?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Quenching studies of CrB-Al, CrB-Al-B and ß-FeB-Al mixtures revealed that all three undergo a topotactic transformation in the presence of Al to form Cr2AlB2, Cr3AlB4, and Fe2AlB2 MAB phases, respectively. The formation mechanism is intercalation for the Cr-based compounds, and reconstructive for the ß-FeB based mixture.
GRAPHICAL ABSTRACT
KEYWORDS:
Introduction
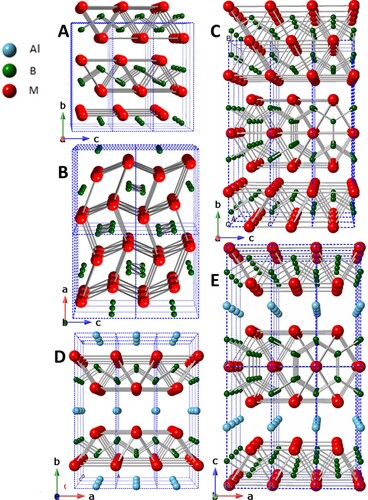
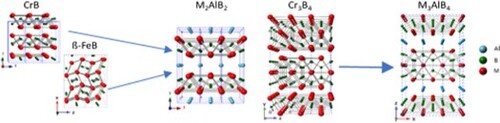
The MAB phases are a group of atomically laminated, transition metal borides (TMB), wherein M is a transition metal, A is typically Al, and B is boron. Their atomically laminated crystal structures are comprised of transition metal boride layers (M-B) interleaved with Al layer(s). Depending on the number of transition metal planes comprising the M-B layers and whether Al monolayers or bilayers separate them, the MAB phases take on the M2AlB2, M3AlB4, M4AlB6, M4AlB4 or M2Al2B2-type structures (Figure ) [Citation1]. One of the hallmarks of the MAB phases, and the leitmotiv of this work, is their close structural relationship to their binary borides. The germane binaries are the CrB, Cr3B4 and ß-FeB-type structures, the other boride structures are not relevant to MAB phase formation. The former crystallizes in the Cmcm space group wherein the B chains are aligned along the a-axis (Figure (A)). The latter crystallizes in the Pnma space group, where the MB layers are comprised of singly-capped trigonal prisms [Citation2] (Figure (B)). Furthermore, the two binaries are structurally related, as the ß-FeB-type structure can be obtained from the CrB-type by transposing the MB slabs of the latter by 1/2 < 001 > [Citation3]. The Cr3B4 binary crystallizes in the Immm space group and is shown in Figure (C).
Regardless of the structure type, the BM6 trigonal prisms making up the M-B layers strongly resemble, and in most cases are identical to, those found in their corresponding binary TMBs with the same M:B ratio [Citation4]. This is exemplified by comparing the crystal structures of CrB and Cr3B4 with those of M2AlB2 (Figure (D)) and M3AlB4 (Figure (E)), respectively.
A notable consequence of having isolated Al layer(s) is that they tend to be relatively more weakly bonded to the M-B layers than the strong covalent M-B bonds. This fact is demonstrated by their mechanical behavior, high-temperature thermal decomposition, and dissolution behavior in acids [Citation4–7]. For example, the MAB phases are softer than their corresponding binaries [Citation1]. At high temperatures, the Al atoms tend to de-intercalate and evaporate/sublimate from the MAB phases, precipitating the corresponding binary borides [Citation6–9]. In the case of MoAlB, the Al atoms react with ambient air at high temperatures to form well-adhered alumina layers that are protective in air up to 1300°C. The same compound can oxidatively self-heal cracks and has excellent ablation resistance even at oxyacetylene flame temperatures [Citation10,Citation11]. Zhou et al. observed that Al selectively dissolved from Cr2AlB2 particles at ambient temperatures when exposed to hydrochloric acid which in turn led to the precipitation of CrB particles [Citation12]. Similarly, Alameda et al. [Citation5] and Kim et al. [Citation13] found that only one of the Al layers in the Mo2Al2B2 crystal structure can be topochemically de-intercalated upon exposure to LiF and HCl solutions to from the metastable Mo2AlB2 phase, whose structure had previously only been observed as stacking faults in MoAlB grains.
