ABSTRACT
High-quality cubic yttrium iron garnet (YIG) nano powders have been rapidly synthesized by a chemical co-precipitation plus microwave heating method. The average particle size of the synthesized YIG nano powders were about 30 nm. The atoms in the particle were near completely orderly arranged by microwave activation, resulting in a higher saturation magnetization of 29.7 emu/g and a lower coercive field of 21 Oe compared with those of conventional heating treatment. This method provides a time-saving and easily controlled route for the synthesis of high-quality YIG nano materials in large-scale production.
GRAPHICAL ABSTRACT
IMPACT STATEMENT
We present a novel method to rapidly fabricate high-quality YIG nano powders with a small particle size and enhanced magnetic properties.
1. Introduction
Yttrium iron garnet (YIG) with the chemical formulation of Y3Fe5O12 is considered to be one of the important soft ferrimagnetic materials, which has been widely used in various magnetic devices, owing to its static and dynamic magnetic properties including its low ferromagnetic resonance linewidth, high electrical resistivity and low dielectric loss compared with all other ferrite materials over the lower end of the RF and microwave spectrum [Citation1–3]. With the rapid development and wide applications of YIG-related novel devices, YIG nano materials have attracted increasing attention [Citation1,Citation4,Citation5]. The high specific surface of YIG nanoparticles may alter the magnetic behaviors due to the anisotropy and spin disorder of the surface [Citation4] but gives opportunity to establish low-temperature sintering [Citation3,Citation6] or to design novel spintronic devices. Therefore, YIG nano powders with controllable particle size, high crystalline and low surface state are of great interest. It is no longer a very difficult problem to fabricate single-phase YIG nano powders with controllable particle size. Several chemical techniques are available for the fabrication of YIG nanoparticles, including hydrothermal [Citation7], chemical co-precipitation [Citation8,Citation9], sol–gel auto-combustion [Citation10] and micro-emulsion methods [Citation11]. However, it is a big problem to prepare YIG nano powders consisting of high-crystalline particles. Obviously, to improve the crystallization of YIG to make the atoms in the particle completely orderly arranged, high sintering temperature or long durations may be required. Nevertheless, conventional heating at a high temperature for long durations remains problematic. That may easily lead to the coarsening and sintering adhesion of the synthesized nanoparticles. The microwave technique is supposed to be a potential way to overcome this obstacle. Teiichi Kimura et al. used a special microwave with the frequency of 28 GHz to fabricate the YIG phase. The results indicated that the YIG phase with a particle size of several micro-meters started to form after only 70 s of irradiation [Citation12]. Although it was not easy to form the single-phase YIG powders, the microwave may activate the re-arrangement and promote the solid-state reaction. Therefore, we present a chemical co-precipitation plus the common industrial microwave heating method to establish the formation of the YIG phase with nano-sized and high-crystalline particles. Such a method may prove suitable for synthesizing high-crystalline YIG nano powders with fine magnetic properties at the industrial scale.
2. Material and methods
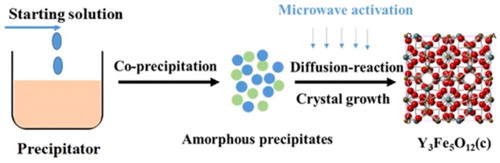
The procedure for microwave-accelerated rapid synthesis of high-quality YIG nano powders was divided into two steps: step 1: yttrium nitrate hexahydrate and ferric nitrate nonahydrate were used as starting materials, weighed according to the stoichiometric ratio and dissolved into deionized water to form an aqueous nitrate solution. An ammonia solution of a concentration of 28 wt% was chosen as the precipitator with the starting solution to ammonium volume ratios of 1:2. Under vigorous stirring, the starting solution was introduced into the ammonium solution and allowed to react for 1 h. Subsequently, the as-formed precipitates were collected from the solution by vacuum filtration followed by two rinsing operations with ethanol before drying at 60°C for 12 h. Afterwards, the dried precipitates were lightly ground to form the precursor powders. Step 2: the precursor powders were placed in a microwave material workstation (MobileLab Workstation, Tangshan Hugh Source Microwave Co.) and calcined at 800°C for 10 min with a temperature ramp rate of 80° C/min. The total processing time was about 50 min.
