?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
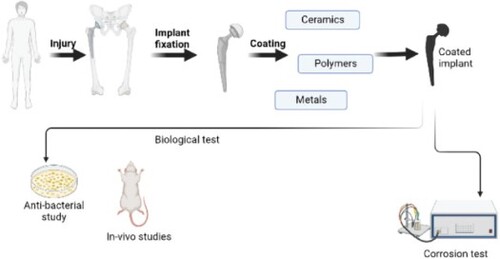
Multifarious materials are used in the biomedical domain to relieve the distress of patients due to underlying diseases or injuries by replacing or augmenting any tissues or organs. Specific metallic substrates used as implants have the probability of failure due to corrosion resulting from direct contact with body fluid. Therefore, we aim to conduct a thorough review of numerous coatings currently available to prevent the implants from corroding. The coatings that are fabricated by various techniques are discussed for their effects on mechanical behaviour. It was deduced that the mechanical behaviour relied on the microstructure of the coating surface. Different surface treatment techniques of coatings offered different microstructures to the coatings. We meticulously review the mechanical and biological behaviour of coatings and this comprehensive literature review serves as evidence that the effective fabrication of an ideal coating has remained elusive for researchers, motivating an ongoing endeavour to attain this goal.
IMPACT STATEMENT We meticulously review the mechanical and biological behaviour of coatings and this comprehensive literature review serves as evidence that the effective fabrication of an ideal coating along with discussions on biological characteristics shown by composites.
Abbreviations: ALP, alkaline phosphatase activity, bFGF, basic fibroblast growth factor, CHAp, Carbonated Hap, CNT, carbon nanotubes, Ecorr, corrosion potential, ECM, extracellular matrix, EIS, Electrochemical Impedance Spectroscopy, EPD, electrophoretic deposition technique, F-Hap, fluorinated hydroxyapatite, f-MWCNT, functionalized multi-walled carbon nanotubes, GO, Graphene Oxide, Hap, hydroxyapatite, HAp/PE, hydroxyapatite/polyethylene, HOS, human osteosarcoma cells, Icorr, corrosion current, MWCNT, Multi-walled carbon nanotubes, PEO, plasma electrolyte oxidation, PLGA, poly(lactic-co-glycolic acid), PMMA, Poly(methyl methacrylate), PSZ, partially stabilized zirconia, rGO, reduced graphene oxide, SBF, Simulating body fluid, SWCNT, Single-walled carbon nanotubes, TCP, Tri calcium phosphate, TZP, tetragonal zirconia polycrystal, YSZ, Yttria-stabilized Zirconia, ZTA, zirconia toughened alumina
GRAPHICAL ABSTRACT
1. Introduction
Biomaterials are substances that are introduced to a living body to supplant an organ or improve the efficiency of bodily function. They have been a topic of research starting from the early 1900s. They were of utmost significance since they aided humankind by relieving the distress caused by ailments and injuries. The very first structured definition for biomaterials was given by Williams [Citation1] as ‘a non-viable material used in medical devices, intended to interact with biological systems’. The adaptations in the definition for biomaterials are given by Williams [Citation2] and the definition put forward in 1999 persists for biomaterials, keeping in mind that the definitions are all related to the health and biomedical domain. The evolution of biomaterials brings forth an idea of how natural bones were first substituted using first-generation materials which were inert and eventually evolved for the betterment of healthcare. Firstly, being used in hard tissue replacements, and finally developing scaffolds for tissue engineering involving soft tissues, drastic progress was made. Alumina was first used in the 1960s as orthopaedic implants for load-bearing applications. Hydroxyapatite was later developed as a bone substituent. Hydroxyapatite-Polyethylene (HAp-PE) composite was used as a hip and middle ear implant in the 1980s [Citation3,Citation4]. From thereon, the researchers made every effort to fabricate an ideal biomaterial and are still engrossed in the process of developing one. Biocompatibility, non-immunogenicity, non-cytotoxicity, non-genotoxicity, non-corrosive nature, adequate mechanical strength, etc. are the basic attributes presumed to be shown by biomaterials. Biocompatibility is not just a mere property of a biomaterial but an interaction that occurs between an implant and the surrounding cells or tissues. Biomaterials could be categorized as metals, polymers, ceramics and composites. When metallic implants are used alone, despite providing high mechanical strength, there are chances of toxicity or bacterial infections that might occur. Later polymers emerged as a result of the quest for a better material. The analogy shown towards macromolecules, the least toxicity and adequate applications in permanent prostheses and tissue engineering made biopolymers a relevant material in the biomaterial domain [Citation5]. Although they show good biocompatibility, they do not provide great wear and tear resistance and have comparatively lower mechanical attributes. Hence researchers were forced to bring to light a new alternative for long-term applications in the biomedical field. Coatings over implants are considered one of the alternatives to surpass the difficulties in biomedical applications. Coatings were expected to provide corrosion resistance, wear and tear resistance, non-inflammatory actions, non-cytotoxicity, osseointegration enhancement, etc. An inorganic polycrystalline material has gained so much attention in the biomaterial field due to its numerous contributions to biomedical fields. The hard and brittle, corrosion-resistant non-metallic material that could alter shape on heating is the so-called ceramics. The word ‘ceramics’ was derived from the Greek language ‘keramicos’ which meant pottery. The wide range of ceramics in different disciplines such as electronics, mechanical engineering, aerospace, dentistry, etc. is paving the way for the alarmingly increasing interest in ceramics in biomaterials. Ceramics have remained a material of curiosity since ancient times. Modern medicine now depends on advanced ceramics due to their higher utilization in dental implants, orthopaedic implants, prosthetic joints and many more. The unique electrical properties, optical properties, long-lasting ability, chemical inertness, great strength and mechanical properties are the cause of attention towards advanced ceramics. On top of that, they gained attention in the biomaterial domain as a therapeutic or diagnostic one.
Ceramics are basically of three types: bioinert, bioactive and biodegradable. Bio-inert ceramics are non-absorbable and have abilities to retain their composition for the long term and show the least interface reactions between the host and the coating due to the formation of a non-adherent fibre surrounding the implant. They could be defined as materials that induce the least responses in the host [Citation6]. Most of the commonly known ceramics fall under this category including Alumina, Zirconia, Pyrolytic Carbons, Sintered hydroxyapatite, etc. [Citation7]. They find applications in hip and knee replacements, certain joints and dental implants. To avoid the limitations that occurred due to osseointegration, various surface modifications were carried out and finally, biomimetic apatite coatings were also introduced. Bioactive ceramics, another class of ceramics, displays surface reactivity due to the origin of bone bonding with the living tissue and the implant material. The introduction of bioactive ceramics to the field was a remedy to avoid the setback caused by bio-inert materials allied to fibrous encapsulation. They take advantage of the bone tissue’s cellular composition and are associated with osteoconduction. A few of them include bio-glasses, glass-ceramics, etc. [Citation8]. Bioactive ceramics are proven to repair soft tissues and regenerate skeletal tissues which owe to the bioactive nature and hence forming interfacial bonds with host tissues. 45S5 Bio-glass was quite successful as a middle ear implant [Citation9]. Biodegradable ceramics also known as bioresorbable are capable of undergoing gradual hydrolytic breakdown in the body and simultaneously healed by the regeneration process. The merit is that the degradation product will be either metabolized by the body or eliminated by the body safely. The degradation mechanism is presumed to occur when material degrades eventually and is replaced by host cells due to cellular degradation which could take place through either phagocytosis or extracellular acidification. Since they have great abilities of resorbing, they find applications in skull restorations, ligament restorations and many more [Citation10]. The porous structure that results in osteoconduction and the resorbable rate is advantageous whereas the disadvantages are the poor tensile strength due to which it cannot be used as load-bearing implants. Examples include Tri calcium phosphates (TCP), Hydroxyapatite, Calcium phosphate with Fe2O3, etc. [Citation11]. Distinct ceramics show distinct mechanical behaviours owing to their physical and chemical properties, structures and phases. Bioinert materials like Zirconia toughened ceramics possess good mechanical strength and hence are used in knee and hip joints and disk replacements. The tetragonal phase and its metastable nature account for the toughening displayed by Yttria-stabilized Zirconia (YSZ) [Citation6]. Bioactive ceramics, on the other hand, can be modified desirably to obtain soft or hard materials depending upon their mechanical strength. Apatite formation is the key for these materials to show bioactivity. Titanium metal and its alloys on providing certain treatments at specific conditions form apatite layers and eventually trail to bone-bonding. Combining a material with an organic or polymeric compound would often lead to better mechanical characteristics that are similar to those of human bones [Citation12]. Conversely, the mechanical behaviour of biodegradable ceramics is inferior to other ceramics since they display lesser tensile strength and brittleness owing to their degradation factors [Citation11]. The biological characteristics are also affected by distinct materials accounting for their in vitro bioactivity. Apart from the physical and chemical attributes that affect the bioactivity, the synthesis methods, precursors used, quantity and quality of dopant also have a net effect on the bioactivity. The incorporation of various dopants in hydroxyapatite through a single fabrication technique imparted different properties to the parent material. Different dopant ions at different concentrations confer distinct characteristics on the material. If one element might impart better osseointegration properties, the other might provide enhanced hardness or tensile strength. Hence the dopant to be used is completely dependent upon the necessity of the patient as to which property needs to be given importance [Citation13]. Bioactive glasses have also been revealed to have excellent tissue regeneration abilities and bone-bonding capabilities. However, their fast degradation rate paved the way for doping them with different elements and improving their osteogenic properties along with better bioactivity [Citation14].