In this work, topotactical reactions are paramount. By definition, topotaxy requires that one crystalline phase transforms into another with a well-defined crystallographic orientational relationship between them [Citation14]. Topotactic transformations can range from simple intercalation reactions to complex reconstructive ones [Citation15]. As indicated above, the M-B bonding in the crystal structures show virtually unchanged bond lengths relative to their binary counterparts. For example, it has been noted that the Cr2AlB2 crystal structure can be visualized as an intercalation of Al atoms into the CrB structure while causing each the Cr-B blocks in the latter to shift by 0.5 <a + c > along [101] [Citation16]. Combined with the absence of binary Cr-Al and Al-B phases after CrB + Al or CrB + Al + B mixtures were heated to synthesize Cr2AlB2 and Cr3AlB4, respectively [Citation17], we hypothesized that these MAB phases can form by a topotactic intercalation of Al atoms into CrB and Cr3B4, respectively [Citation16].
The purpose of this work was to understand how the binary borides transform into their respective MAB phases. Given their close structural resemblances, our working hypothesis was that there is a topotactic relationship exists between them. To test this hypothesis and to shed light on the reaction mechanisms, mixtures of CrB and Al or CrB, Al and B powder mixtures were heated and quenched from various temperatures. Using X-ray diffraction, XRD, we thus followed how the reactions developed at intermediate temperatures. Since Fe2AlB2 is isostructural with Cr2AlB2 and ß-FeB is considered a twinned, distorted form of CrB [Citation3], we carried out analogous experiments with ß-FeB and Al mixtures to assess the effect of precursor structure on the reaction paths. Herein we show that for both CrB cases, the Al simply intercalates in between the Cr-B blocks [Citation12] converting them to their MAB counterparts. However, in the FeB case, the Al first reacts with the Fe, forming an intermetallic before completing the reaction to form Fe2AlB2.
Methods
All samples for quenching were prepared via a powder metallurgical route starting with CrB (H.C. Starck, 1-2 μm average particle size), ß-FeB (See [Citation18], <44 µm), amorphous B (Alfa Aesar, 98%, <44 µm) and Al (Alfa Aesar, 99.5%, <44 µm) in molar ratios of 2CrB:1.5Al, 3CrB:1B:1.5Al, or 2FeB:1.5Al. The large excess of Al was used to facilitate a more uniform reaction at the lower temperatures. The mixtures were mechanically ball-milled at 60 rpm in 8 g batches for 24 h in a plastic jar using an equal mass of 5 mm diameter yttria-stabilized zirconia media. Green bodies were cold pressed at a load corresponding to a stress of 200 MPa into 2 g pellets, with a diameter of 13 mm and a height of ≈ 10 mm. Pellets were heated one at a time in a horizontal tube furnace under a flowing Ar atmosphere. The pellets were heated at a heating rate of 5°C/min to the temperatures indicated in Table , and allowed to equilibrate for 3–5 min before quenching directly into distilled water at ambient temperature. The quenching temperatures were chosen to evaluate the status of the reaction as it progressed from reactants to the final products. The lowest temperature, 680°C, was chosen to evaluate the beginning of the liquid phase reaction between Al and the transition metal borides. The maximum temperature reflects typical reaction temperatures used for the synthesis of various MAB phase [Citation1,Citation16,Citation17]. Lastly, one sample of each composition was reacted at the highest temperatures used for an extended time (see Table ) in an attempt to achieve equilibrium and/or a fully reacted state.
Table 1. Initial compositions and quenching temperatures.
The surfaces of all samples were lightly sanded to remove any surface oxides and drilled using a cobalt steel bit to produce a fine powder. This powder was characterized by using a X-ray diffractometer (Rigaku SmartLab) with a Cu Kα source (λ=0.0154 nm) and a detector-side graphite monochromator. The quantitative analysis was performed using MDI Jade 6.5 with the Easy Quant package using the RIR method [Citation19].
