Abstract
Despite having never been synthesized, the MAX phase Zr2AlC attracts a lot of interest owing to its foreseen properties. A possible way to circumvent this obstacle is to stabilize Zr2AlC by partially substituting one of its constituting elements. Here we report on attempts to synthesise quaternary MAX phases (Zr,M)2AlC and Zr2(Al,A)C where M = Cr, Ti or Mo and A = S, As, Sn, Sb and Pb. We were notably able to produce Zr2(Al0.2Sn0.8)C, Zr2(Al0.35Pb0.65)C, and Zr2(Al0.3Sb0.7)C, with the latter representing the first antimony-based MAX phase ever reported.
Impact Statement: Numerous syntheses of Zr2AlC derived compositions were attempted. Zr2(Al0.2Sn0.8)C, Zr2(Al0.35Pb0.65)C and Zr2(Al0.3Sb0.7)C were notably produced and reported for the first time.
Keywords:
Introduction
The MAX phases are a family of carbides and nitrides crystallizing with the hexagonal P63/mmc structure in the general formula Mn+1AXn with n being an integer, M being an early transition metal, A being a group 13–16 element and X being C and/or N.[Citation1] Around 70 ternary MAX phases can be synthesized as a bulk phase. More than half of known MAX phases with n = 1 were first reported in the 1960s by Nowotny et al. [Citation2] and named ‘H-phases’. They were almost completely ignored for two decades until Barsoum and El-Raghy renewed interest in 1996 by reporting the remarkable properties of Ti3SiC2,[Citation3] then demonstrating these properties were shared by the other MAX phases [Citation4,Citation5] and by finally defining the current ‘MAX phase’ appellation and definition.[Citation6] Owing to their structure consisting of the stacking of n ‘ceramic’ layer(s) of MX interleaved by an A ‘metallic’ plane, MAX phases show a combination of both ceramic and metallic features (high thermal shock resistance, good machinability and high thermal and electrical conductivities, alike most metals; high decomposition or melting temperature, high elastic stiffness, and high oxidation and corrosion resistance as common for ceramics).[Citation1] Since the mid-2000s, discoveries of new stable bulk ternary MAX phases for n values of 1–3 have been rare [Citation7] presumably inferring that the true achievable list is close to be completed.[Citation8] More recently research aimed to: (i) identify MAX phases with higher n,[Citation8] (ii) produce thin films [Citation9,Citation10] and (iii) investigate the formation of quaternary MAX phases (i.e. the partial substitution of either the M, A or X element by a fourth element).[Citation11,Citation12] The latter is almost limitless in terms of possible combinations. Recently, Naguib et al. listed 24 quaternary MAX phases for which at least one composition was already reported in the open literature,[Citation11] some of them also existing with different n values. This approach also offers the possibility of including new elements that are not reported to form alone a bulk ternary MAX phase, such as Mn incorporated in (Cr1−xMnx)2GaC (0 ≤ x ≤ 0.3).[Citation13] While also adding Bi in the list of MAX phase forming elements we were able to synthesize Zr2(Al0.42Bi0.58)C, despite the fact that the two corresponding end-members (Zr2AlC and Zr2BiC) seemingly cannot be.[Citation14] Similar effect is given by V substitution as noticed by Zhou et al. for (Cr0.5V0.5)3AlC2.[Citation15] While the mechanism driving the structural stability of Zr2(Al0.42Bi0.58)C is still unclear, Caspi et al. [Citation16] Anasori et al. [Citation17] and Lin et al. [Citation18] recently demonstrated by neutron diffraction or high-resolution transmission electron microscopy that respectively (Cr0.5V0.5)3AlC2, (Mo2/3Ti1/3)3AlC2 and (Cr2/3Ti1/3)3AlC2 were stabilized thanks to ordering of the two M atoms of each of these compounds onto the two different Wyckoff sites with n = 2 (e.g. Cr is exclusively detected on the 4f sites while Ti is predominantly on the 2a sites in (Cr2/3Ti1/3)3AlC2). These examples outline that quaternary MAX phases could be synthesized to approach as close as possible the composition and properties of non stable or metastable ternary MAX phases of interest.