The ceramics or composites are used as coatings over implants to supplement certain existing properties and also to reduce the detrimental effects caused by bare implants due to exposure to the biological medium. Henceforward, we contemplate ceramic coatings and their attributes. The mechanical properties of the coatings, which are already evaluated by a few authors, are reviewed and an interrelation between the ceramic coating technique and mechanical parameters is established. Various parameters, such as adhesion strength, bending strength, Young’s modulus, hardness and toughness of coating, fatigue resistance, etc., are studied. Adhesion strength is the ability of the coating to stick onto the implant surface without peeling off. Young’s modulus that determines the resistance towards deformation of a coating, hardness which is the strength of the coating to resist breakage and bending strength which is the resistance the materials possess until the fracture point, etc. are further reviewed. Several ceramics are available as coatings for orthopaedic joints and additional purposes. Fig. shows the various ceramic-coated orthopaedic hip joints.
Figure 1. (a) Alumina-coated dental implants [Citation15], (b) Hydroxyapatite-coated hip prosthesis [Citation16], (c) Bio-glass coated Titanium dental implant [Citation17] and (d) Plasma-sprayed ceramic coated acetabular cups [Citation18].
![Figure 1. (a) Alumina-coated dental implants [Citation15], (b) Hydroxyapatite-coated hip prosthesis [Citation16], (c) Bio-glass coated Titanium dental implant [Citation17] and (d) Plasma-sprayed ceramic coated acetabular cups [Citation18].](/cms/asset/862fe500-aaba-458c-b92c-04804108a2a7/tmrl_a_2250110_f0001_ob.jpg)
Corrosion resistance studies are also reviewed for observing any valid relation between the same and mechanical properties. Besides the mechanical behaviour studies and corrosion studies, the biological studies, which include in vitro and in vivo studies, stem cell studies and tissue regeneration studies, are reviewed for an in-depth understanding of coatings. Since each coating exhibits different characteristics, the coating to be chosen for a particular application needs to be very specific. On that account, excessive studies on mechanical behaviour alone cannot be considered for a coating to be chosen as the finest. The biocompatibility, the osseointegration, the new-bone-formations, etc. also need to be studied meticulously.
To the best of our knowledge, there is no state-of-the-art literature that interrelates the mechanical and biological properties of different fabrication techniques of coatings. We meticulously examined various ceramic coatings, composites and ion doping, while critically assessing their respective coating techniques in this comprehensive review paper, with the ultimate objective of elucidating the intricate correlations between the mechanical behaviour and the application of these innovative surface modification techniques. Apart from the mechanical evaluation, biological evaluation studies are also reviewed for a thorough understanding of the coatings.
2. Ceramic coatings
2.1. Hydroxyapatite
Hydroxyapatite (HAp) embraces a lot of advantages such as providing amplified adhesion strength along with aiding osteoconduction, osseointegration and improved biocompatibility [Citation19]. It does not exhibit very great load-bearing capability due to its mediocre mechanical properties like hardness and toughness. A vast range of studies was carried out on HAp coatings to learn more about their mechanical attributes, biocompatibility, microstructure, corrosion behaviour and the possibilities of improving their mechanical properties. Figure shows hydroxyapatite-coated acetabular cups for orthopaedic applications. He et al. [Citation20] explained the role of porosity and the microstructure on the mechanical properties exhibited by sintered HAp. The porous microstructure of the sintered HAp could mimic the natural bone structure extremely well and proved that the dependence of porosity was very significant on mechanical behaviour and showed that HAp resembles the elastic modulus of bone. Apart from these findings, they also figured out that porosity and hardening were not directly related, but the creep ability and the energy absorbance were related directly to the porosity. Obada et al. [Citation21] had something to showcase related to the effect of the sintering temperature of natural HAp. The grain size, the pore shape and the microstructure are criteria that determine the mechanical behaviour of the coating. While carrying out sintering, observations were made that the grain size was reduced which, in turn, improved the mechanical properties. Increased sintering temperature, hardness values and crystal structure are correlated to mechanical characteristics. Lacefield [Citation22] documented clearly the different coating methods of HAp on different metals in 1988. Sufficient adherence to implant and the least expense is an advantage. The criteria selected for the fabrication methods should ensure that HAp should not irreversibly alter the structural composition and the mechanical properties related to the implants. Among the four techniques such as dip coating-sintering, Immersion coating, Hot Isocratic Process and Sputter coating, the latter was found to be effective by Lacefield. Aarthy et al. [Citation23] explain the effect of sintering temperatures on mechanical and biological characteristics of natural HAp, obtained from goat bones. HAp is subjected to different sintering temperatures and 1300°C was observed to be the optimum temperature that helps in apatite formation. Biological evaluation studies show hemocompatibility with improved apatite growth at the optimal sintering temperature. Recently, substituted HAp has gained profound attention due to the structure and composition that permits ionic substitutions which, in turn, imparts a new quality or additional benefits such as osteointegration, bioactivity or anti-microbial effect. The structure of hydroxyapatite makes the substitutions occur unhindered through substitutive and interstitial mechanisms in both cationic and anionic sites by existing synthesizing techniques which are critically reviewed by Arcos et al. [Citation24]. Lately, advancements are made in coatings as they are grabbing intense attention. Metallic biomaterials which can offer good mechanical strength but not excellent bioactivity can be coated with HAp. The adhesion between the implant-coating surface needs to be taken care of, which arises due to the presence of attractive chemical forces [Citation25]. HAp over Mg alloy was a great innovation since it imparted great uses in the biomedical industry. Due to the degradable nature of Mg, HAp was coated to obtain implants with better bioactivity and the least corrosion. It was later found that instead of coating HAp alone, the incorporation of polymeric additives and substituted HAp like Fluorinated Hydroxyapatites (F-HAp) found more applications in these sectors [Citation26]. Nano-indentation studies of HAp over Mg alloy showed hardness, toughness and wear resistance. Lemoine et al. [Citation27] also found out that annealing at lower temperatures does not affect the coating adversely. Adequate research has been dedicated to hydroxyapatite coatings and has yielded notable advancements, driving the field forward.
Figure 2. Hydroxyapatite-coated acetabular cups for orthopaedic application [Citation28].