As a comparison, the precursor powders were also placed in a muffle furnace and calcined at 800°C for 2 h. The phase compositions of the as-synthesized composites were analyzed by the X-ray diffraction technique using CuKa radiation (XRD, Bruker D8 advance) at room temperature. The chemical bonding state of the synthesized powders was characterized by X-ray photoelectron spectroscopy (XPS, ThermoFisher Scientific, ESCALAB250Xi). The particle size and morphology were observed by TEM (FEI Tecnai G2 F30 S-TWIN) and their magnetic properties were measured using a vibrating sample magnetometer (ADE EV7 VSM).
3. Results and discussions
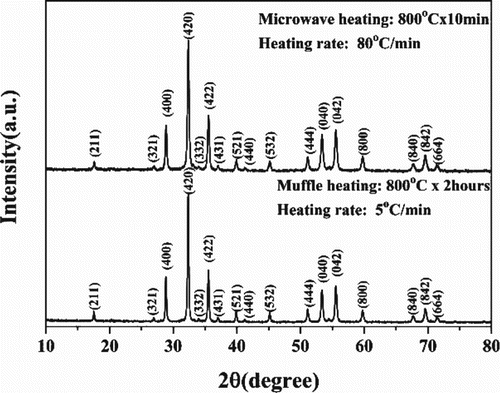
A representative XRD pattern of the as-synthesized powders is illustrated in Figure . All diffraction peaks are indexed to the cubic YIG phase without any obvious peaks from secondary impurity phases. A similar result is obtained from the sample processed by the muffle furnace heating approach that included a much longer soak time of 2 h at 800°C. The phenomenon of rapid phase formation by microwave heating allows for the acceleration of elemental diffusion in the precursor powders and activation of the solid-state reaction resulting in fast formation of the YIG phase while retaining a nanostructure. This is similar to that reported in the sol–gel auto-combustion of barium ferrite nano powders by Liu et al. [Citation13]. More systematic experiments are required to determine the activation energy to provide a clear interpretation of microwave-assisted processing—these studies are ongoing.
Figure 1. XRD patterns of processed powders by conventional heating treatment and microwave sintering with chemical co-precipitated precursor nano powders as starting materials.
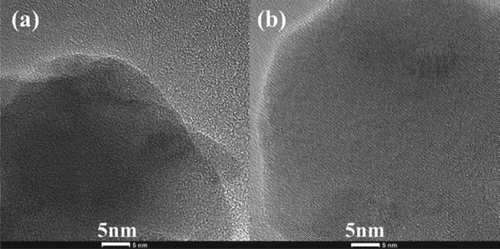
The TEM images of the as-synthesized YIG nano powders are shown in Figure . The particles are characterized by near spherical shape with the average particle size being about 30 nm. The agglomeration between small particles is due to the high specific surface tension and sintering adhesion. The individual YIG crystallites appear to have a good crystallinity due to their clear lattice fringes. Moreover, the HR-TEM images of the synthesized powders using conventional and microwave heating are demonstrated to identify the differences in the surface structures of the synthesized powders by using conventional and microwave heating (as shown in Figure ). The particles synthesized by the microwave heating approach crystallized well without the obvious disarrangement. Clear lattice fringes with a uniform direction were obtained, indicating the atoms in the particle were near completely orderly arranged by microwave activation. In comparison, there are surface layers consisting of small micro-domains on the particles synthesized by conventional muffle heating, which gave the lattice fringes the different directions. Therefore, it is reasonable to believe that the atoms in the particle synthesized by microwave heating are arranged more orderly than those in the particles synthesized by conventional muffle heating. Interestingly, the shorter treating time under microwave irradiation leads to better atomic arrangement especially in the surface layer of the nanoparticles, which indicates that the microwave activates the atomic diffusion besides the heating effect.
Figure 2. TEM images of powders processed by microwave sintering with chemical co-precipitated precursor powders as the starting materials.
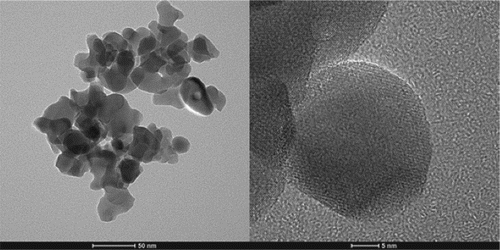
Figure 3. HR-TEM images of YIG powders synthesized by conventional heating (a) and microwave sintering (b).
