Abstract
Adding nanoparticles to soft matter and liquids is known to provide remarkable control in the processing of novel materials. Here, we demonstrate a similar potential in crystalline solids. Specifically, we show that the addition of a high density of W nanoparticles dramatically alters the coarsening behavior of precipitate-hardened Cu–Ag alloys. First, the nanoparticles suppress precipitate growth, but far more surprisingly, they induce non-equilibrium Ag wetting layers on grain boundaries. This observation is explained using kinetic Monte Carlo simulations, which show that caging of Ag precipitates by the W nanoparticles suppresses their growth and drives the formation of wetting layers.
The use of nanoparticles in the processing of complex materials systems has become a powerful method for developing materials with unique microstructures and enhanced properties. In the well-established area of precipitate strengthened alloys, for example, new nano-precipitate aluminum alloys use core-shell structures to gain improved strength and high-temperature microstructural stability.[Citation1–3] For solidification processing, Chen et al. have recently shown that the addition of oxide nanoparticles to Sn–Al alloys melts can result in much finer microstructures than previously possible, and with substantial reduction of dendrite growth.[Citation4] Similarly, the use of nanoparticles for controlling coarsening in two-phase, soft matter systems has been explored both theoretically [Citation5,Citation6] and experimentally.[Citation7] The theoretical work showed in particular that, in an equiatomic A–B phase-separating system, non-wetting particles could break the symmetry of the connectivity of the co-existing domains, and suppress or even arrest the coarsening kinetics. Experimentally, the addition of nanoparticles to polymers makes it possible to synthesize nanocomposites with novel mechanical, optical, and electrical properties, as discussed in Ref. [Citation8]. In this context, the present work considers a novel processing scheme using non-wetting nanoparticles to control microstructural evolution of two-phase alloys during thermal processing—first, maintaining an ultrafine precipitate structure, and second changing altogether the morphology of this precipitate structure. Specifically, we show that the addition of 1.5 at. % W, in the form of 1–2 nm nanoparticles, to a Cu-15 at. % Ag alloy restricts the growth of the Ag precipitates to the dimension of the inter-nanoparticle spacing and that it eventually leads to the dissolution of the Ag nanoparticles in favor of the formation of an Ag wetting layer on the grain boundaries (GBs). This second effect is rather surprising since GB wetting layers are rarely observed in binary alloys, and not before seen in Cu–Ag alloys. We show that this wetting layer, in fact, is a non-equilibrium structure that results from the constraints imposed on the Ag precipitates by the W nanoparticles.
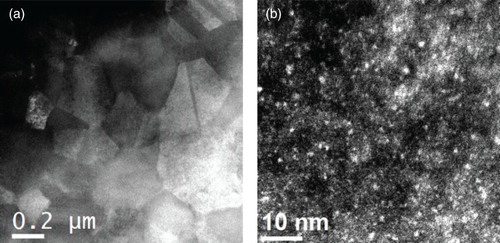
The alloy specimens employed in the experiments, Cu83.5Ag15W1.5 and Cu85Ag15, were prepared by physical vapor deposition growth on oxidized Si wafers at room temperature using DC magnetron sputtering. Film thicknesses were ≈300 nm, with a 2–3 nm W capping layer on the surface to prevent Ag segregation during annealing. Details of the growth process can be found elsewhere.[Citation9] As-grown films were solid solutions, which were subsequently irradiated with 1.8 MeV Kr ions at room temperature to a dose of . This processing step has two important functions. First, it increases the average grain size in the present case from ≈30 nm for the as-grown samples, to ≈200 nm, as seen in (a), while keeping the Ag atoms in solution.[Citation10] Secondly, the irradiation step induces the selective precipitation of small W nanoparticles in the ternary alloy,[Citation11] shown in (b). The W nanoparticles are 1–2 nm in diameter, and have a density of
(in atomic fraction). Details of this microstructure are reported in Ref. [Citation9].
Figure 1. HAADF STEM images showing the microstructure of Cu83.5Ag15W1.5 alloy following room-temperature irradiation. (a) The grain morphology; (b) the uniform distribution of W nanoparticles (indicated by small bright dots in the image).