![Figure 2. Hydroxyapatite-coated acetabular cups for orthopaedic application [Citation28].](/cms/asset/bde5802d-a42b-41aa-b766-7a625decf700/tmrl_a_2250110_f0002_ob.jpg)
2. 2. Alumina
Alumina (Al2O3) is advantageous for its low cost of production, great mechanical strength, stiffness and hardness, wear resistance and adhesion properties. Alumina was the first ceramic to be used as an implant in orthopaedics. Fatigue resistance and fracture toughness are noteworthy among their properties. The low pore size of materials would increase the elastic modulus and henceforth the mechanical strength [Citation29]. Their mechanical properties are observed using various techniques such as dynamic fatigue, stress relaxation, internal friction measurement, etc. [Citation30]. Moreover, the bending strength increases with increased purity. Purity is the most important criterion for determining the characteristic of mechanical hardness and strength of Alumina. Figure represents the Alumina-coated dental implants. Tallon et al. [Citation29] confirmed that the mechanical behaviour improved with highly porous alumina coating synthesized by the gel-casting method. The elastic moduli were assessed by the authors to portray that lesser pore size and porosity manifest higher elastic moduli. The tensile and compressive strengths come around 5 and 16 MPa for those with larger porosities and those with lower porosities gave values of 12 and 57 MPa, respectively. Apart from the values evaluated, a mentionable finding was that the sintered samples showcased mechanical properties, at least twice more than the raw samples. Hou et al. [Citation31] put forward the findings that alumina coatings on Al alloys can be carried out by plasma-arc heat treatment which tends to improve the interfacial bonding and wear resistance. Conventionally coated Al2O3 has poor cohesive and adhesive strength. However, the research revealed that plasma spraying could enhance the interfacial bonding of Al2O3 coatings. This accounts for good mechanical characteristics and wear resistance of Al2O3 on Al alloys which were believed to have fewer wear properties. Alumina was added to CoCr alloy to be used as an orthopaedic implant due to its wear-resistant nature. It was showcased that the material exhibited great tribological behaviour with the addition of Al2O3. This was believed to be caused due to the grain-like microstructure and the presence of the Al2O3 phase in the CoCr metal alloy [Citation32]. Walpole et al. [Citation33] discuss the non-porous alumina material that shows excessive biological performance which, in turn, is highly favourable for osteoblastic activity. They deduced that the resultant coating over Titanium substrate in a prosthesis will come up with highly excellent mechanical properties. Nano-porous alumina appeared to provide finer results before loading with bioactive material. Mechanical properties measured disclosed that shear and tensile strengths are in excess. The mechanical measurements also showed very little adhesion between the coating and the implant after anodization since a smooth surface was created. However, the layer of alumina on the Titanium substrate showed increased mechanical properties despite less adhesion of alumina. It might be possibly due to the interlayers that would have direct contact, thereby resulting in a weak interface. Finally, conclusions were made that nano-porous alumina on Ti-6Al-4V alloy furnishes smooth surfaces when synthesized by the Anodization technique and imparts increased shear and tensile values. Al2O3 was combined with Tricalcium phosphate and TiO2 to obtain a coating that offers great wear resistance, fracture toughness and microhardness. These mechanical behaviours are attributed to the Al2O3 phase present in the mixture in the optimum amount provided that other ceramics are also at their optimum level. Any alterations in their proportion tend to reduce their characters [Citation34]. It was also observed by Cheng et al. [Citation35] that Alumina alone showed the least mechanical behaviours compared to materials that are combined. Conventional Al2O3, nanostructured by-layered Al2O3 with TiO2 offered greater surface roughness and better microstructure that may consequently provide better mechanical properties.
Figure 3. Implants with alumina over the surface [Citation36].
![Figure 3. Implants with alumina over the surface [Citation36].](/cms/asset/60e0b8bf-7da0-4344-bbab-5c439a4d468e/tmrl_a_2250110_f0003_ob.jpg)
2. 3. Zirconia
The great chemical and dimensional stability together with much hardness and strength made Zirconia (ZrO2) a great ceramic far better than Alumina [Citation37]. They exhibit excellent mechanical properties including bonding strength, hardness, thermal conductivity, etc. due to the toughening mechanism exhibited by the Zirconia microstructure. Zirconia is ascertained to show the rhombohedral structure and it takes other forms such as Partially Stabilized Zirconia (PSZ), Tetragonal Zirconia Polycrystal (TZP), etc. As a consequence of the transition from the metastable tetragonal phase to the monoclinic phase, degradation occurs in the mechanical properties. The mechanical properties exhibited by TZP ceramics are high compared to normal tissues and it depends on the microstructure and the stability of the implant. In addition, the mechanical properties rely on the precursors of the Zirconia manufacturing process. Zirconia toughened Alumina (ZTA) is another distinct class of zirconia ceramics. The hardness of TZP and ZTA was examined to be slightly lesser than that of biodegradable alumina [Citation38]. ZTA ceramics was also studied by Pobloth et al. [Citation39] to investigate whether cancellous bone integration occurs in ZTA implants. The research group observed that even a thin bioactive coating in ZTA was providing good osteointegration, but, thicker coatings hardly provided any benefits. The pre-crystallized and crystallized Zirconia-reinforced lithium silicate glass ceramics serve applications in dentistry together with clinical radiation studies. They show pronounced fracture toughness compared to conventional ZrO2 due to their microstructure [Citation40]. Figure shows zirconia-coated dental implants. The reason for the increased importance of zirconia over alumina is the improved mechanical aspects, biocompatibility and improved aesthetic which is similar to bone. Rajan, et al. [Citation41] looked into Zirconia coatings on Titania implants. By adopting nano-indentation techniques, Young’s modulus was low and higher in nano-hardness. Also, the surface characterization was observed for ZrO2 as crystalline. This was carried out to study the bone affinity shown towards different coatings and the conclusions paved applications for ZrO2-coated Ti and Zr composite. Luo et al. [Citation42] put forward the UV-curable ZrO2 nanoparticle coating on implants. Nanoindentation was used for evaluating the mechanical properties and found that nonetheless, they were not pre-heated, they displayed considerable mechanical properties. They possess high refractive index, hardness and Young’s modulus due to the ZrO2 fraction in the nano-particle coating. Huang et al. [Citation43] investigated the osseointegration properties of Zirconia coating which was plasma sprayed, which resulted in nano-structured ZrO2 coating over the Titania implant. The conclusions led to the observation of in-vivo osseointegration improvement when plasma-sprayed ZrO2 was coated, with enhanced surface roughness and wettability which aided in osseointegration. Hydrophilicity was also enhanced by a nano-porous Zirconia-coated ZrO2 implant but observed a reduced bonding strength due to the process of Anodization used for coating. It also displayed improved biological characteristics like cell adhesion and osteoinduction [Citation44]. Wang et al. [Citation45] investigated that Monoclinic Zirconia coating fabricated by plasma-spraying over the Ti-6Al-4V alloy retained mechanical properties which were comparatively more than HAp coatings fabricated by the same technique. The elastic modulus was 139.72 GPa for monoclinic ZrO2 which was slightly lesser than Y2O3- ZrO2 which came to around 136.41 GPa and CaO-reinforced ZrO2 which was 178.53 GPa. The mechanical strength of ZrO2 is compared to that of stainless steel because ZrO2 is frequently utilized in coatings and orthopaedic implantations.
Figure 4. (a): Zirconia dental implant [Citation46]. (b) Microstructure of Zirconia coating [Citation47].