Among all apparently non-synthesizable ternary MAX phases, Zr2AlC is of prime interest, especially for the nuclear industry [Citation14] mostly because of the high neutron transparency of Zr2AlC constituting elements but also its potentially good irradiation resistance behaviour, as found in other MAX phases.[Citation19,Citation20] In particular, Zr2AlC is strongly considered as a protective material in modified Accident Tolerant Fuel (ATF;[Citation21]) assembly grids [Citation22] since as demonstrated for other Al-based MAX phases, a good high temperature oxidation resistance is expected.[Citation1]
In the present paper we report syntheses attempts of numerous (Zr0.75M0.25)2AlC, (Zr0.5M0.5)2AlC and Zr2(Al1-xAx)C (0.25 ≤ x ≤ 0.6), compositions, with M = Mo, Ti or Cr and A = S, As, Sn, Sb or Pb. The M and A elements were chosen primarily with consideration for their neutron cross-section [Citation23] and further selected by the fact that the M2AlC and Zr2AC ternary MAX phases were reported to be synthesizable.[Citation1] For their closeness in term of properties with their MAX phase former neighbours in the Periodic Table, Mo, As and Sb were also selected despite not passing the second selection criterion. This report complements a recent paper focused on the Zr2(Al0.42Bi0.58)C MAX phase [Citation14] that also contained a non-regular A-element and also reports on (Zr0.4Nb0.6)2AlC and (Zr0.2Nb0.8)2AlC syntheses by Reiffenstein [Citation24] et al. and Naguib et al. [Citation11] respectively.
Experimental Details
Commercial reactants (powders) used were: ZrH2 (>99.7%, −325 mesh), ZrC (>99.5%, excluding <200 ppm Hf, −325 mesh) TiH2 (>99%, ∼40 µm), Cr (>99%, −325 mesh), Mo (>99.9%, −200 mesh), Al (>99.5%, −325 mesh), Al2S3 (>99%, millimetric granules), As (>99%, −70 mesh), Sn (>99.8%, −325 mesh), Sb (>99.5%, −200 mesh) and Pb (>99.9%, −200 mesh) all provided by Alfa Aesar, and graphite (>99.9%, <20 µm) purchased from Sigma-Aldrich. To limit as much as reasonably possible oxygen impurities, the reactants were kept and weighed in Nylon milling jars filled with 10 mm ZrO2 balls in an argon (Ar) filled glove box. The jars were sealed and placed for 0.5 h at 360 rpm in a rotary mill (Nanjing University Instrument Plant, China). The mixed powders were then transferred in the glove box and kept in sealed plastic bottles until used for the synthesis.
As is the custom for most MAX phase syntheses, stoichiometries were experimentally adjusted to 2/1.05/0.95 for (Zr + M), (Al + A) and C, respectively (Table ). This is in order to compensate for the usual partial sublimation of the A element(s) and the possible partial uptake of carbon from the graphite crucibles and dies used. Reactions were carried out by pressureless heating of the powders under Ar. Minutes before the thermal treatment, the sealed Ar-filled bottles were opened and the powders were poured in graphite crucibles (provided by Almath Crucibles Ltd., UK) which were then placed in a hot press here used as a graphite furnace in presureless mode (FCT Systeme HP W/25/1, Rauenstein, Germany) filled with Ar (static). Four different synthesis temperatures were used: 1900°C (10 min dwelling, then 1 h at 1600°C), 1450°C (1 h dwelling), 1300°C (10 h) and 1150°C (10 h). Heating and cooling rates were set at ∼20°C min−1. When needed, the obtained solids were crushed into powder in an agate mortar using an agate pestle (usually for compounds obtained at reaction temperatures ≥1300°C). The obtained powders were then analysed by X-ray diffraction, XRD, using a Bruker D2 Phaser SSD160 (Karlsruhe, Germany) equipped with a Cu source (Kα1/α2 = 1.5406/1.5444 Å). Routine analyses consisted of 6–105o 2θ scans with a 0.03o 2θ step and 0.4 sec step−1. Crystalline phase determination was done with the help of X'pert HighScore Plus software (PANalytical B.V. Almelo, the Netherlands) using ICDD (International Centre for Diffraction Data) database. Existing Ti2AlC (29–0095), Cr2AlC (29–0017), Zr2PbC (28–0546), Zr2SnC (29–1355) and Zr2SC (16–0848) datasheets were used as references for tentative matching. Similarly, the presence of higher order MAX phase (n = 2 or 3) or other non-MAX phases ternary layered carbides was ruled out respectively by comparing to powder diffraction simulated patterns created using Crystal Maker and Crystal Diffract softwares (CrystalMaker Software Ltd., Oxford, UK) and by comparison with reported XRD data ([Citation25] and references therein,[Citation26]). Refinement of unit cell parameters was done by full-pattern matching (Le Bail function) using the Fullprof Suite program.[Citation27] When required, Rietveld refinement was also utilized using X'pert HighScore Plus software Rietveld tool. Scanning Electron Microscopy imaging and coupled Energy Dispersive X-ray Spectroscopy (EDX) were carried out in a JEOL JSM-6400 (Tokyo, Japan) scanning electron microscope (SEM), equipped with an EDX detector (ultra-thin polymer window, INCA, Oxford Instruments, Oxford, UK). The powders were spread and stuck onto a conductive carbon tape and analysed without any further preparation.
