ABSTRACT
Nanostructured oxy-hydroxide materials with varied compositions of aluminum and gallium were synthesized in an environmentally friendly way with water as the only solvent. The tunability of optical properties of these materials through concentration ranges is particularly of interest for organic/inorganic hybrid materials in optoelectronic devices. Size comparisons of the materials were performed using dynamic light scattering measurements. The measured size of the particles decreased with increasing Al. This supports the blue shift seen from the GaOOH emission wavelength to the Al0.5Ga0.5OOH emission wavelength in the photoluminescence spectra. The X-ray diffraction showed the formation of a possible amorphous layer in Al0.5Ga0.5OOH and Al0.2Ga0.8OOH.
GRAPHICAL ABSTRACT
IMPACT STATEMENT
A water-based synthesis for mixed aluminum–gallium nanostructured oxides is presented with size comparisons and optical characteristics demonstrating emission wavelength tunability.
KEYWORDS:
Investigation into mixed aluminum and gallium containing oxides has seen increased attention due to their uses in catalysis and hydrogenation reactions. The mixed Al and Ga system with water has been researched, with the findings indicating that a boehmite structure of Al2O3·H2O exists up to the composition of approximately 70Al2O3·H2O, 30Ga2O3·H2O [Citation1]. A one-step precipitation reaction was followed to form mixed binary oxides of γ-Al2O3-α-Ga2O3 for catalysis applications [Citation2]. The characteristics and properties of mixed (AlxGa1−x)2O3 have been reported using precursor nitrate materials [Citation3]. A solvothermal reaction has been used to produce spinel structured γ-Ga2O3–Al2O3 mixed materials [Citation4].
The optical properties of these mixed oxide materials have, as of yet, not been extensively researched. Work in this field has focused mainly on the optical properties of mixed Al–Ga materials with various other metals. Examples of this are Co(Al1−xGax)2O4 [Citation5], Co3−xMxO4 (M = Al, Ga, In) [Citation6], ZnX2O4 (X = Al, Ga, In) [Citation7] and BaO–M2O3 (M = Ga, Al, In)–P2O3 glasses [Citation8]. The wide bandgap semiconductor GaOOH has recently been identified as a suitable material for the covalent attachment of the phosphonic group containing organic dye molecules [Citation9]. These composites are especially suitable for use in dye-sensitized solar cells (DSSCs) [Citation10]. It is hypothesized that by incorporating or substituting Al ions in GaOOH using varied concentrations, we can tune the optical properties, in particular the luminescent wavelength. This provides a unique advantage for DSSCs and other dye-based optoelectronics. Band matching of the inorganic semiconducting material with the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO) of the organic dyes could be done with added precision in conjunction with computational models. Another advantage to investigating AlOOH for this application is the increased stability seen in aluminum containing oxide materials when functionalized with organic molecules [Citation11].
In this study, aluminum nitrate nonahydrate and gallium nitrate hydrate precursors dissolved at various concentrations in DI water were titrated with sodium hydroxide and a previously reported hydrothermal microwave synthesis (GaOOH) was performed [Citation12]. This titration and hydrothermal reaction has been done with AlOOH using an autoclave in previous work [Citation13]. The advantages of using a microwave to replace this are numerous, including the ability to perform a reaction which could take as long as a day within a 20-min period. Corresponding ratios of mol:mol of Al:Ga were used to form the hypothesized compounds of AlOOH, Al0.8Ga0.2OOH, Al0.5Ga0.5OOH, Al0.2Ga0.8OOH and GaOOH. The Al-containing materials were more colloidal in nature as well as smaller in size than pure GaOOH, therefore, these solutions were centrifuged to remove solvent instead of just being filtered. We found it easier to increase the yield using centrifugation rather than filtration. The effects of Al incorporation and concentration on the material diameter were investigated with dynamic light scattering (DLS).