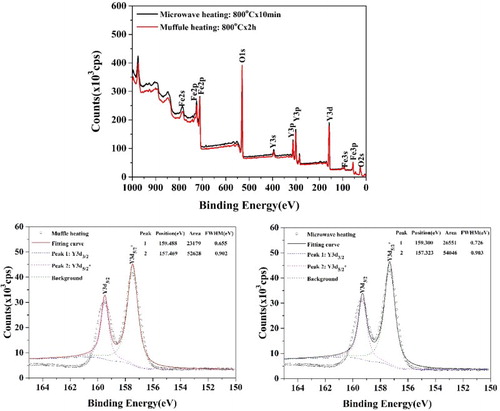
The atomic arrangement in the surface layers may result in the changes of the coordination polyhedrons of the YIG phase, which must be reflected in the valence of the atoms. Considering the ratio of the surface layer to be small in the particles, XPS was used as it is sensitive to the surface structure. The XPS curves of YIG nano powders synthesized by conventional heating and microwave sintering are given in Figure . The curve in wide range is quite similar for the high content of interior body phases in the particles. The slight differences should be ascribed to the atomic arrangements in surface layers. Focusing on Y3d, the hyperfine curves show the different valence states of Y3d in the two YIG nano powders from the slight peak shift and the relative intensity ratios of Y3d5/2* and Y3d3/2. This result indirectly confirms the conclusion from HR-TEM.
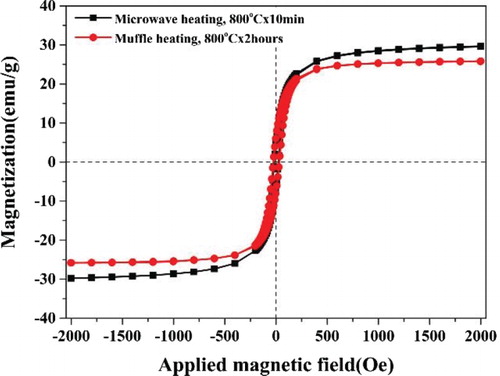
Figure illustrates the magnetic hysteresis loops of YIG nano powders synthesized by microwave (black) and muffle hearting (red) routes. Both loops demonstrate typical easy magnetic behaviors with small coercive fields (Hc). The saturation magnetization of the synthesized powders processed by microwave heating is about 29.7 emu/g and is much higher than that of powders synthesized by conventional muffle heating for longer durations, indicating the complete crystallization and atomic ordering of magnetic moments of the powders synthesized by microwave heating. Moreover, the coercive field of the synthesized powders by microwave heating is about 21 Oe and is markedly lower than that of the powders synthesized by conventional muffle heating (i.e. 30 Oe). We conjecture that the coercivity tracks linearly with increasing particle size for ferrimagnetic single domain particles and the critical size of YIG single domain particles is about 190 nm [Citation8,Citation14,Citation15]; therefore, the particle size of the powders synthesized by microwave heating should be smaller than that of those synthesized by muffle heating. Interestingly, the synthesized powders processed using microwave heating are of smaller particle size as well as resulting in superior magnetic properties in comparison to those synthesized by muffle heating. This seems to be contradicted. The saturation magnetization of nano powders generally decreases with a decrease in particle size, due to the higher surface to volume ratio in the smaller particles (contribution from the non-magnetic surface layer). This changing trend is in agreement with the hypothesis that the particle microstructures of the nano powders are similar. The saturation magnetization decreases with the ratios of the non-magnetic or weak-magnetic surface layer. Referring to the two kinds of the YIG nano powders synthesized by microwave heating and conventional muffle heating, they were quite different in particle microstructures especially in the surface layers. The atoms in surface layers of the YIG nanoparticles synthesized by microwave heating were near completely orderly arranged so that they approached a higher saturation magnetization even if the particle size was a little smaller than that of the nano powders synthesized by using conventional muffle heating.