Table 1. Summary of syntheses results. Dwelling times are given in the experimental section. In the last column, obtained phases are given in descending order with respect to their relative amount according to XRD and the MAX phases are rendered in bold. The compositions of the phases identified in this table are that of the closest matching with reference datasheets. For actual MAX phases compositions measured by EDX, refer to Table .
Results and Discussion
The synthesis results are summarized in Table . Several Zr2AlC and Zr2AlN syntheses were first attempted. As expected by the absence in the literature of an experimental report of these potential MAX phases, no MAX phases were obtained. Heating to 1900°C, resulted in only ZrC or ZrN, while for lower temperatures, ZrC or ZrN were combined with different Zr-Al alloys.
All the Zr substitution attempts by Ti, Cr and Mo were also unsuccessful (Table ). A limited MAX phase presence was determined for the (Zr0.5Cr0.5)2AlC composition, however experimental XRD and EDX investigations strongly suggest—analogously to the case of Zr2(Al1−xSx)C compounds detailed in the next paragraph—that the MAX phase component is Cr2AlC, with no quantifiable amount of Zr in it. This is notably confirmed by the measured a and c lattice parameters for the herein produced MAX phase (a = 2.863(2) Å, c = 12.83(1) Å) which both fall right into the range of values reported for Cr2AlC (2.844 ≤ a ≤ 2.865 Å and 12.814 ≤ c ≤ 12.857 Å) ([Citation12] and references therein).
On the basis of experimental conditions employed for synthesis of Zr2SC in the literature,[Citation28,Citation29] the composition temperatures chosen to synthesize Zr2(Al1−xSx)C (0.25 ≤ x ≤ 0.6) were 1300°C and 1450°C. From EDX, as submicron grains were obtained (not shown), only global analyses on large areas (≈10.000 µm2) were performed. They reveals Zr/(Zr +Al + S) ratios of 0.8 to 0.85, indicating the loss of both Al and S. Al2S3 normally evaporates at 1500°C, but it is reasonable to assume some Al-S compounds richer in S (or Al-S-C compounds) can volatilize below 1300°C.[Citation30] Despite this partial loss of Al and S, a MAX phase was detected by XRD in all Zr2(Al1−xSx)C powders. Lattice parameter refinement (summarized in Table ) always resulted in values close to those reported for Zr2SC. That does not indicate per se that the Al content in the formed MAX phases is low as S substitution by Al in the structure could potentially be achieved without notable unit cell parameter changes. This is, however, unlikely considering that DFT calculations[Citation14,Citation31–35] and existing lattice parameters values for other Zr2AC and M2AlC MAX phases allow to predict that a hypothetical Zr2AlC compound should fall in the following ranges: 3.1 < a < 3.4 and 13.6 < c < 14.7 Å. Therefore, even considering the lowest projected value for Zr2AlC (13.6 Å) the c lattice parameter (12.1 Å for Zr2SC [Citation1,Citation28,Citation36]) is expected to strongly increase even for limited S by Al substitution. Lastly, Al-rich phases ZrAl2 and ZrAl3 are present in significant amounts as secondary phases (Table ). This all suggests that Al has very little, or no, solubility in Zr2SC.
Table 2. Unit cell parameters of the produced Zr2(Al1-xAx)C quaternary MAX phases and comparison with existing ternary 211 MAX phases.
Attempts to make a Zr2(Al0.5As0.5)C MAX phase at 1150°C and 1300°C were also unsuccessful according to XRD. Similarly, the synthesis of Zr2(Pb0.45Bi0.55)C was attempted at 1300°C, motivated by the possible usefulness of such a potential MAX phase in the lead-bismuth eutectic cooled nuclear reactor design.[Citation39] A MAX phase was detected as a secondary phase, however based on EDX no quantifiable amount of Bi was found in the MAX phase, suggesting Zr2PbC was formed with little or no Bi in it.