![Figure 4. (a): Zirconia dental implant [Citation46]. (b) Microstructure of Zirconia coating [Citation47].](/cms/asset/1633497a-ee6a-46fa-ad32-1ea63d4daa9a/tmrl_a_2250110_f0004_ob.jpg)
2. 4. Silicate-based coatings
Silica, being a trace component in human and animal tissues and aiding in bone formation applications, is increasingly used in silicate-based coatings. However, the high quantity of Si inside the body is toxic to the human body and cells. Former studies have confirmed death in mice by nephrosis after high amounts of silicate-ceramic powder by Kawanabe et al. [Citation48] by injecting different ceramic powders by intramuscular or subcutaneous injections. Silicate-based coatings have a beneficial chemical composition and mechanical properties. Their chemical composition makes them aid in bone-tissue interaction due to the surface reactivity, as described by Hench et al. [Citation49]. The apatite layer formed results in natural bioactive fixation which consequently leads to stronger tissue-implant bonding. Surface roughness and porosity determine the mechanical behaviour. Keeping aside mechanical properties, the bio-functional properties such as osseointegration, anti-bacterial properties, etc. are highly noteworthy properties of silicate-based coatings. The mechanical behaviour of SiO2-based coatings, including bending strength, fracture toughness and Young’s modulus, is way better than hydroxyapatite or any other calcium-phosphate ceramics, as inferred by Mohammadi et al. [Citation50]. It is also to be noted that the inclusion of trace elements improves mechanical characteristics. Biodegradability turns out to be a very significant advantage of these coatings and is far better than calcium phosphates and tricalcium phosphates. The downside is the dissolution rate which rapidly increases and alters the pH levels that may be detrimental to the bone tissues [Citation51]. Bioactive silicate-based coatings with various substitutions are compared for their mechanical behaviour by Mohammadi et al. [Citation50] to obtain an inference that Mg and Zn substituted coatings show more analogy to the mechanical properties of bones. Silicon excretion also remains a drawback of silicate-based coatings. The poor value of bonding strength will potentially maximize the chance of implant failure. Zhou et al. [Citation52] studied and demonstrated that calcium silicates are incredibly significant in promoting osteogenesis or juvenile bone formation; in fact, better than β-Tri Calcium Phosphate (β-TCP). They also showcased effectiveness in bone marrow mesenchymal stem cell proliferation compared to calcium-based coatings. Better osteogenesis was reported by the high concentration of Silica in CaSiO3 coating. The elastic modulus of Silicate-based coatings is similar to bone [Citation50]. Brunello et al. [Citation53] found out that the silicate ceramic showed high efficiency of the apatite mineralization compared to HAp in SBF. Copper- and Gallium-doped Bioactive silica glass coatings are investigated for their mechanical properties and in vitro characteristics. It was observed that an improvement in bonding strength was obtained which was similar to that of bones. Furthermore, the cytocompatibility was great for which SiO2 is the sole reason [Citation54]. Vijayalakshmi et al. [Citation55] studied the effect of bone formation on calcium silicate and silica glass by SBF immersion. The effect of sintering temperatures on the surface of the glasses was clearly investigated. Figure represents pure silica and calcium silicate coatings developed by spin coatings sintered at 600°C. It has been observed that silica gel played a significant role in hydroxycarbonate apatite formation due to the presence of silanol groups present on the surface. From the observation, it was evident that the bioactivity of silica glass is dependent on the sintering temperatures.
Figure 5. SEM image for the Silica glass coatings. (a) Pure silica and (b) calcium silicate sintered at 600°C [Citation55].
![Figure 5. SEM image for the Silica glass coatings. (a) Pure silica and (b) calcium silicate sintered at 600°C [Citation55].](/cms/asset/49ad98a2-5ba0-4f6a-bc46-341ff151ba9d/tmrl_a_2250110_f0005_ob.jpg)
3. Importance of composite coatings
The amalgamation of two different substances with entirely dissimilar properties to induce a synergistic effect is termed composites. They are combined by enhancing their inherent individual properties. They are synthetic multi-phase materials. Ceramic matrix composites generally have favourable corrosion resistance, thermal stability, creep resistance, wear resistance, prolonged fatigue life, increased specific stiffness, etc. The matrix and the reinforcing phase are of equal significance in determining the biological response.
While focusing on the merits, we observe that ceramic matrix composites are the principal cause of the increased applications in the field. They possess far better strength than other composites. The resistance offered towards everyday wear and tear together with long life is yet another advantage. Composites being advocated widely, have desirable orthopaedic applications but have challenging problems due to their design and fabrications. The reinforcing material and the matrix could be altered to attain optimized mechanical and biological properties, inclusive of the environment or the surface effects. These factors in conjunction with the appropriate fabrication methods are the criteria for determining the better composites. The utilization of composite materials allows upgraded mechanical compatibility with surrounding tissues, with long-term fixation [Citation56]. Smith [Citation57] showed a porous ceramic that was combined with the reaction products of Alumina, Silica, etc. They found out that the resultant had comparable bone strength and similar modulus of elasticity, inertness, etc. Examples of composites include HAp reinforced with collagen fibres and nanosized HAp coatings with poly(lactic-co-glycolic acid) PLGA on Mg alloys to provide a dual function [Citation58].
3.1. TiO2 composites
Titania (TiO2) ceramic nanoparticles are notable for their biocompatibility, bio-inertness, antimicrobial properties and photocatalytic activity. It is also capable of diminishing bacterial growth. TiO2 coatings at different nanostructures were studied for their bioactivity which concluded that the bactericidal actions are good and effective enough [Citation59]. TiO2 layers have a lot of advantages when combined with HAp. Figure represents the interface of HAP and 20% TiO2 coatings. It can enhance the adhesion of HAp along with restraining the decomposition of HAp, improve corrosion resistance and biocompatibility, etc. The likeliness of the crack formation is downsized in coatings and also inhibits the release of toxic ions from implants. The mechanical properties, after combining these two, will be enhanced. The superior the bonding strength, the smaller the thickness of the coating. The inter-layer thickness can influence the final corrosion performance. The anti-corrosive properties are very crucial to protect the implants from body fluids. The HAp-TiO2 combination improved the corrosion resistance of the implants [Citation60]. When HAp grains are introduced to titania surfaces, the osseointegration is similar to that of pure HAp which is significantly high [Citation61]. Araghi et al. [Citation62] evaluated the HAp/TiO2 adhesion strength by coating HAp and TiO2 over Ti alloy implants using the Electrophoretic Deposition (EPD) technique. Amid some other factors, sintering temperature plays an influential role and hence compels us to select the optimized sintering temperature. Elseways, while the sintering temperature is high, it offers high-density coatings, but might probably cause HAp decomposition and when it’s low, it may give rise to weakly bonded HAp. Amid EPD coating, more cracks were examined when high voltages were applied. Functionally graded HAp with TiO2 evinced ample adhesion strength than HAp solely [Citation63]. Lee et al. [Citation64] also accentuate, in particular, that HAp-TiO2 combined conjointly would offer favourable adhesion properties, mechanical stability and osteoblastic response. They substantiated that TiO2 buffered HAp could be employed as a remarkably desirable alternative to HAp directly on Ti. Similarly, they observed that HAp/Ti has a lower strength at the interface than HAp/TiO2 on Ti. Rath et al. [Citation65] investigated TiO2/HAp bilayer coating and it appeared that they had upgraded adhesion strength set against individual ones. This was ascribed to an assortment of reasons including high mechanical interlocking, improved wetting and intermixing elements at the HAp/TiO2 interface. Also, high corrosion resistance is ascertained due to the presence of a dense interlayer between the HAp/TiO2 composite and Ti implant. It was affirmed by Li [Citation61] that due to HAp, TiO2 also showed exceptional osteoconductivity. Implants of TiO2/HAp coatings carry sound bonding strength despite three months of implantation.
Figure 6. (a): Cross-sectional view of HAp and 20% TiO2 sol-gel composite coating [Citation63].
![Figure 6. (a): Cross-sectional view of HAp and 20% TiO2 sol-gel composite coating [Citation63].](/cms/asset/9c7a28e4-0661-4bb3-8490-28a550223fd9/tmrl_a_2250110_f0006_ob.jpg)
3.2. ZrO2 composites
Zirconia is normally stabilized using Yttria (Y2O3), manifesting dynamite mechanical properties, corrosion resistance, wear resistance, etc. and hence used in orthopaedic applications. When zirconia and hydroxyapatite are incorporated together, they can complement the bonding strength between the implant and the coatings in addition to boosting the adhesion strength. The inflated temperature would deteriorate the adhesion due to the dilation mismatch that may arise at the HAp-ZrO2 interface. HAp in aggregation with ZrO2 expressed better bonding strength than pure HAp owing to more crystalline and fewer porous traits of the microstructure. It was established that by adding Zirconia to HAp, the samples displayed morphology that was distinct and transformed from the former. Figure represents a porous Zirconia/ HAp composite. Furthermore, the grain size and the porosity were altered in the composites without any crack being inspected in the microstructure of the composites. Incorporating Zirconia and HAp promoted the bonding strength, function and mechanical properties of the coating conjointly [Citation66]. ZrO2 exhibits prominent fracture toughness making it essential for implants. Zirconia combined with Y2O3 can attain the stabilized tetragonal phase which has appreciated properties. Mechanical properties are also elevated when partially stabilized Zirconia is used owing to its biocompatibility. Zirconia can even be reinforced with HAp by the method of co-precipitation for load-bearing applications and safer orthopaedic applications [Citation67]. Microwave sintering is an alternative method for ZrO2 and HAp composite fabrication. Curran et al. [Citation68] with the assistance of Microwave sintering discussed that a scarce difference in mechanical properties was observed with Microwave and conventional sintering. Moreover, the high ZrO2 loadings reduced the mechanical strength since the TCP was formed, and hence porosity increased. Nevertheless, since the microstructure is observed to be different for different methods, microwave sintering aided in osteointegration application. Sung et al. [Citation69] used Y2O3 stabilized ZrO2 and detected high crystallinity and uniform distribution by TEM studies. They also evaluated the increased modulus of elasticity, rupture and fracture toughness. On that account, it was available for safer use in load-bearing applications. When HAp with PSZ was combined by Matsuno et al. [Citation70], the mechanical properties showed an enhancement and were greater than those of the human bone. They have high fracture toughness so far compared to other bioactive ceramics. Kmita et al. [Citation71] proceeded with the hot-pressing method, for reinforcing ZrO2 with HAp and succeeded in determining the influence of the mechanical strength. The increase in bending strength and fracture toughness was observed to be high instead of normal HAp coatings. The different layers of coatings also improved the mechanical properties. ZrO2/Al2O3 composites over Zr alloy implant using Plasma Electrolytic Oxidation (PEO) technique were known to exhibit great mechanical properties along with corrosion behaviour compared to individual coatings of ZrO2 or Al2O3. While the composite was coated over the implant, it was observed that the Al2O3 layer was coated at the topmost layer and delamination was noticed with better wear resistance [Citation72].