4. Conclusion
Single-phase cubic YIG nano powders have been rapidly synthesized by microwave heating by using chemically precipitated precursor powders as the starting materials. Brief exposure to microwave radiation is shown to accelerate element diffusion and activate the solid-state reaction, resulting in the rapid formation of a pure YIG phase with the atoms in the particle near completely orderly arranged. The as-synthesized powders were characterized as having a smaller particle size of about 30 nm and superior magnetic properties when compared with those synthesized by conventional muffle heating. The microwave-accelerated method provides a time-saving and easily controlled route for the synthesis of YIG in large-scale production.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Collet M, de Milly X, d’Allivy Kelly O, et al. Generation of coherent spin-wave modes in yttrium iron garnet microdiscs by spin-orbit torque. Nat Commun. 2016;7:10377. doi: 10.1038/ncomms10377
- Zheng H, Zhou JJ, Deng JX, et al. Preparation of two-dimensional yttrium iron garnet magnonic crystal on porous silicon substrate. Mater Lett. 2014;123:181–183. doi: 10.1016/j.matlet.2014.03.006
- Jia N, Huaiwu Z, Li J, et al. Polycrystalline Bi substituted YIG ferrite processed via low temperature sintering. J Alloy Compd. 2017;695:931–936. doi: 10.1016/j.jallcom.2016.10.201
- Sharma V, Saha J, Patnaik S, et al. Synthesis and characterization of yttrium iron garnet (YIG) nanoparticles – microwave material. AIP Adv. 2017;7:056405. doi: 10.1063/1.4973199
- Sharma V, Kumari S, Kuanr BK. Bam/YIG nano-composites: a microwave material for C to U band application. AIP Adv. 2017;7:056417. doi: 10.1063/1.4974495
- Yang Q, Zhang H, Liu Y, et al. The magnetic and dielectric properties of microwave sintered yttrium iron garnet (YIG). Mater Lett. 2008;62:2647–2650. doi: 10.1016/j.matlet.2008.01.040
- Cho YS, Burdick VL, Amarakoon VRW, et al. Hydrothermal. Preparation and morphology characteristics of Y3Fe5O12. J Am Ceram Soc. 1997;80:1605–1608. doi: 10.1111/j.1151-2916.1997.tb03025.x
- Zhang W, Guo C, Ji R, et al. Low-temperature synthesis and microstructure-property study of single-phase yttrium iron garnet (YIG) nanocrystals via a rapid chemical coprecipitation. Mater Chem Phys. 2011;125:646–651. doi: 10.1016/j.matchemphys.2010.10.004
- Fernandez-Garcia L, Suarez M, Menendez JL. Synthesis of mono and multidomain YIG particles by chemical coprecipitation or ceramic procedure. J Alloy Compd. 2010;495:196–199. doi: 10.1016/j.jallcom.2010.01.119
- Hosseini Vajargah S, Madaah Hosseini HR, Nemati ZA. Preparation and characterization of yttrium iron garnet (YIG) nanocrystalline powders by auto-combustion of nitrate-citrate gel. J Alloy Compd. 2007;430:339–343. doi: 10.1016/j.jallcom.2006.05.023
- Vaqueiro P, Lpez-quintela MA, Rivas J. Synthesis of yttrium iron garnet nanoparticles via coprecipitation in microemulsion. J Mater Chem. 1997;7:501–504. doi: 10.1039/a605403j
- Kimura T, Takizawa H, Uheda K, et al. Microwave synthesis of yttrium iron garnet powder. J Am Ceram Soc. 1998;81:2961–2964. doi: 10.1111/j.1151-2916.1998.tb02720.x
- Liu J, Zeng Y, Guo C, et al. One-step synthesis of barium hexaferrite nano-powders via microwave-assisted sol–gel auto-combustion. J Euro Ceram Soc. 2010;30:993–997. doi: 10.1016/j.jeurceramsoc.2009.10.019
- Sánchez RD, Rivas J, Vaqueiro P, et al. Particle size effects on magnetic properties of yttrium iron garnets prepared by a sol-gel method. J Magn Magn Mater. 2002;247:92–98. doi: 10.1016/S0304-8853(02)00170-1
- Vaqueiro P, López-Quintela MA, Rivas J, et al. Annealing dependence of magnetic properties in nanostructured particles of yttrium iron garnet prepared by citrate gel process. J Magn Magn Mater. 1997;169:56–68. doi: 10.1016/S0304-8853(96)00728-7