Results & discussion
Cr2AlB2
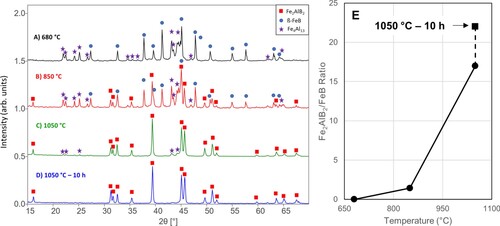
XRD patterns of the quenched samples, starting with CrB and 1.5 Al to produce Cr2AlB2, are shown in Figure . Quenching from 680°C, there is no evidence of the formation of the MAB phase. This is not too surprising since this temperature is just over Al's melting point and had insufficient time to react with the CrB (Figure A). Quenching from 850°C, a large quantity of Cr2AlB2 forms, though unreacted CrB and Al still remain in the sample (Figure B). Quenching from 970 °C shows the continued consumption of the CrB and Al precursors, as shown by the decreased relative intensities of the CrB peaks relative to the 850°C quench (Figure C). After reacting at 970°C for 15 h, a further reduction of the CrB peak intensities indicate that the Al was able to further intercalate between the CrB layers (Figure D). The ratio of intensities between the (130) peak of Cr2AlB2 and the (021) peak of CrB shows the evolution of the MAB phase with increasing temperatures and after reacting for 15 h (Figure E). Based on these results, it is reasonable to conclude that the following single-step reaction is operative:
(1)
(1)
Figure 2. XRD patterns for 2 CrB + 1.5 Al quenched from, (A) 680°C, (B) 820°C, and (C) 970°C. For reference, (D) 970°C for 15 h. (E) The ratio of Cr2AlB2 to CrB over the range of quenching temperatures and after a 15 h at 970°C.
The Al present after 15 h at 970°C is simply due to the large molar excess of Al present in the initial reagent mixture. Note that this result implies that Cr2AlB2 is in most probably in equilibrium with molten Al.
Cr3AlB4
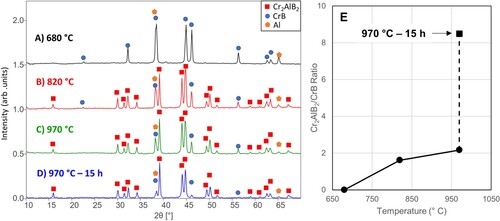
XRD patterns of the 3 CrB, 1.5 Al and 1.0 B mixture after quenching from different temperatures and after holding at the maximum temperatures for 10 h are shown in Figure . XRD of the samples evidences a three-step evolution. First, the elemental B reacts with CrB to form CrB2, followed by the formation of Cr3B4 at higher temperatures. The last reaction is one with Al to form Cr3AlB4. At 680°C, there is some indication that CrB2 has started to form, but a majority of the CrB and all the Al remains unreacted (Figure A). It should be noted that B is not detected by XRD because it remains amorphous at temperatures below 1100°C [Citation20]. At 750°C, the CrB reacts further with the B forming more CrB2 (Figure B). However, no peaks corresponding to Cr3AlB4 or Cr2AlB2 are present in the pattern, despite the fact that Cr2AlB2 showed signs of formation between 680–820°C in Figure . Once the sample reaches 820°C, the CrB and CrB2 react and form Cr3B4, which in turn reacts with Al and the formation of Cr3AlB4 begins (Figure C). At this temperature, Cr3B4 and Cr3AlB4 are the majority phases present, with some CrB and Cr2AlB2 impurities present. At 1020°C, only small amounts of CrB and Cr3B4 impurities exist with almost all the binary borides reacted to form Cr3AlB4 (Figure D).