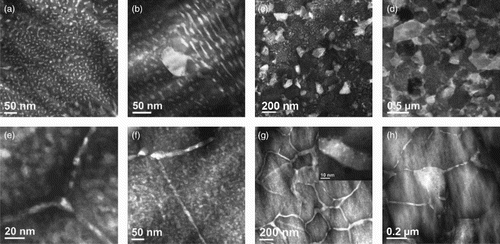
The effect of the high density of W nanoparticles on the microstructural evolution of the ternary alloy during thermal annealing can be observed in the series of high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM) micrographs shown in , which compares coarsening in binary Cu85Ag15 (Figure. (a–d)) with that in ternary Cu83.5Ag15W1.5 ((e–h)). The bright regions in these images reveal the Ag precipitates. Dramatic differences are indeed observed. As shown in (a), the binary alloy undergoes predominantly homogeneous nucleation of Ag precipitates following low-temperature annealing at 300°C for 0.5 h, possibly by spinodal decomposition (the alloy lies within the coherent spinodal [Citation12]). Some coarsening of these precipitates at the GB is evident. As the annealing time is increased to 2 h, the growth of the GB precipitates begins to dominate the microstructural development, accompanied by precipitate depletion in the surrounding regions, see (b). At higher annealing temperature, 400°C ((c)), these GB precipitates begin to grow to a size comparable to that of the Cu grains, and by 500°C only these large Ag precipitates remain, (d). For the ternary alloy, small Ag precipitates are also observed nucleating both within the Cu grain interiors and at the Cu GBs after annealing at 400○C for 1 h (see (e)). As the annealing time is increased, however, the GB precipitates do not grow, but rather they begin to form a continuous Ag wetting layer within the GB that thickens with increased annealing time, as shown in (f) and 2(g) for specimens annealed at 400°C and 500°C, respectively (see also figure S1 in the Supplemental materials for more details). A homogeneous distribution of Ag precipitates remains within the Cu grain interiors, although a denuded zone does develop near the GBs (see figures S2 and S3 in the Supplemental materials). For the annealing at 500○C, moreover, a high density of W nanoparticles can now be found within the thick GB wetting layer, as shown in the inset of (g). Finally, at 600°C, the Ag wetting layers continue to thicken at first, but eventually they transform from two-dimensional GB wetting layers to a three-dimensional grain-like morphology, as shown in (h). Our results thus reveal that the W nanoparticles do not only retard the kinetics for precipitate coarsening in this alloy but also fundamentally alter the kinetic pathway the system follows in moving toward its equilibrium state.
Figure 2. HAADF STEM images showing microstructural evolution during annealing following the room-temperature irradiation in Cu85Ag15 (a–d) and Cu83.5Ag15W1.5 (e–h): (a) 300°C for 0.5 h; (b) 300°C for 2 h; (c) 400°C for 1 h; (d) 500°C for 10 h; (e) 400°C for 1 h; (f) 400°C for 10 h; (g) 500°C for 10 h. The inset in (g) shows uniformly dispersed W nanoparticles in the Ag wetting layer. (h) 600°C for 10 h.
Before discussing the reasons for the above findings, we point out that while second-phase wetting layers have been observed in binary alloys previously, they occur rather infrequently during solid state processing of metals; we are aware of such layers only in Zn-5 wt.% Al, initially reported by work Lopez et al.,[Citation13] and in a few other metal systems.[Citation14,Citation15] Far more common are GB complexions,[Citation16,Citation17] but in the present work, the Ag is clearly forming a wetting layer and not a complexion, that is, the thick Ag layer is an equilibrium bulk phase and not an equilibrium GB phase. The lack of GB wetting layers in most metals is not surprising since close to equilibrium, a wetting layer requires the interface energy to be less than half of the GB energy of the matrix. Our results reported in (a–d) clearly demonstrate that such a wetting layer does not occur in dilute binary Cu85Ag15 alloys at the processing temperatures employed here.