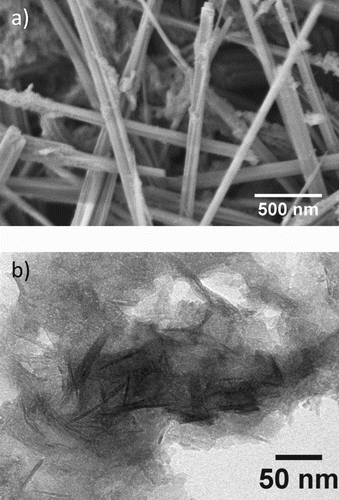
The size characteristics of the nanocrystalline materials are important considerations for optical properties. The optical bandgap of materials is heavily influenced by size at nanometer scales. DLS provides a diameter measurement based on a spherical particle, the size measurements were still enough to provide size comparison data for increasing Al concentrations. In conjunction with photoluminescence (PL) spectroscopy, these sizes can be used to explain any shifts in the emission wavelengths seen in the spectra which do not correspond to bandgap differences. The morphology of the GaOOH was investigated with SEM (Figure (a)). Microscopy measurements are a more reliable method of assessing the size of the synthesized materials. TEM was used to image the morphology of the hypothesized Al0.5Ga0.5OOH material (Figure (b)). The materials are observed to conform to rod-like morphologies, as expected from previous reports [Citation12,Citation13].
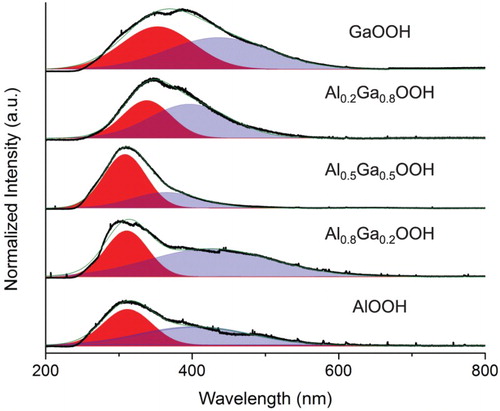
The underlying goal of the synthesis of these mixed oxides was to provide a level of tunability to the optical properties of the materials for enhanced band matching with organic dye molecules. To explore the possibility of using these mixed oxides for this, PL data were obtained. This provided specific information about the luminescent emission of these materials and how this was affected by the composition. A homemade PL setup was used with an excitation wavelength of 193 nm. The laser used was a Coherent Xantos XS Argon Fluoride Eximer laser and different lenses were used to collimate and focus the beam on the sedimented samples in quartz cuvettes. In Figure the PL spectrum of each of the five materials is shown going from top to bottom with increasing Al concentrations. Blue shifting is evident with increasing Al concentrations going from the high Ga concentrations to Al0.5Ga0.5OOH. The peaks present for each of the samples contained broad shoulders due to the expected high level of defects within the materials.
Peak fitting was done on individual spectra using Origin 8.0 to determine specific emission wavelengths. The main peaks of the hypothetical compounds AlOOH, Al0.8Ga0.2OOH, Al0.5Ga0.5OOH, Al0.2Ga0.8OOH and AlOOH were observed at 311.62, 306.39, 308.24, 337.94 and 352.85 nm, respectively. Blue shifting is seen from the high Ga concentration samples to the equal concentration samples and wavelength differences become negligible between peaks with added Al concentration. The band gap difference between AlOOH (4.51 eV) [Citation14] and GaOOH (4.75 eV) [Citation12] would suggest a red shift; however, the effects of size at nanometer scales must be accounted for. In this study, it is evident that size effects overcome the material bandgap considerations.