Figure 7. Porous Zirconia/HAp composite with 91% porosity [Citation73].
![Figure 7. Porous Zirconia/HAp composite with 91% porosity [Citation73].](/cms/asset/b60ce6d9-c31f-495e-bb73-bb35c69d6db0/tmrl_a_2250110_f0007_oc.jpg)
3.3. Al2O3 composites
Alumina is a well-acknowledged bioinert material that hardly shows any activity against human tissues. The possibility of fabricating a composite to enhance the mechanical properties without retarding the biological properties accelerates research on HAp/Al2O3 composites. Together with Alumina, CaP is also reinforced to improve the bioactivity of CaP ceramics. This is accomplished to meliorate the mechanical properties like compressive strength and retain the bioactivity like osteointegration [Citation74]. Synthesizing a composite of HAp and Al2O3 to augment the mechanical properties of HAp by integrating with alumina that accommodates aspects such as inertness, high toughness, compression strength, etc. without altering the biological properties like osseointegration which is a characteristic of HAp. They are synthesized by different methods such as the precipitation method, hydrothermal method, dry mechanochemical method, etc. [Citation75,Citation76]. Al2O3 and HAp aggregate relies on the concentration by the weight of both components. With added Alumina content, it was noticed that the amorphous phase of the composite increased by 15 to 40% [Citation75]. The nanosized HAp having commendable crystallinity is normally reinforced with Al2O3 by the mechanochemical process that shows mechanical process and chemical conditions. The composition of Al2O3 and HAp was vital in determining the mechanical or biological properties. When the increased concentration of HAp was considered, the lower grain size was obtained and they possessed uniform microstructure and hence agreeable osseointegration. 20 to 80% wt of HAp showed good compressive strength which inhibits damage to the implant [Citation77]. Al2O3 composite with Graphene and hydroxyapatite efficiently shows anti-bacterial properties and better wear performance over Ti alloy implants. The micro-hardness also showed improvement after effective coating. Graphene content present accelerates the apatite formation which is aided by hydroxyapatite and rules out the limitations of pure Al2O3 coatings [Citation78]. Juang et al. [Citation79] concluded that the enhancement of mechanical properties was either due to new phase formation by the reaction that may occur at the interlayer of Al2O3 or due to the small pore size of the structure. Figure represents the coating of the HAp/Alumina composite. In this case, the concentration of Al2O3 was 10%. Raj et al. [Citation75] studied this particular composite and prepared it by the hydrothermal process which could indicate that the increase in temperature favoured the formation of nano HAp/Al2O3 which could exhibit fair thermal stability and serve applications in biomedical applications. Evis et al. [Citation79] figured out that HAp/Alumina composite perhaps becomes more temperature resistant upon small amounts of doping of ions. Also, tensile forces of Hydroxyapatites show up cracks. This was under the betterment of coatings and the better bonding they exhibited with Ti implants.
Figure 8. Microstructures observed in the HAp/alumina composite [Citation80].
![Figure 8. Microstructures observed in the HAp/alumina composite [Citation80].](/cms/asset/5a0ca008-910c-429b-a3f1-56dafc4f8144/tmrl_a_2250110_f0008_ob.jpg)
3.4. SiO2 composites
Silica-based materials are familiar for initiating the mineralization of bones and for various other applications. Si exhibits toxicity when the amount is intensified inside the body. Nonetheless, while Si is combined with calcium and Calcium Silicate composite formation occurs, the toxicity is least and the Si excretion happens through urine without being heaped to any of the organs. When SiO2 and HAp are combined, the bone-apatite layer grows with an increased concentration of SiO2. An appreciable characteristic of HAp is that it effortlessly gets decomposed to Tricalcium phosphates with elevated temperature. But immediately after SiO2 is added, HAp decomposes at a lower temperature. SiO2 regulates the microstructure and builds up bone-like layers on the surface of the scaffolds. As HAp decomposition it occurs at low temperatures, reacts with SiO2 and leaves behind bioactive glass and Ca2SiO4 which are relatively less toxic than Si ions according to the probe by Jia et al. [Citation81]. By the same token, carbonated HAp (CHAp) with SiO2 composition is studied thoroughly. HAp on SiO2 can feasibly be prepared using the sol–gel method by Taha et al. [Citation82]. The mechanical properties such as Young’s modulus, elastic modulus, compression strength, etc. were observed to be enhanced when SiO2 was added to CHAp and acquired sintered nanocomposites. The compression strength is analogous to that of natural bone for SiO2/HAp which implies that it can resist the pushing action. A convincing improvement was attained by the coating when SiO2 was added. Mesoporous silica-HAp nanocomposites are also synthesized using the sol–gel method that results in effective crystal growth of HAp with a longer ageing time [Citation83]. Qiu et al. [Citation84] investigated the betterment of bone formation that is provided by silica-doped HAp. The higher the Si doping, the lesser the crystallinity. The crystallinity and the sintering properties are influenced by the Si content of the composite. They were noted to promote bone-in-growth and osseointegration and lessen the use of autogenous bone grafts. The formation of an apatite layer due to the Si-coated HAp on Ti alloy was analysed by Regi et al. [Citation7]. The ageing period depends on the temperature of the fabricating process which is the sol–gel technique and they display a crack-free surface with better tensile values compared to those without Si. However, apatite formation is a boon and hence it becomes a satisfactory alternative to prevalent HAp coatings. Si with HAp is prepared by hydrothermal methods and the Si0doped HAp reveals superior bioactivity with intense Si concentration. Chellappa et al. [Citation85] were successful in synthesizing different composite powders which are ZrO2/TiO2, SiO2/TiO2 and SiO2/ZrO2 over Ti-6Al-4V alloy. The ZrO2/ TiO2 composite exhibited superior corrosion behaviour and uniform surface structures with desirable densification. The Electrochemical Impedance Spectroscopy (EIS) studies and polarization studies concluded that the corrosion resistance was higher for ZrO2/TiO2 coatings and, therefore, prominently efficient. Consequently, concluding silica composites show lesser corrosion properties. Likewise, SiO2 and ZnO composite powders fabricated over the Ti-6Al-4V alloy show refined mechanical properties and corrosion resistance because they have a more densified surface structure than pure SiO2. The ZnO in the composite is the reason behind the composite being denser and helps in the reduction of pores in the composites. It also improves silica’s low adhesion strength [Citation86]. Recent advances have been made in silica composites by incorporating antibiotics in silica and coating them over implants. Ballarre et al. [Citation87] fabricated a composite of silica inculcating chitosan and gentamicin and a dual layer of coating is given over the Ti alloy to exhibit great antibacterial activity and bioactivity. Another bioactive scaffold of nano-composites formed of sodium alginate, hydroxyapatite, silica and graphene oxide showed excellent apatite formation due to the richness in SiO2 content [Citation88].