The first step in explaining the formation of the Ag wetting layers is drawing attention to the observation that Cu–Ag interfaces are pinned by W nanoparticles. This occurs by a mechanism akin to Zener pinning of GBs. Since the interface energy for Ag–W appears higher than for Cu–W, the pinning force will, in fact, be stronger than that typical of grain-boundary pinning. Moreover, unlike grain-boundary migration where the driving force is the difference in the free energy between the two grains, the driving force here for precipitate growth is the difference in chemical potentials of Ag atoms in small versus large precipitates. This difference reflects that traditional Zener pinning refers to a non-conserved order parameter, grain orientation, whereas in the present case it refers to a conserved order parameter, alloy solute concentration. The details of this pinning are not of primary concern here (the interested reader is referred to Ref. [Citation9]), but we emphasize that the Ag precipitates are highly constrained by the W nanoparticles. As a consequence, the chemical potential of Ag is greatly increased in the alloy owing to capillarity. This excess free energy thus becomes available when atoms flow from small precipitates to a grain-boundary wetting layer. Consequently, the condition for wetting is relaxed from to
where
is the energy recovered when Ag atoms transfer from the caged Ag precipitates to form the wetting layer, that is,
, where r is the radius of the Ag precipitates and
is the areal density of Ag atoms required to form the wetting layer. Note, for
and r = 3 nm (as discussed below), that the effect is large,
.
We can understand the pathway by which the wetting layer forms in the ternary alloy by recalling that in the binary alloy Ag precipitates nearly homogeneously throughout the Cu matrix. This results from the very high supersaturation of Ag in the vapor-deposited thin film, although the precipitates grow most rapidly at the GBs and form a zone denuded of Ag precipitates in the surrounding matrix ((b)). In the alloy containing the W nanoparticles, in contrast, the Ag precipitates on the GBs experience the same caging effect as in the bulk, and hence they cannot continue to grow. Nonetheless, the free energy of Ag precipitates on the GBs is lower than in the bulk, and so small Ag precipitates continue to nucleate and grow on the GBs, until they cover the entire grain-boundary area. This is illustrated in (e) where a very high density of small Ag precipitates is observed at the GBs. These precipitates eventually begin to impinge with one another, and form a continuous layer (see (f)).
While this scenario can well explain why an incipient wetting layer begins to form, it does not explain how the wetting layer in the boundary thickens, to ∼40 nm before transforming to a 3-D structure, that is, why the W nanoparticles do not pin the wetting layer. The HAADF micrograph inset in (g) indeed shows that the Ag wetting layer overruns and envelops the W nanoparticles as it thickens. The wetting layer is thus a transient state imposed on the system by a high density of W nanoparticles. We use the results from kinetic Monte Carlo (KMC) simulations to explain why the growth of the wetting layer is not pinned by the nanoparticles in this case. Our model for examining the pinning of the Ag precipitates and GB wetting layer employs a rigid face-centered cubic (fcc) lattice, initially containing matrix atoms A (ostensibly Cu), one vacancy, and a high density of 1 nm particles comprising C (i.e. W) atoms. Diffusion in this simulation is mediated by the single vacancy. The interspacing of the C particles is ∼4 nm, which are placed on an fcc lattice. The system is annealed at T = 0.055 eV (365°C). We introduce one precipitate of B (Ag) with small radius, and then let it grow by slowly adding B atoms to the solution until the precipitate reaches a given size. The steady-state solubility of B in A matrix is thus obtained as a function of r. Since the B precipitates are not perfectly spherical, r represents their effective radius, that is, the radius of a sphere with the same total volume. The pair interactions between A, B, and C atoms were selected to mimic the properties of Cu, Ag, and W; values for the cohesive energies, saddle-point energies and ordering energies are provided in . Additional details can be found in Ref. [Citation9]. The results are illustrated in , where the solubility limit of B is plotted as a function of radius. Since the excess chemical potential (cB is the measured solubility;