Figure 3. XRD patterns of 3 CrB + 1.5 Al + 1 B after quenching from (A) 680°C, (B) 750°C, (C) 820°C, and (D) 1020°C. For reference, (E) 1020°C for 10 h. (F) The ratio of Cr3AlB4 to CrB over the range of quenching temperatures and after 10 h at 1020°C
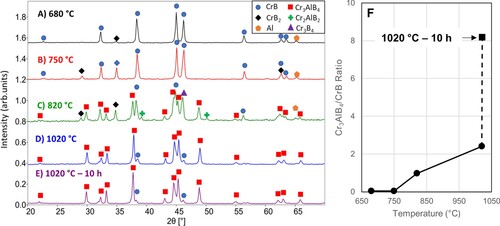
After reacting for 10 h, there is a negligible amount of CrB present, yet Cr3B4 remains at the same levels (Figure E). As the reaction temperature increases, the ratio of relative intensities of the (102) peak of Cr3AlB4 to the (021) peak of CrB illustrates the progression of the reaction (Figure F). Between 820°C and 1020°C, the Cr3AlB4:CrB ratio more than doubles, and after reacting for 10 h there is a fourfold increase in Cr3AlB4. The near absence of Cr2AlB2 at higher temperatures implies that it is most likely an impurity phase, not a step along the reaction pathway. Once the Cr3B4 has formed, the Al intercalates between the layers forming the 314 MAB phase structure. This process can be represented by the following reactions:
(2)
(2)
Fe2AlB2
Unlike the Cr based MABs, the Fe2AlB2 XRD patterns show a different reaction mechanism, as the Al does not directly intercalate into the FeB structure (Figure ). Even at 680°C, the Al reacts with the ß-FeB to form the intermetallic Fe4Al13 (Figure A). This low temperature formation of Fe4Al13 is described by Li et al. [Citation21] as taking place at temperatures as low as 650°C and would result in the formation of free B. As in the Cr3AlB4, this free B required to mass balance the reaction (see Equation 3) is not detected by XRD due to its amorphous nature. At 850°C the reaction shifts to the third step as the ß-FeB peak intensities decrease, and Fe2AlB2 begins to form (Figure B). However, the intensities of the intermetallic peaks remain constant over this temperature range. At 1050°C, all of the ß-FeB has reacted, forming a majority phase of Fe2AlB2 with some remnant intermetallic impurities (Figure C). After reacting for 10 h, there is no discernible presence of the intermetallic phase as the reaction goes to completion (Figure D). This reaction is sequence can be represented by the equations:
(3)
(3)
Discussion
Based on these results, the formation of both Cr2AlB2 and Cr3AlB4 both seem to be a product of a high-temperature intercalation of Al atoms between atomic layers of CrB and Cr3B4, respectively. In this reaction, the CrB and Cr3B4 structures were retained in each M-B layer during this process, as noted by the lack of additional phases.
As discussed previously, a topotactic transformation is solely concerned with the relationship between the initial and final crystal structure. Though the formation of Fe2AlB2 from FeB and Al does not follow the same classic intercalation mechanism as occurs during the formation of Cr2AlB2 and Cr3AlB4, it still undergoes a topotactic transformation. The ß-FeB structure can be considered to be a deformed CrB structure and is therefore related to the final Fe2AlB2 structure. The decomposition of the ß-FeB and subsequent intermediate formation of the Fe3Al14 intermetallic are steps along the reaction pathway.
Summary and conclusions
To investigate the formation mechanisms of Cr2AlB2, Cr3AlB4, and Fe2AlB2, high temperature quenching experiments were carried out. The Cr2AlB2 reaction path was single step as the Al began reacting with the CrB to form the Cr2AlB2 between 680°C and 820°C. The Cr3AlB4 reaction path was three steps: formation of CrB2 from CrB and B, formation of Cr3B4 from CrB and CrB2, and finally the reaction with Al between 750°C and 820°C. Both systems undergo a similar topotactic transformation, as the Cr-B structure is retained after the Al reaction. The final products of the reactions are dependent on the initial stoichiometry, as no Cr3AlB4 formed from 2CrB + 1.5Al case and an insignificant amount of Cr2AlB2 formed when the initial mixture was 3CrB + 1B + 1.5Al.
Unlike the Cr based MABs, the Al did not simply intercalate between the binary boride layers; the system underwent a reconstructive reaction instead. The Al first reacted with the Fe to form an intermetallic at low temperatures. As the temperature increased, the ß-FeB and intermetallic reacted to form Fe2AlB2.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kota S, Sokol M, Barsoum MW. A progress report on the MAB phases: atomically laminated, ternary transition metal borides. Int Mater Rev. 2019;65(4):226–255.