3.5. HAp/Carbon nanotube composites
Carbon nanotubes (CNT) are broadly used in recent years due to the extended degree of benefits they provide. There exist distinct types like multi-walled carbon nanotubes (MWCNT) which show an intense degree of perfection in structure and single-walled carbon nanotubes (SWCNT) which have fair uniformity in diameter. Natural bone, being a composite, embodies various components inclusive of organic components with collagen fibres which are similar to carbon nanotubes. The toxicity studies are still not completely explored. But hitherto both studies showed it was toxic due to cell proliferation, they do not accumulate in organs. Instead, they are being excreted out through urine. Petite nanotubes are the least toxic [Citation89]. HAp is already a proven bioceramic and CNT is focused more in this context. Combining CNT with any other reasonable material would end up in a range of superior properties which advances further research in their field. Their mechanical properties such as great conductivity, stiffness, inertness, etc. are attributed to the perfectly aligned nanotubes and the C–C sp2 bond. Moreover, they can withstand tension since only the layers outside are active during tension and then transformed into the inner layers [Citation90]. Whilst integrating HAp and CNT composite, the porosity needs to be controlled to attain good mechanical properties for the composite since the least porous have mechanical properties to a greater degree. SWCNT/HAp composite coatings manifest stronger bioactivity than pure HAp. The enormous potential for CNT is notably broad in the biomedical field. Pei et al. [Citation91] put forward the finding that SWCNT/HAp composites on Ti implants made a substantial improvement in their properties. They provide a uniform and crack-free surface on SWCNT/HAp and exhibit crystallinity. They also induce superior cell proliferation to pure HAp. Xu et al. [Citation92] considered the optimum sintering conditions of CNT incorporated with HAp. Spark plasma sintering is used for composites with enriched mechanical properties. CNT reinforced with HAp also manifests adhesion properties with osteoblast cells. The biocompatibility of CNT with HAp has also been investigated. Lahiri et al. [Citation93] discussed the CNT/HAp on the grounds of fair modulus and thereby showed increased mechanical properties and improved wear resistance. Thom et al. [Citation94] discussed the composite on the surface of the 316 L stainless steel by the Electrophoretic deposition (EPD) technique expecting the presence of CNT to diminish the solubility and boost the mechanical properties. As CNTs can enhance the mechanical properties by reinforcing into HAp, Pei et al. [Citation91] fabricated SWCNT/HAp composite by the EPD method and observed the bonding strength to be 25.7 MPa which was much more sophisticated than pure HAp coatings. The post-EPD treatments including oxidative treatment were introduced for improving the purity of the coating to such an extent to become more productive. The implementation of thinner coatings, specifically with a thickness of 10µm, serves as a crucial strategy for minimizing crack formation. Improved bonding strength ranging from 15.3 to 25.7 MPa indicated superior mechanical properties of the composite. Stango et al. [Citation95] fabricated HAp/f-MWCNT composites by the sol–gel method. With different concentrations of f-MWCNTs, the properties varied. Figure shows HAp@3% f-MWCNT composite coating developed on 316L SS substrate by a spin coating method. When f-MWCNT concentration was increased from 0 to 5%, the adhesion strength was 25.71 to 43.19 MPa. Also, due to the close bond between HAp and f-MWCNT which has an effect on interactions of Ca2+ and COO- ions, the coating was dense and compact. Similarly, Sivaraj et al. [Citation96] investigated the composite coating of Cu-doped hydroxyapatite with functionalized MWCNT for its corrosion and anti-bacterial properties. The fabricated composite was coated over the 316L SS alloy implant and noted high barrier properties which meant higher corrosion resistance. Apart from the prevention from corrosion they turned out to be effective against both gram-positive and gram-negative bacteria and showed enhanced bioactivity through cell-membrane integrity assay, MTT assay and haemolytic assay.
Figure 9. SEM image and cross-sectional image of Hap/3% f-MWCNT composite coating on the 316L SS substrate [Citation97].
![Figure 9. SEM image and cross-sectional image of Hap/3% f-MWCNT composite coating on the 316L SS substrate [Citation97].](/cms/asset/ff4f4b7c-3a56-4b71-8966-a764cbef612c/tmrl_a_2250110_f0009_oc.jpg)
3.6. HAp/Graphene derivative composites
HAp thus far has been proven as an exceptional implant coating. That being the case, here we explore the properties of the graphene family. The remarkable characteristics of graphene and its derivatives include osteogenic activity, chondrogenic activity, bone-tissue regeneration inducing differentiation of stem cells, etc. and have high mechanical properties due to its satisfying elasticity, crystallinity, flexibility and high surface area. Due to their unique and remarkable properties, they have been extensively used as biomaterials or implant coatings. Henceforth, toxicity studies also need to be conducted to ensure that it is not cytotoxic. There are quite a few works of literature that acknowledge that graphene and its derivatives could be functionally modified with the intention that they do not exhibit any toxicity and can be excreted through safe means without affecting any tissues/organs. Graphene-based derivatives are remarkably efficient and have a low biological barrier. Nevertheless, they are non-biodegradable and long-term toxicity is also under concern [Citation98]. Cytotoxicity of graphene is concentration dependent and with lower concentration, they are biocompatible. Graphene shows extremely good mechanical properties because the sp2 C–C bonds have honeycomb structures [Citation99]. The two derivatives of graphene are Graphene oxide (GO) and reduced-graphene oxide (rGO). The microhardness of HAp escalates with the Graphene derivative and similarly elastic modulus and fracture toughness also are improved inclusive of compressive strength. Moreover, graphene can withstand harsh conditions during synthesizing, like high temperature and pressure. The apatite layers are thicker and more uniform than pure hydroxyapatites [Citation100]. The composites are highly efficient in improving the adhesion strength of HAp on the metal. They have a higher Young’s modulus for GO than pure Graphene due to multiple stacking [Citation101]. Even though uniform distribution, inherent brittleness, hardness to cut into shape, etc. persist as a problem, graphene incorporated with HAp offers superior mechanical characteristics, greater potential biological characteristics and upgraded corrosion resistance [Citation102]. The mechanical efficiency was evaluated by varied parameters counting the inherent behaviour of the matrix, the synthesizing method, etc. considering that incorporating graphene over HAp showed a smaller grain size and also resulted in the formation of the thick bone-like apatite layer. The grain size and porosity were similar and hence graphene was more reliable for strength [Citation103]. Baradaran et al. [Citation104] concluded that even an infinitesimal amount of graphene derivative added to the synthesized composite can effectively improve their mechanical properties and they exhibit higher elastic modulus and fracture toughness than pure HAp. They further validated that the reduced Graphene Oxide (rGO) addition raised the porosity of the GO/HAp composite. Moreover, the addition of the rGO alters the density and when the rGO content is more, the density takes a leap which proceeds to the deterioration of mechanical properties but improved biocompatibility. Stango et al. [Citation105] revealed the use of HAP/GO composite with better mechanical strength and bone mineralization by forming more adhesion strength for more beneficial load-bearing applications. Figure represents the coating morphology of HAP/3% GO developed on 316L SS by a spin coating method. GO surface is composed of chemical moieties such as –COOH, -OH and epoxy forming a covalent link with 316L SS. The combination of GO with HAP supports its association with the implant surface by increasing the corrosion and wear resistance. The hardness measurement values suggested that the reinforcement of Graphene oxide enhanced the toughness of the composite from 2.59 to 4.22 GPa just as the concentration of GO increased from 0 to 3% [Citation105]. Li et al. [Citation106] also gave an explicit idea of Graphene/HAp composite synthesized by EPD and they found it to be superior to HAp coating. It was observed that graphene had adhesion to human-osteoblast-like cell lines and induced proliferation in vitro for their adhesion and corrosion resistance. Cell adhesion and proliferation showed significant improvement in the prominently utilized Graphene oxide-hydroxyapatite nanoparticle composites, with observed improvements correlating with an increase in graphene oxide content [Citation107]. Due to their immense contribution to cell proliferation, graphene oxides are of extreme importance in biomedical research. Al-Arjan et al. [Citation108] included Apple pectin to Graphene oxide/hydroxyapatite composite to notice better compressive strength, Young’s modulus and porosity and uniform pore distribution which is regulated by GO. The physical cross-linking due to the presence of GO attributes to the better microstructure and hence better mechanical properties.