Three compositions were eventually determined to produce positive results matching the initial goal of synthesizing a MAX phase as compositionally close to Zr2AlC as possible: Zr2(Al0.5Sn0.5)C, Zr2(Al0.5Sb0.5)C and Zr2(Al0.5Pb0.5)C. According to XRD, all three presented better results (lower unwanted phase contents) at 1300°C than at 1450°C or 1150°C. This is in agreement with El-Raghy et al. [Citation40] who determined that for Sn and Pb-based MAX phases (including Zr2SnC and Zr2PbC), the onset temperature of decomposition and also the best temperature for time–effective formation of such MAX phases were around 1200–1300°C. They also understood that finding the optimal reaction temperature is difficult due to ZrC duality, being both a Zr2AC precursor and its main decomposition product. The closeness of decomposition and necessary reaction temperatures likely explains why the syntheses 10 h at 1300°C were more successful than those for the same dwell time at 1150°C presumably because of slower kinetics of formation, while temperature as high as 1450°C promoted the competing decomposition process.
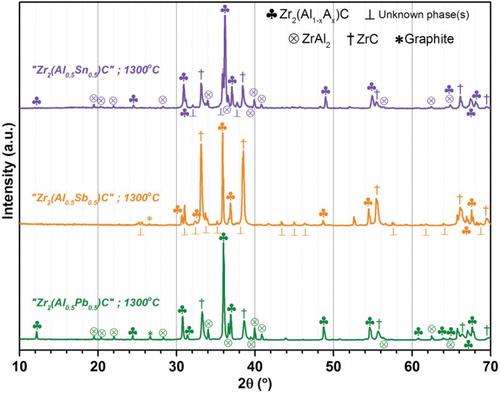
X-ray diffractograms of these 3 compounds of interest (prepared at 1300°C) are presented in Figure ; the results of refinement are summarized in Table . The Sb-based composition contains significantly more ZrC than the other two compositions. Rietveld refinement was additionally performed to estimate the phase mass ratios and confirmed the 211 MAX phase content in the 1300°C Zr2(Al0.5Sb0.5)C sample was low (less than 30 wt %, even disregarding for refinement purpose the unassigned diffraction lines) compared to the 57 wt % of Zr2(Al0.5Sn0.5)C and the 65 wt % of Zr2(Al0.5Pb0.5)C.
Figure 1. (Colour online) X-ray diffractograms of the powder samples obtained after heating the Zr2(Al0.5Sn0.5)C, Zr2(Al0.5Sb0.5)C and Zr2(Al0.5Pb0.5)C compositions to 1300°C for 10 h.
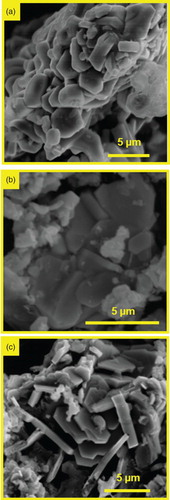
SEM (Figure ), coupled with EDX characterization, revealed that the MAX phase grains were 1–10 µm large and were agglomerated in clusters of a few tens of micrometres, while the grains of the unwanted phases (ZrAl2 and ZrC notably) were mostly isolated or formed smaller clusters. Additionally, to allow phase identification in the SEM, EDX was used to determine the A/(Al + A) ratio in the MAX phases. From about 20 independent point scans in each sample, the compositions were determined to be Zr2(Al0.20Sn0.80)C, Zr2(Al0.30Sb0.70)C and Zr2(Al0.35Pb0.65)C, with standard deviations of below ±0.05 for all of the elements (except carbon as it is not quantifiable with EDX). Such low standard deviations between the measurements, indicate all the MAX grains in each sample maintain identical or very close compositions, as recently demonstrated for Zr2(Al0.42Bi0.58)C.[Citation14] In the aforementioned chemistries full occupancy of two was assumed for the Zr atomic sites.
Figure 2. SEM images (secondary electron mode) of, (a) Zr2(Al0.2Sn0.8)C, (b) Zr2(Al0.3Sb0.7)C and (c) Zr2(Al0.35Pb0.65)C. In (a) according to EDX the shown grains only consist of Zr2(Al0.2Sn0.8)C (secondary phases present elsewhere). In (b) and (c) the darker contrast plate grains are respectively Zr2(Al0.3Sb0.7)C and Zr2(Al0.35Pb0.65)C, while the lighter surrounding grains are various secondary phases (see Table ). All images are for a reaction temperature of 1300°C (10 h dwelling).