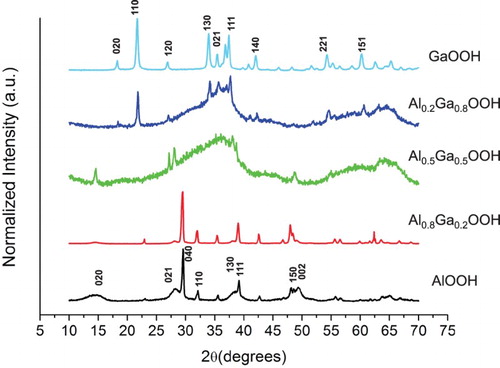
The crystallinity of the various compounds was investigated with X-ray diffraction (XRD) (Figure ). These data were obtained on a Rigaku SmartLab X-Ray Diffractometer. The spectra belonging to Al containing compounds indicated some leftover precursor material. The broad nature of the AlOOH peaks is indicative of nano-scale particles. At equal concentrations of Ga and Al, the nature of the peaks in the XRD spectrum suggests a possible amorphous region in these samples. This is observed in the TEM image of this sample (Figure (b)), which shows nanostructured materials embedded in an amorphous matrix. This is also observed in the theoretical Al0.2Ga0.8OOH sample. However, this sample contained well-resolved peaks associated with GaOOH. The well-resolved peaks from the GaOOH are consistent with the highly crystalline nanostructures seen in the SEM image (Figure (a)). It is possible that the amorphous nature of these samples is due to the incomplete substitution of Ga into the boemite structure of AlOOH. Some microcrystal peaks are found in both the Al0.2Ga0.8OOH and Al0.5Ga0.5OOH spectra, with the broad, amorphous peaks. The peaks found in the Al0.2Ga0.8OOH spectra can be attributed to GaOOH, whereas the peaks in the Al0.5Ga0.5OOH spectra are unique. It is expected that this material is likely mostly amorphous with a small amount of microcrystals within the matrix.
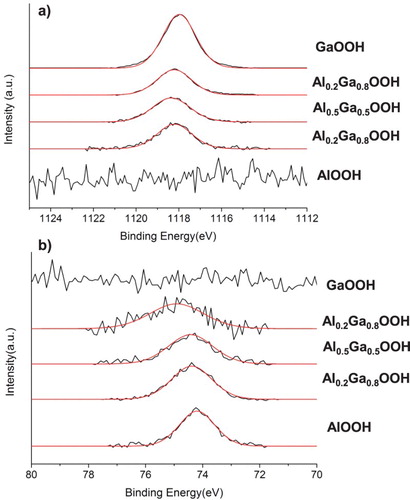
In order to obtain high-resolution atomic ratios of Al:Ga for each of the compounds, inductively coupled plasma-mass spectrometry (ICP-MS) was employed. The samples were prepared by dissolving less than 0.1 mg of each sample in a dilute nitric acid solution. A lab blank of just nitric acid was used as a control. The average Al:Ga ratio of the three mixed compounds is given in Table . The AlOOH and GaOOH sample contained no Ga and Al, respectively, which was confirmed by XPS (Figure ). The XPS data were obtained on a Kratos Analytical Axis Ultra XPS. Reaching exact values of molar ratios for Al and Ga in these samples is unlikely due to the inability to control the amount of unreacted precursors following the solution-based reaction. The Al:Ga ratio of Al0.8Ga0.2OOH, Al0.5Ga0.5OOH and Al0.2Ga0.8OOH were calculated to be 6.32 ± 0.72, 1.687 ± 0.050 and 0.48 ± 0.026, respectively. These are believed to be reasonable representations of the theoretical values of 4, 1 and 0.25. It is noted that each sample contained higher levels of Al than the theoretical values, suggesting the possibility that the AlOOH reaction left less unreacted precursor than the GaOOH reaction.
Table 1. The DLS measurements of the average diameter of the mixed oxides.
Figure 3. (a) Ga 2p high-resolution spectra and (b) Al 2p high-resolution spectra of the mixed oxides.
The AlxGa1−xOOH system was studied to determine its viability as an optically tunable inorganic for use in hybrid organic/inorganic optoelectronic devices. An example of this is DSSCs, where the efficiency of the device is directly impacted by the band matching between the HOMO of the organic dye and the conduction band of the inorganic material. Varied concentrations of precursor Al and Ga(NO3)3 were added and a microwave hydrothermal technique was used to synthesize nanostructured samples. Difference concentrations were used for this study in order to investigate the size differences measured with DLS based on the varied concentrations. In conjunction with these size differences and concentrations, PL data were taken which indicated a blue shift in the materials from GaOOH to Al0.5Ga0.5OOH. The emission wavelengths beyond this concentration of Al did not show a significant change. The XRD spectra of each of the mixed oxides provided crystallographic information about the materials. Based on the collected data, the method does not produce phase pure materials. These results indicate the possibility of tunability of the optical emission properties of this material system. This would prove to be valuable for a number of organic/inorganic hybrid materials for use in optoelectronics and bioelectronics. It would be of interest to investigate the properties of these materials as synthesized by conventional hydrothermal reaction to determine differences between the two synthetic routes. The results of these syntheses suggest some reproducibility; however, the concentration and crystallinity of the samples were uncontrolled (Table and Figure ).