Figure 10. SEM image and cross-sectional image showing dense coating morphology of HAP/3% GO on 316L SS [Citation109].
![Figure 10. SEM image and cross-sectional image showing dense coating morphology of HAP/3% GO on 316L SS [Citation109].](/cms/asset/6aa4acde-1b16-4e7d-8a93-2216c9067b52/tmrl_a_2250110_f0010_oc.jpg)
4. Doping of ions
Doping is regarded as the deliberate incorporation of a highly distinct component into a substance, for enhancing its potential outcomes. Ionic substitutions or doping is a very good alternative for optimizing the properties of ceramic coatings. Different ions show diverse properties on doping even with minute amounts. It was demonstrated that F− ions doped with CaP enhanced the mineralization whereas SiO2 retards the mineralization [Citation110]. Thence the selection is completely based on suitability and necessity. Table tabulates some dopants and their effect on various ceramic coatings.
Table 1. Doping of various ions in different ceramics and the advantages.
5. Effect of different coating types on the mechanical behaviour
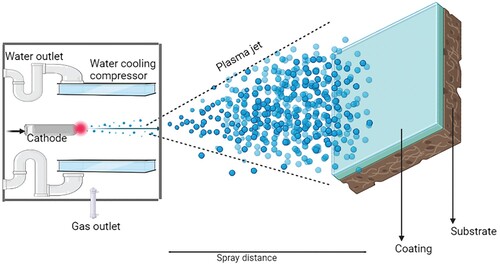
5.1. Plasma spraying technique
Plasma spraying stands out as a highly favourable coating technique due to its intrinsic qualities and desirable characteristics, making it a promising option for a range of coating applications. The technique fundamentally uses plasma gas and introduces the substrate to the plasma gas and further the particles are deposited on the substrate. They exhibit decent adhesion properties with enhanced biocompatibility. The spraying technique has advantages such as controllable porosity, controlled microstructure and simpleness in the process which is complex to execute [Citation140]. Figure represents the schematic representation of the plasma spraying method. A variety of parameters also needs to be considered such as the particle size, the power setting, the post-treatments, the degree of crystallinity, etc. Aside from the prevailing parameters, it also depends upon the decomposition of HAp; nonetheless, the strong influence of the parameter can transform HAp into tricalcium phosphate [Citation141]. If the pore size is large, it would deteriorate the mechanical properties. Hence taken into account, plasma spraying is obligated to be carried out to improve bioactivity [Citation142]. Porous tantalum was coated over the Ti alloy by plasma spraying technique to observe good mechanical properties for the implants than the bare Ti alloy due to their homogenous porosity. The surface characteristics and micro-structure were improved with these coatings due to the effective coating technique with an optimized selection of coating parameters [Citation143]. Yang et al. [Citation144] discussed the different parameters that have an impact on the plasma spraying technique of HAp coating. Furthermore, as the plasma spraying is irreversible, they display alterations in the crystallinity of HAp. Plasma spraying usually gives thin and dense layers. Bansal et al. [Citation145] used a plasma spraying technique for coating hydroxyapatite reinforced with Zinc Oxide to enhance the corrosion resistance of the Mg alloy. With different weight proportions of ZnO, it was observed that the microhardness improved with uniformity and hardly any cracks. Singh et al. [Citation146] studied the titania-reinforced hydroxyapatite over Ti alloy by plasma spraying and observed fair surface roughness owing to the particle size of sprayed powders. The plasma-sprayed coating has to be monitored for the micro-size voids present due to the material nature as we see here for hydroxyapatite which has an amorphous nature. By analysing the plasma-sprayed coating surface, it is possible to deduce valuable information pertaining to microcracks, porosity, porosity, micro-voids, as well as the uniformity or roughness of the coating. As the mechanical properties depend on microstructures, the plasma spraying method is effective enough to obtain better mechanical attributes [Citation147].
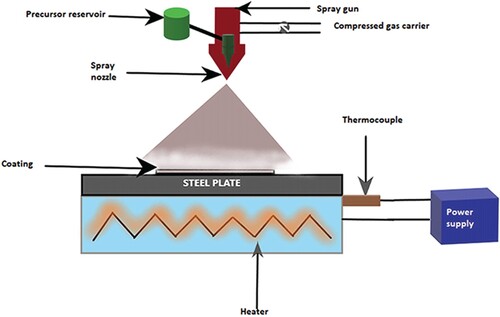
5.2. Spray pyrolysis
The spray pyrolysis method imparts thin films with larger particle sizes which is attributed to the agglomeration of particles. A uniform spraying could be attained by spray pyrolysis [Citation148]. They also have the advantage of relatively less contamination. Figure is the schematic representation of the spray pyrolysis method. The method is time also efficient. The process is carried out by an ultrasonic nebulizer, as discussed by Chou et al. [Citation149] at the 1.6 MHz frequency of equipment. They studied the Ag-Zn doped β-TCP for its antibacterial properties. Uskokovic and Jokonavic [Citation150] used ultrasonic spray pyrolysis for coating HAp over a Titanium alloy implant. The drawback of plasma techniques, which is less control over stoichiometry and composition, was eliminated by spray pyrolysis. They observed a homogenous coating supported by EDX analysis. While the coating thickness increased, the coating roughness decreased. Spray pyrolysis appears a time-consuming process since it did not give a uniform coating with 1.5 h; rather, it took 5 h to complete monolithic films. Vijayalakshmi and Sivaraj [Citation151] studied a Zn-HAp over CNT on 316L stainless steel using the former technique. They revealed a phase formation due to Zn and the morphology was studied by different methods such as Fourier Transmission Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy (SEM). It was finally observed that they have enhanced antibacterial activity due to the superior surface reactivity, surface area and other properties attained through the fabrication technique. Cho et al. [Citation152] studied the nanostructured α-TCP coatings by flame pyrolysis which was slightly different from the procedure of normal spray pyrolysis. Flame spray pyrolysis was attained by an externally applied energy source. With this technique, the microstructure of the ceramic was altered tremendously by the high temperature and hence exhibited a porous structure. They provide monolayers with spherical shapes. Visentin et al. [Citation153] fabricated a bilayer of TiO2 and HAp using the spray pyrolysis technique. The bilayer is achieved with fewer cracks and more uniformity by optimizing the parameters. Due to the fabrication of the bilayer, it is possible to minimize cracks and obtain a uniform coating, thereby improving mechanical characteristics.
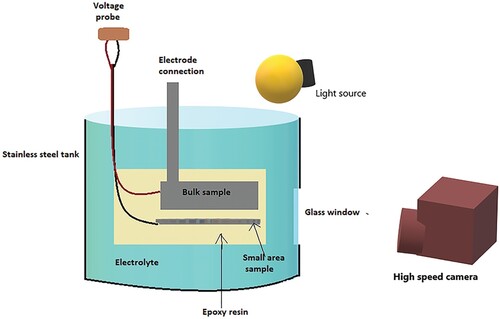
5.3. Plasma electrolyte oxidation
Figure illustrates a schematic representation of the Plasma Electrolyte Oxidation (PEO) method. The plasma electrolyte oxidation technique is used as coatings even for non-adhesive compounds. Thick coatings show more corrosion resistance properties than thin coatings. The processing time of coating can affect the efficiency of the coating [Citation154]. In 2018, a review on PEO by Hryniewicz [Citation155] suggested that metals such as Ti, Ta, Nb, Zr, Al, etc. were fabricated over substrates by the same process. Kesik et al. [Citation156] studied the plasma electrolyte oxidation carried out over the Ti alloy for observing the improved bioactivity. The alloy later showed high bioactivity and anti-bacterial properties due to the surface modification. Rokosz et al. [Citation157] studied how different parameters like voltage influence the PEO process. It was found that the voltage had effects on the thickness of the coating and the thickness of semi-porous layers. Sieber et al. [Citation158] put forward the idea of using PEO for wear resistance applications. They coated ceramics over Al alloys using PEO. The defective microstructure in the coating layers on pure Al was greater than Al alloy by the PEO process which was suspected to damage the implant substrate. However, coating over Al alloy improves wear resistance and corrosion resistance. Mg alloys are coated using bioceramics for the betterment of biodegradability. PEO is an excellent method for these kinds of coatings due to the thickness and bi-layered structure that can be obtained which could prevent degradation to a certain level. The morphology of the coating can be observed to be altered by altering parameters such as electrolyte concentration, composition, pH, process time, etc. Other parameters are voltage, current density, frequency and anode-to-cathode distance. Varying these parameters tends to provide an optimized coating with efficient characteristics [Citation159,Citation160]. Similarly, coatings over Zr alloys by PEO technique were also studied. ZrO2, which is chemically inert, is prone to show bioactivity only if certain materials are coated over them. The oxide layers formed will have better cell adhesion properties accounting for the PEO method [Citation161].