Note that EDX revealed the targeted ratio x = 0.5 was not reached; in all cases the compounds contained less Al than expected. This may be explained by partial volatilization of Al, although this is improbable as ∼15 wt % of ZrAl2 was detected by XRD in Zr2(Al0.5Sn0.5)C and Zr2(Al0.5Pb0.5)C samples and this counterbalances the deviation of x = 0.5 targeted in the MAX phases. Furthermore, the absence of significant Al volatilization during synthesis was indirectly ruled out by reacting at 1300°C a new Zr2(Al0.5Pb0.5)C powder batch with an excess of +15 mol % of both Al and Pb. The X-ray diffractogram and MAX phase lattice parameters obtained were similar to the first attempt with the usual +5 mol % excess and the MAX phase grain compositions were still determined by EDX to be Zr2(Al0.35Pb0.65)C. As a result, it is more likely that the Zr2(Al0.2Sn0.8)C, Zr2(Al0.3Sb0.7)C and Zr2(Al0.35Pb0.65)C compositions form over the targeted Zr2(Al0.5A0.5)C composition because they are more stable.
Furthermore, for the three compounds, little or no Pb, Sb or Sn were found in the non-MAX phase grains. For Pb and Sn based compositions, this suggests the MAX phases were formed up until consumption of the operational limiting reactant - Pb or Sn—respectively leaving the excess Zr, Al and C to form ZrC and ZrAl2. This likely explanation is also supported by the phase weight ratios determined by Rietveld refinement (65 wt % Pb-MAX/21 wt % ZrC/14 wt % ZrAl2 and 57 wt % Sn-MAX/25 wt % ZrC/18 wt % ZrAl2) which sum fairly well to the initial elemental ratios.
In contrast the Zr2(Al0.5Sb0.5)C composition differs as the relative amount of MAX phase is much lower (<30 wt % when performing Rietveld and disregarding unassigned phase(s)) and the rarity of Sb in the non-MAX grains (determined by EDX) means a substantial fraction of the initial Sb escaped before completion of the MAX phase formation, presumably by volatilization of a Sb-rich binary or ternary compound; Garcia and Corbett,[Citation41] for example, reported quantitative Zr-Sb volatilization can occur below 1000°C.
Table compares the quaternary MAX phase unit cell parameters obtained here with those of experimental values of their most relevant ternary MAX phases. Additionally, Figure displays the relative change of unit cell parameters and volume in respect to Zr2AlC values recently estimated by DFT.[Citation14] For the same targeted MAX phase, the different reaction temperatures resulted in close lattice parameters (typically within the range of associated errors), for this reason only the values for the 1300°C samples are given. This suggests that the obtained A/(Al + A) ratios are not influenced by the reaction temperature, at least over the 1150–1450°C temperature range. The unit cell parameters obtained for Zr2(Al0.2Sn0.8)C and Zr2(Al0.35Pb0.65)C are lower than those reported for Zr2SnC [Citation4,Citation37] and Zr2PbC respectively,[Citation1,Citation38] which is not too surprising given the smaller atomic radius of Al compared to Sn or Pb. From Figure , it is first worth noting that the progressive replacement of Al by another heavier and larger element (Sn, Sb, Pb or also Bi [Citation14]) mainly impacts the a lattice parameter (up to ∼+1.7% relative expansion from Zr2AlC [Citation14] to Zr2PbC [Citation38]) and much lightly the c parameter (up to ∼+0.6%); in other words the Zr2AC unit cell expand (or shrink) relatively more along the (0001) plane than along the c-axis when the A element atomic size increases (or decreases). In Figure , Vegard's law is followed for both a and c lattice parameters of Zr2(Al0.2Sn0.8)C and Zr2(Al0.35Pb0.65)C when compared to their respective end-members. For Zr2(Al0.3Sb0.7)C, lattice parameters greater than those estimated for Zr2AlC are, as expected, obtained. More importantly, it is worth noting that both a and c parameters for Zr2(Al0.3Sb0.7)C (Table ) are in the narrow ranges of reported values for the other experimentally synthesized Zr2AC and Zr2(A,A’)C MAX phases: 3.34 ≤ a ≤ 3.38 and 14.51 ≤ c ≤ 14.91 Å (Table and [Citation1]). This is true except for Zr2SC, which presumably has a peculiar structural stability.[Citation14,Citation42] It is therefore suggested that the stabilization of the Zr2AC and Zr2(A,A’)C phases can only be reached in the above-defined lattice parameter narrow ranges and that steric constraints could (partially) drive the formation of Zr2(Al0.2Sn0.8)C, Zr2(Al0.3Sb0.7)C and Zr2(Al0.35Pb0.65)C instead of the targeted Zr2(Al0.5A0.5)C compositions which would fall out of this steric stability region. For such a hypothesis to be confirmed would require additional investigations, both computational (e.g. DFT) and experimental.