is the equilibrium solubility for a flat interface), (a) is essentially a plot of chemical potential versus precipitate radius for a given density of C particles. At small r, the chemical potential of B atoms decreases rapidly with increasing r; this is the usual Gibbs–Thomson effect, which is indicated by the dashed curve; this curve was obtained from simulations using the binary alloy. The net effect of the pinning particles on solubility of B, obtained by subtracting the Gibbs–Thomson fitting from the KMC measurements, is shown in the inset of (a). When r approaches the inter C-particle spacing, the chemical potential begins increasing with r, giving rise to the Zener-like pinning. Notice that for the second peak in the inset of (a) (effective r ≈ 4.4 nm), the solubility corresponds to a spherical precipitate of size r ≈ 3 nm. We have thus used this value in our estimate of
, above. The increased interface curvature due to the nanoparticles can be observed in (b). At very large r in this plot,
is still increased by the C nanoparticles, but owing to Gibbs–Thomson effect, the chemical potential for a large B precipitate is still relatively small. This is illustrated in (c), where the interface curvature is smaller compared to that in (b). B atoms in the small caged precipitates, therefore, will flow to the GB wetting layer (
), enabling the layers to overrun the W nanoparticle obstacles and thicken. (g) (and Supplemental materials figure S3), indeed, clearly illustrates that the region close to the wetting layer is being depleted of Ag precipitates. Eventually this wetting layer becomes sufficiently thick that it is unstable to transformation to a 3-D morphology, with dimensions on the order of the Cu grain size, and thus similar to the binary case, compare, for example, (d) and 2(h). The formation of these large Ag precipitates in the ternary alloy, however, has been delayed by several hundred degrees, and they form by a completely different mechanism.
Figure 3. (a) Solid line: KMC measured solubility of B for the system with high-density C pinning particles. Dashed line: the solubility fitting using Gibbs–Thomson equation for the system without C pinning particles. The inset shows the solid line subtracted by the dashed line. (b) A slice of the microstructure of the system with an effective radius of r ≈ 4.4 nm (pointed by an arrow in (a)), showing a pinned B precipitate developing large positive and negative curvatures. (c) A slice of the microstructure of the system with an effective radius of r ≈ 6 nm (also pointed by an arrow in (a)), showing smaller curvatures. Red: A, blue: B, yellow: C.
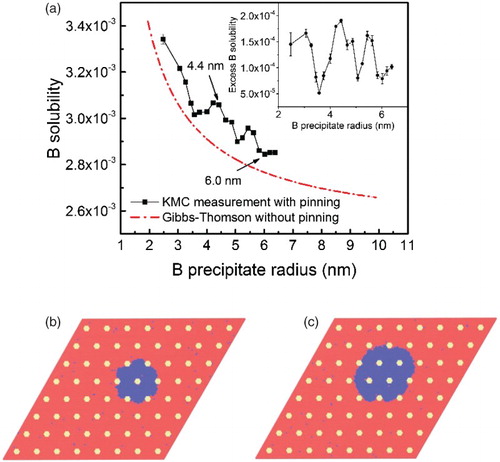
Table 1. Thermokinetic parameters used in the KMC simulation.
In summary, we have shown that the addition of W nanoparticles to a dilute Cu–Ag alloy dramatically alters the kinetic pathway followed by this system during annealing. The Ag precipitates become encaged by the nanoparticles as they grow, and this suppresses their growth, which in turn drives the formation and growth of a non-equilibrium Ag GB wetting layer. This finding suggests a promising scheme for synthesizing novel high-strength nanostructured alloys, as highly immiscible nanoparticles can be incorporated into alloys not only by ion beam irradiation but also by severe plastic deformation.[Citation18]
Supplementary online material
A more detailed information on experiments is available at http://dx.doi.org/10.1080/21663831.2015.1090496http://dx.doi.org/10.1080/21663831.2015.1090496
Supplemental_Material.docx
Download MS Word (1.4 MB)Acknowledgements
This research was supported by US National Science Foundation under Grant Number DMR-1306475. The work was carried out in part in the Frederick-Seitz Materials Research Laboratory Central Facilities, University of Illinois at Urbana-Champaign.
Disclosure Statement
No potential conflict of interest was reported by the authors.