- Akopov G, Yeung MT, Kaner RB. Rediscovering the crystal Chemistry of borides. Adv Mater. 2017 Jun;29(21):1604506.
- Shirae K, Ueda Y, Kachi S, et al. Phase diagram and magnetovolume effect of pseudo-binary CrB-FeB system. Mater Res Bull. 1987;22(4):521–526.
- Ade M, Hillebrecht H. Ternary Borides cr2alb2, Cr3AlB4, and Cr4AlB6: The first members of the series (CrB2)nCrAl with n = 1, 2, 3 and a unifying concept for Ternary Borides as MAB-phases. Inorg Chem. 2015 Jul 6;54(13):6122–6135.
- Alameda LT, Moradifar P, Metzger ZP, et al. Topochemical deintercalation of Al from MoAlB: stepwise etching pathway, layered intergrowth structures, and two-dimensional MBene. J Am Chem Soc. 2018;140(28):8833–8840.
- Kota S, Agne M, Zapata-Solvas E, et al. Elastic properties, thermal stability, and thermodynamic parameters of MoAlB. Physical Review B. 2017;95(14):144108.
- Levin EM, Jensen BA, Barua R, et al. Effects of Al content and annealing on the phases formation, lattice parameters, and magnetization of AlxFe2B2(x=1.0,1.1,1.2) alloys. Physical Review Materials. 2018;2(3):034403.
- Kota S, Chen Y, Wang J, et al. Synthesis and characterization of the atomic laminate Mn2AlB2. J Eur Ceram Soc. 2018;38(16):5333–5340.
- Liu J, Li S, Yao B, et al. Thermal stability and thermal shock resistance of Fe2AlB2. Ceram Int. 2018;44(13):16035–16039.
- Bei G, van der Zwaag S, Kota S, et al. Ultra-high temperature ablation behavior of MoAlB ceramics under an oxyacetylene flame. J Eur Ceram Soc. 2019;39(6):2010–2017.
- Lu X, Li S, Zhang W, et al. Crack healing behavior of a MAB phase: MoAlB. J Eur Ceram Soc. 2019;39(14):4023–4028.
- Zhang H, Dai F-Z, Xiang H, et al. Phase pure and well crystalline Cr2AlB2: a key precursor for two-dimensional CrB. J Mater Sci Technol. 2019;35(8):1593–1600.
- Kim K, Chen C, Nishio-Hamane D, et al. Topochemical synthesis of phase-pure Mo2AlB2 through staging mechanism. Chem Commun (Camb. 2019 Aug 14;55(63):9295–9298.
- Bailey SW. Report of the IMA-IU Cr. joint committee on nomenclature. Am Mineral. 1977;62(5-6):411–415.
- Figlarz M, Gerand B, Delahaye-Vidal A, et al. Topotaxy, nucleation and growth. Solid State Ionics. 1990;43:143–170.
- Lu J, Kota S, Barsoum MW, et al. Atomic structure and lattice defects in nanolaminated ternary transition metal borides. Materials Research Letters. 2016;5(4):235–241.
- Kota S, Wang W, Lu J, et al. Magnetic properties of Cr2AlB2, Cr3AlB4, and CrB powders. J Alloys Compd. 2018;767:474–482.
- Hanner LA. Quaternary Solid solutions in the (M1-x M’x)2 AlB2 and (Mo1-x M’x) AlB systems. Drexel University; 2020.
- Hubbard CR, Snyder RL. RIR-measurement and use in quantitative XRD. Powder Diffr. 1988;3(2):74–77.
- Shalamberidze S, Kalandadze G, Khulelidze D, et al. Production of α-rhombohedral boron by amorphous boron crystallization. J Solid State Chem. 2000;154(1):199–203.
- Li X, Scherf A, Heilmaier M, et al. The Al-rich part of the Fe-Al phase diagram. J Phase Equilib Diffus. 2016;37(2):162–173.