Figure 3. (Colour online) Lattice parameters a (top), c (middle) and unit cell volume V (bottom) variations in the Zr2(Al1-xSnx)C, Zr2(Al1-xSbx)C and Zr2(Al1-xPbx)C MAX phase systems as a function of x. Hollow symbols are for literature values (see Table for references). Zr2AlC values (x = 0) were selected from the most recent DFT calculation estimates [Citation14].
![Figure 3. (Colour online) Lattice parameters a (top), c (middle) and unit cell volume V (bottom) variations in the Zr2(Al1-xSnx)C, Zr2(Al1-xSbx)C and Zr2(Al1-xPbx)C MAX phase systems as a function of x. Hollow symbols are for literature values (see Table 2 for references). Zr2AlC values (x = 0) were selected from the most recent DFT calculation estimates [Citation14].](/cms/asset/b64b35c0-aa19-410a-9eb8-fe29bcad377d/tmrl_a_1143053_f0003_c.jpg)
Conclusions and Perspectives
Numerous synthesis attempts were performed with the objective of stabilizing Zr2AlC by the partial substitution of either Zr or Al. Although (Zr,Nb)2AlC solid solutions have been reported,[Citation11,Citation24] partial substitution attempts of Zr by Mo, Ti and Cr in Zr2AlC did not lead to the formation of any Zr-containing MAX phases. Similarly, substitution of Al by As or S did not give any favourable result.
However, partial substitution of Al by the heavier MAX formers Sn, Sb and Pb (and Bi [Citation14]) led to formation respectively of Zr2(Al0.2Sn0.8)C, Zr2(Al0.3Sb0.7)C and Zr2(Al0.35Pb0.65)C (and Zr2(Al0.42Bi0.58)C). Notably, this represents the first report of the synthesis of an antimony-bearing MAX phase. Even though Al amounts in these compounds are low, it nonetheless confirms the desired Zr2AlC phase can be approached by partial substitution(s) on the A-sites. The authors thus suggest that new stabilizing elements and/or combination of two stabilizing elements should be tried to achieve a compound having a composition as close as possible to Zr2AlC. These experimental efforts might also take advantage of computational calculation methods such as DFT simulations to narrow the list of promising candidates.
Finally it also worth mentioning that MAX phases are also of interest for MXenes preparation [Citation43] and the new compositions prepared here should therefore be considered to attempt the preparation of the so far non-synthesisable [Citation44] Zr2C slabs.
Acknowledgements
D.H. is grateful to D. Shepherd (National Nuclear Lab) and the CARAT consortium managed by Westinghouse for their support and fruitful discussions. Dr Ben Milsom is acknowledged for his help in the access and use of QMUL equipment. Prof. M.W. Barsoum is also warmly thanked for his help and advice.
Disclosure Statement
No potential conflict of interest was reported by the authors.
ORCID
Denis Horlait http://orcid.org/0000-0002-2645-6896
Alexander Chroneos http://orcid.org/0000-0002-2558-495X
Additional information
Funding
References
- Barsoum MW. MAX phases: properties of machinable ternary carbides and nitrides. Weinheim: Wiley-VCH; 2013.
- Nowotny H. Strukturchemie einiger verbindungen der ubergangsmetalle mit den elementen C, Si, Ge, Sn. Prog Solid State Chem. 1970;2:27–70. doi: 10.1016/0022-4596(70)90028-9
- Barsoum MW, El-Raghy T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2. J Am Ceram Soc. 1996;76:1953–1956. doi: 10.1111/j.1151-2916.1996.tb08018.x
- Barsoum MW, Yaroschuck BG, Tyagi S. Fabrication and characterization of M2SnC (M = Ti, Zr, Hf and Nb). Scr Met Mater. 1997;37:1583–1591. doi: 10.1016/S1359-6462(97)00288-1
- Barsoum MW, Brodkin D, El-Raghy T. Layered machinable ceramics for high temperature applications. Scr Met Mater. 1997;37:535–541. doi: 10.1016/S1359-6462(96)00418-6
- Barsoum MW, El-Raghy T. The MAX phases: unique new carbide and nitride materials. Am Sci. 2001;89:336–345. doi: 10.1511/2001.28.736
- Cuskelly DT, Richards ER, Kisi EH, Keast VJ. Ti3GaC2 and Ti3InC2: first bulk synthesis, DFT stability calculations and structural systematics. J Solid State Chem. 2015;230:418–425. doi: 10.1016/j.jssc.2015.07.028
- Hu C, Zhang H, Li F, Huang Q, Bao Y. New phases’ discovery in MAX family. Int J Refract Met Hard Mater. 2013;36:300–312. doi: 10.1016/j.ijrmhm.2012.10.011
- Eklund P, Beckers M, Jansson U, Hogberg H, Hultman L. The Mn+1AXn phases: materials science and thin-film processing. Thin Solid Films. 2010;518:1851–1878. doi: 10.1016/j.tsf.2009.07.184
- Meshkian R, Ingason AS, Dahlqvist M, Petruhins A, Arnalds UB, Magnus F, Lu J, Rosen J. Theoretical stability, thin film synthesis and transport properties of the Mon+1GaCn MAX phase. Physica Status Solidi. 2015;9:197–201. doi: 10.1002/pssr.201409543
- Naguib M, Bentzel GW, Shah J, Halim J, Caspi EN, Lu J, Hultman L, Barsoum MW. New solid solution MAX phases: (Ti05,V05)3AlC2, (Nb05,V05)2AlC, (Nb05,V05)4AlC3 and (Nb08,Zr02)2AlC. Mater Res Lett. 2014;2:233–240. doi: 10.1080/21663831.2014.932858
- Horlait D, Grasso S, Al Nasiri N, Burr PA, Lee WE. Synthesis and oxidation testing of MAX phase composites in the Cr–Ti–Al–C quaternary system. J Am Ceram Soc. 2016; 99. doi:10.1111/jace.13962.