ORCID
Shipeng Shu http://orcid.org/0000-0003-3859-5014
References
- Fuller CB, Seidman DN, Dunand DC. Mechanical properties of Al(Sc,Zr) alloys at ambient and elevated temperatures. Acta Mater. 2003;51:4803–4814. doi: 10.1016/S1359-6454(03)00320-3
- Karnesky RA, Dunand DC, Seidman DN. Evolution of nanoscale precipitates in Al microalloyed with Sc and Er. Acta Mater. 2009;57:4022–4031. doi: 10.1016/j.actamat.2009.04.034
- Radmilovic V, Ophus C, Marquis EA, Rossell MD, Tolley A, Gautam A, Asta M, Dahmen U. Highly monodisperse core-shell particles created by solid-state reactions. Nat Mater. 2011;10:710–715. doi: 10.1038/nmat3077
- Chen L-Y, Xu J-Q, Li X-C. Controlling phase growth during solidification by nanoparticles. Mater Res Lett. 2014;3:43–49. doi: 10.1080/21663831.2014.956264
- Ginzburg VV, Peng G, Qiu F, Jasnow D, Balazs AC. Kinetic model of phase separation in binary mixtures with hard mobile impurities. Phys Rev E. 1999;60:4352–4359. doi: 10.1103/PhysRevE.60.4352
- Balazs AC, Ginzburg VV, Qiu F, Peng GW, Jasnow D. Multi-scale model for binary mixtures containing nanoscopic particles. J Phys Chem B. 2000;104:3411–3422. doi: 10.1021/jp993356+
- Tanaka H, Lovinger AJ, Davis DD. Pattern evolution caused by dynamic coupling between wetting and phase separation in binary liquid mixture containing glass particles. Phys Rev Lett. 1994;72:2581–2584. doi: 10.1103/PhysRevLett.72.2581
- Balazs AC, Emrick T, Russell TP. Nanoparticle polymer composites: where two small worlds meet. Science. 2006;314:1107–1110. doi: 10.1126/science.1130557
- Zhang X, Shu S, Bellon P, Averback RS. Precipitate stability in Cu–Ag–W system under high-temperature irradiation. Acta Mater. 2015;97:348–356. doi: 10.1016/j.actamat.2015.06.045
- Chee SW, Stumphy B, Vo NQ, Averback RS, Bellon P. Dynamic self-organization in Cu alloys under ion irradiation. Acta Mater. 2010;58:4088–4099. doi: 10.1016/j.actamat.2010.03.039
- Zhang X, Wen JG, Bellon P, Averback RS. Irradiation-induced selective precipitation in Cu-Nb-W alloys: an approach towards coarsening resistance. Acta Mater. 2013;61:2004–2015. doi: 10.1016/j.actamat.2012.12.020
- Pandey OP, Ojha SN, Lele S. On spinodal boundaries of the Ag-Cu. Scr Metall Mater. 1993;29:1131–1134. doi: 10.1016/0956-716X(93)90190-4
- Lopez GA, Mittemeijer EJ, Straumal BB. Grain boundary wetting by a solid phase; microstructural development in a Zn-5 wt% Al alloy. Acta Mater. 2004;52:4537–4545. doi: 10.1016/j.actamat.2004.06.011
- Straumal AB, Yardley VA, Straumal BB, Rodin AO. Influence of the grain boundary character on the temperature of transition to complete wetting in the Cu-In system. J Mater Sci. 2015;50:4762–4771. doi: 10.1007/s10853-015-9025-x
- Straumal BB, Kogtenkova OA, Kolesnikova KI, Straumal AB, Bulatov MF, Nekrasov AN. Reversible “wetting” of grain boundaries by the second solid phase in the Cu-In system. JETP Lett. 2014;100:535–539. doi: 10.1134/S0021364014200107
- Kaplan WD, Chatain D, Wynblatt P, Carter WC. A review of wetting versus adsorption, complexions, and related phenomena: the Rosetta stone of wetting. J Mater Sci. 2013;48:5681–5717. doi: 10.1007/s10853-013-7462-y
- Cantwell PR, Tang M, Dillon SJ, Luo J, Rohrer GS, Harmer MP. Grain boundary complexions. Acta Mater. 2014;62:1–48. doi: 10.1016/j.actamat.2013.07.037
- Suryanarayana C. Mechanical alloying and milling. Prog Mater Sci. 2001;46:1–184. doi: 10.1016/S0079-6425(99)00010-9
