2)C2. J Amer Ceram Soc. 2014;97(2):570–576. doi: 10.1111/jace.12696
- Dahlqvist M, Alling B, Abrikosov IA, Rosen J. Magnetic nanoscale laminates with tunable exchange coupling from first principles. Phys Rev B. 2011;84(22): 220403. doi: 10.1103/PhysRevB.84.220403
- Dahlqvist M, Alling B, Rosén J. Stability trends of MAX phases from first principles. Phys Rev B. 2010;81(22):220102. doi: 10.1103/PhysRevB.81.220102
- Liu BX, Cheng XY. A metastable Cr carbide of NaCl structure formed by carbon-ion implantation into chromium films. J Phys Condens Matter. 1992;4(16):L265. doi: 10.1088/0953-8984/4/16/003
- Lipatnikov VN, Gusev AI, Ettmeier P, Lengauer W. Order-disorder phase transformations and specific heat of nonstoichiometric vanadium carbide. Physics Solid State. 1999;41(3):474–480. doi: 10.1134/1.1130806
- Fries RJ, Wahman LA. Effect of stoichiometry on the thermal expansion of TaCx. J Amer Ceram Soc. 1967;50(9):475–477. doi: 10.1111/j.1151-2916.1967.tb15165.x
- Haglund J, Grimvall G, Jarlborg T, Guillermet A. Band-structure and cohesive properties of 3d-transition-metal carbides and nitrides with the NaCl-type structure. Phys Rev B. 1991;43(18):14400–14408. doi: 10.1103/PhysRevB.43.14400
- Baranov AN, Bell AMT, Solozhenko VL. Low-temperature thermal expansion of rock-salt ZnO. Solid State Commun. 2014;177:65–67.