- Mockute A, Lu J, Moon EJ, Yan M, Anasori B, May SJ, Barsoum MW, Rosen J. Solid solubility and magnetism upon Mn incorporation in bulk Cr2AlC and Cr2GaC MAX phases. Mater Res Lett. 2015;3:16–22. doi: 10.1080/21663831.2014.944676
- Horlait D, Middleburgh SC, Chroneos A, Lee WE. Synthesis and DFT investigation of new bismuth-containing MAX phases. Sci Reports. 2016;6:18829.
- Zhou Y, Meng F, Zhang J. New MAX-phase compounds in the V–Cr–Al–C system. J Am Ceram Soc. 2008;91:1357–1360. doi: 10.1111/j.1551-2916.2008.02279.x
- Caspi EN, Chartier P, Porcher F, Damay F, Cabioc'h T. Ordering of (Cr,V) layers in nanolamellar (Cr0.5V0.5)n+1AlCn compounds. Mater Res Lett. 2014;3:100–106. doi: 10.1080/21663831.2014.975294
- Anasori B, Halim J, Lu J, Voigt CA, Hultman L, Barsoum MW. Mo2TiAlC2: a new ordered layered ternary carbide. Scr Mater. 2015;101:5–7. doi: 10.1016/j.scriptamat.2014.12.024
- Liu Z, Wu E, Wang J, Qian Y, Xiang H, Li X, Jin Q, Sun G, Chen X, Wang J, Li M. Crystal structure and formation mechanism of (Cr2/3Ti1/3)3AlC2 MAX phase. Acta Mater. 2014;73:186–193. doi: 10.1016/j.actamat.2014.04.006
- Nappe JC, Grosseau P, Audubert F, Guilhot B, Beauvy M, Benabdesselam M, Monnet I. Damages induced by heavy ions in titanium silicon carbide: effects of nuclear and electronic interactions at room temperature. J Nucl Mater. 2009;385:304–307. doi: 10.1016/j.jnucmat.2008.12.018
- Tallman DJ, Hoffman EN, Caspi EN, Garcia-Diaz BL, Kohse G, Sindelar RL, Barsoum MW. Effect of neutron irradiation on selected MAX phases. Acta Mater. 2015;85:132–143. doi: 10.1016/j.actamat.2014.10.068
- Bragg-Sitton S. Development of advanced accident tolerant fuels for commercial LWRs. Nucl News. March 2014; 83–91.
- Xu P, Lahoda EJ, inventor; Westinghouse Electric Company Llc, assignee. High temperature strength, corrosion resistant, accident tolerant nuclear fuel assembly grid. United States patent US20150098546 A1. 2015 Apr 9.
- Sears VF. Neutron scattering lengths and cross sections. Neutron News. 1992;3:26–37. doi: 10.1080/10448639208218770
- Reiffenstein E, Nowotny H, Benesovsky F. Strukturchemische und magnetochemische untersuchungen an komplexcarbiden. Monatsh für Chem verw Teile anderer Wiss. 1966;97:1428–1436. doi: 10.1007/BF00902593
- Wang J, Zhou Y. Recent progress in theoretical prediction, preparation, and characterization of layered ternary transition-metal carbides. Annu Rev Mater Res. 2009;39:415–443. doi: 10.1146/annurev-matsci-082908-145340
- Hu C, Lai CC, Tao Q, Lu J, Halim J, Sun L, Zhang J, Yang J, Anasori B, Wang J, Sakka Y, Hultman L, Eklund P, Rosen J, Barsoum MW. Mo2Ga2C: a new ternary nanolaminated carbide. Chem Comm. 2015;51:6560–6563. doi: 10.1039/C5CC00980D
- Roisnel T, Rodriguez-Carvajal J. Winplotr: a windows tool for powder diffraction patterns analysis. Mater Sci Forum. 2011;118–123.