Table 2. ICP-MS data of Al and Ga leaching of the mixed oxides.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hill VG, Roy R, Osborn EF. The system alumina gallia water. J Am Ceram Soc. 1952;35(6):135–142. doi: 10.1111/j.1151-2916.1952.tb13087.x
- de Leon JND. Binary gamma-Al2O3-alpha-Ga2O3 as supports of NiW catalysts for hydrocarbon sulfur removal. Appl Catal B-Environ. 2016;181:524–533. doi: 10.1016/j.apcatb.2015.08.028
- Escribano VS, Amores JMG, Lopez EF, et al. Solid state characterization of coprecipitated alumina-gallia mixed oxide powders. J Mater Sci. 2005;40(8):2013–2021. doi: 10.1007/s10853-005-1225-3
- Nakatani T, Watanabe T, Takahashi M, et al. Characterization of gamma-Ga2O3-Al2O3 prepared by solvothermal method and its performance for methane-SCR of NO. J Phys Chem A. 2009;113(25):7021–7029. doi: 10.1021/jp901569s
- Shakiba A, Jamison AC, Lee TR. Poly(L-lysine) interfaces via dual click reactions on surface-bound custom-designed dithiol adsorbates. Langmuir. 2015;31(22):6154–6163. doi: 10.1021/acs.langmuir.5b00877
- Newhouse PF, Parkinson BA. Combinatorial optimization of spinel Co3-xMxO4 M=(Al, Ga, In) alloyed thin films prepared by ink jet printing: photoelectrochemical, optical, and structural properties. J Mater Chem A. 2015;3(11):5901–5907. doi: 10.1039/C4TA05671J
- Karazhanov SZ, Ravindran P. Ab initio study of double Oxides ZnX2O4 (X=Al, Ga, In) having spinel structure. J Am Ceram Soc. 2010;93(10):3335–3341. doi: 10.1111/j.1551-2916.2010.03864.x
- Prasad S, Reddy MS, Kumar VR, et al. Specific features of photo and thermoluminescence of Tb3+ ions in BaO-M2O3 (M = Ga, Al, In)-P2O5 glasses. J Lumines. 2007;127(2):637–644. doi: 10.1016/j.jlumin.2007.03.011
- Pearce BL, Berg NG, Rahn MS, et al. In situ and ex situ functionalization of nanostructured gallium oxy-hydroxide with a porphyrin dye. Scanning. 2016. doi: 10.1002/sca.21315.
- Hagfeldt A, Boschloo G, Sun LC, et al. Dye-sensitized solar cells. Chem Rev. 2010;110(11):6595–6663. doi: 10.1021/cr900356p
- Vogelson CT, Koide Y, Alemany LB, et al. Inorganic-organic hybrid and composite resin materials using carboxylate-alumoxanes as functionalized cross-linking agents. Chem Mat. 2000;12(3):795–804. doi: 10.1021/cm990648e
- Sun M, Li DZ, Zhang WJ, et al. Rapid microwave hydrothermal synthesis of GaOOH nanorods with photocatalytic activity toward aromatic compounds. Nanotechnology. 2010;21(35):355601-1–355601-7. doi: 10.1088/0957-4484/21/35/355601
- Yang Q. The reaction conditions influence on hydrothermal synthesis of boehmite nanorods. Inorg Mater. 2010;46(9):953–958. doi: 10.1134/S0020168510090062
- Alemi A, Hosseinpour Z, Dolatyari M, et al. Boehmite (gamma-AlOOH) nanoparticles: Hydrothermal synthesis, characterization, pH-controlled morphologies, optical properties, and DFT calculations. Phys Status Solidi B-Basic Solid State Phys. 2012;249(6):1264–1270. doi: 10.1002/pssb.201147484