- Kulkarni SR, Phatak NA, Saxena SK, Fei Y, Hu J. High pressure structural behavior and synthesis of Zr2SC. J Phys Condens Matter. 2008;20:135211. doi: 10.1088/0953-8984/20/13/135211
- Opeka M, Zaykoski J, Talmy I, Causey S. Synthesis and characterization of Zr2SC ceramics. Mater Sci Eng A. 2011;528:1994–2001. doi: 10.1016/j.msea.2010.10.084
- Sharma SC, Chang YA. The Al−S (Aluminum-Sulfur) system. J Phase Equilib. 1987;8:128–131. doi: 10.1007/BF02873197
- Khazaei M, Arai M, Sasaki T, Estili M, Sakka Y. Trends in electronic structures and structural properties of MAX phases: a first-principles study on M2AlC (M = Sc, Ti, Cr, Zr, Nb, Mo, Hf, or Ta), M2AlN, and hypothetical M2AlB phases. J Phys Condens Matter. 2014;26;505503. doi: 10.1088/0953-8984/26/50/505503
- Yakoubi A, Beldi L, Bouhafs B, Ferhat M, Ruterana P. Full-relativistic calculation of electronic structure of Zr2AlC and Zr2AlN. Solid State Comm. 2006;139:485–489. doi: 10.1016/j.ssc.2006.06.044
- Bouhemadou A, Khenata R, Chegaar M. Structural and elastic properties of Zr2AlX and Ti2AlX (X = C and N) under pressure effect. Eur Phys J B. 2007;56:209–215. doi: 10.1140/epjb/e2007-00115-6
- Kang DB. Influence of different a elements on bonding and elastic properties of Zr2AC (A = Al, Si, P, S): a theoretical investigation. Bull Korean Chem Soc. 2013;34:609–614. doi: 10.5012/bkcs.2013.34.2.609
- Kanoun MB, Goumri-Said S, Reshak AH, Merad AE. Electro-structural correlations, elastic and optical properties among the nanolaminated ternary carbides Zr2AC. Solid State Sci. 2010;12:887–898. doi: 10.1016/j.solidstatesciences.2010.01.035
- Kudielka H, Rohde H. Strukturuntersuchungen an carbosulfiden von titan und zirkon. Zeit Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie. 1960;114:447–456. doi: 10.1524/zkri.1960.114.1-6.447
- Utili M, Agostini M, Coccoluto G, Lorenzini E. Ti3SiC2 as a candidate material for lead cooled fast reactor. Nucl Eng Des. 2011;241:1295–1300. doi: 10.1016/j.nucengdes.2010.07.038
- El-Raghy T, Chakraborty S, Barsoum MW. Synthesis and characterization of Hf2PbC, Zr2PbC and M2SnC (M = Ti, Hf, Nb, or Zr). J Eur Ceram Soc. 2000;20:2619–2625. doi: 10.1016/S0955-2219(00)00127-8
- Garcia E, Corbett JD. A synthetic and structural study of the zirconium-antimony system. J Solid State Chem. 1988;73:440–451. doi: 10.1016/0022-4596(88)90130-2
- Barsoum MW. The Mn+1AXn phases: a new class of solids; thermodynamically stable nanolaminates. Prog Solid State Chem. 2000;28:201–281. doi: 10.1016/S0079-6786(00)00006-6
- Naguib M, Kurtoglu M, Presser V, Lu J, Niu J, Heon M, Hultman L, Gogotsi Y, Barsoum MW. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater. 2011;23:4248–4253. doi: 10.1002/adma.201102306
- Lei JC, Zhang X, Zhou Z. Recent advances in MXene: preparation, properties, and applications. Front Phys. 2015;10:107303. doi: 10.1007/s11467-015-0493-x
- Jeitschko W, Nowotny HN, Benesovsky F. Kohlenstoffhaltige ternaere verbindungen (H-Phase). Monatshefte Chemie. 1963;94:672–676. doi: 10.1007/BF00913068
- Jeitschko W, Nowotny H, Benesovsky F. Carbides of formula T2MC. J Less-Comm Met. 1964;7:133–138. doi: 10.1016/0022-5088(64)90055-4