5.4 Frequently used common coating techniques
5.4.1. Electrophoretic deposition
EPD is a coating technique that has broad application in coatings, with complex microstructures and nanostructures that are functionally graded [Citation162]. The gains achieved from the EPD were more vital causing the wide use of the EPD technique including the ease of the procedure, being the simplest technique and possible scaling up too. Cost-effectiveness, flexible microstructure, high degree of control over the complex shapes and uniform distribution of particles are noteworthy advantages [Citation163]. The process is attained by the migration of charged particles towards an electrode in an electric field and the deposition is achieved by the agglomeration of particles. Usually, in the post-EPD process, an appropriate heat treatment has to be applied for enhancing the mechanical properties. The parameters such as voltage and time of EPD are important and they are conducted usually under constant voltage/current. A variety of nanostructures could be fabricated using EPD techniques including nanotubes, nanowires, etc. Boccaccini et al. [Citation164] reviewed the fabrication of CNT with SiO2, TiO2, MnO2, Fe2O3 and other composites. The technique can also be used for manipulating the structures or layers of CNT for obtaining thin coatings. Kwok et al. [Citation165] already characterized HAp fabricated by EPD on the Ti-6Al-4V due to the homogeneity they showed while deposition. The thickness of coatings was preferred to be 10µm and found to be crack free also exhibiting high adhesion strength of 6.8 to 10.7 MPa. Boron-doped hydroxyapatite was used to coat over implant material to have better corrosion resistance, implant-tissue interface interaction and cell adhesion. The morphology displayed after EPD is also a fair reason behind the better properties exhibited by the coatings [Citation166]. Drevik et al. [Citation167] proposed the fabrication of nano-HAp over the Ti-6Al-4V using EPD. They also were tested for various tests including mechanical tests, scratch tests, nanoindentation, etc. and showcased their improved properties. Chellappa et al. [Citation85] investigated various composite coatings involving ZrO2/TiO2, SiO2/TiO2 and SiO2/ZrO2 on the Ti-6Al-4V by the EPD method by varying voltage and time duration to improve the mechanical strength of the coatings. All the composite coatings derived by EPD were found to have better corrosion resistance behaviour with enhanced scratch resistance. Moreover, EPD is combined with another coating technique to improve their efficiency. Nawaz et al. [Citation168] combined EPD with Radiofrequency sputtering techniques to obtain coatings of great anti-bacterial efficiency and biocompatibility.
5.4.2. Sol–gel coating
A procedure that dates back to 1842, involves the production of a sol which is subsequently deposited on the substrate or implant through methods such as dipping, spinning or spraying. The aids provided by sol–gel were similar to that of EPD which include chemical stability, simple proceeding techniques, easiness of synthesizing, cost-effectiveness and the capability to coat complex microstructures and geometrical shapes. Dense coatings with more than 10µm thickness could be achieved by sol–gel coatings which yield high quality, crack-less and thicker coatings [Citation169]. They also furnish desirable bioactivity when organic/inorganic precursors are used. It was concluded that the bioactivity described by them was exceedingly higher than those techniques that use high temperatures. There were different parameters related to the quality of the coating; one such is the dipping process into the solution. Earlier in 1992, it was found by Gugliemi et al. [Citation170] that they dealt with protection against oxidation, and thermal fluctuations by acting as barriers, scratch resistance, etc. They observed it by coating four metals using SiO2/TiO2/ZrO2 coatings by the sol–gel technique. The osteointegration was also improved by sol–gel coatings over stainless steel implants. This is a proven environment-friendly technique, which can leave behind toxic processes, cease waste formation and increase corrosion resistance. A lot of parameters influence the sol–gel-coated substances including the sol concentration, precursors, solvents, pH level, viscosity, temperature, composition, etc. Except for the longer process time, the purity and adhesion offered by the sol–gel coating method are worth mentioning [Citation171]. Nanostructured coatings could also be fabricated by the sol–gel technique. Advincula et al. [Citation172] studied the biocorrosion properties of nanostructured TiO2 over Ti-6Al-4V. A TiO2 stable layer was observed and it gradually controlled the resistance of elements other than Ti. Gugliemi [Citation170] further suggested that the cracking of the coating depended on the thickness. An increase in additional layers of coatings implied more defects in the coatings. Vijayalakshmi et al. [Citation173] spun the sol on the polished 316L SS by the spin coating method at the constant speed of 2000RPM/Min at room temperature. They studied the effect of single- and triple-layered coatings to increase the coating thickness with high adhesive strength. The insulating aspect of sol–gel coatings indisputably increased the corrosion resistance with a better mechanical strength. Priyadarshini et al. [Citation174] have developed sol–gel Ce-HAP coating on Ti-6Al-4V by a spin coating method. Increased adhesion strength between the coating and substrate interface likely arises owing to the formation of chemical bonds at the interface. Anti-bacterial resistance was improved for coatings by inculcating gentamicin with hydroxyapatite over Ti alloy for controlled release of antibiotics. Sol–gel techniques are used for enhanced anti-bacterial effects due to their potential for controlled release of drugs from the coatings [Citation175].
6. Characteristics of coatings
6.1 Mechanical evaluation
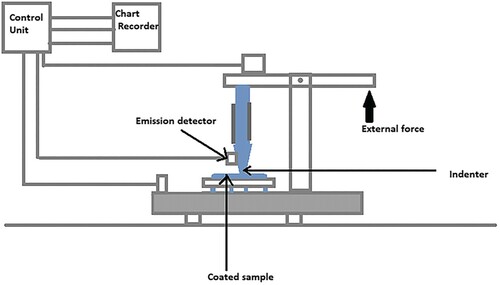
To ensure a clear and explicit evaluation, specific tests are employed to completely understand how the coatings over the implants are achieved and to assess their quality both in vitro and in vivo. Figure portrays the schematic representation of the Scratch test apparatus. For many years, the scratch test has been a relevant method for evaluating the mechanical stability of coatings. The adhesive property displayed by the coating to the surface of the implant was substantiated by a scratch test which was influenced by assorted parameters such as the hardness and thickness of coatings, roughness of the surface hardness of the metallic implant, lubrication, etc. It was corroborated that the roughness value should be less than 0.25 µm for the desired result [Citation176]. Schwarzer et al. [Citation177] perform the macro- and micro-scratch tests. The coating thickness and the surface roughness exerted an impact on the test. One parameter essential for the scratch test is hardness. A scratch test could be used only when the shear stress between surface layers is lesser than that of the softer compound. Buckling and wedge spallation seemed to be the failure modes of scratch test with hard coatings, as shown by Bull and Berasetegui [Citation178]. Drevet et al. [Citation179] examined the mechanical properties by scratch test and nanoindentation technique. The conclusion from the scratch test of nanostructured HAp coated over the Ti-6Al-4V by EPD pointed out that post-EPD treatments improved the adhesion, before the treatment, under the circumstance that, the load was 3.8N and afterwards showed the rapid change in load from 3.3 to 7.9N. The results suggested that the coatings were less porous and compact due to Young’s modulus measured from 5.2 to 19 GPa. Ceramic coatings through the EPD technique are carried conducive to hinder the cracking. EPD coating shows good results against cracking as a result of the densification of the coating. When EPD coatings are carried out twice to enhance their thickness, the crack of the first coating is filled and hence reduces the overall cracking of the coating [Citation180].
Nanoindentation is another aforementioned method for mechanical testing for coatings which is a technique for observing the mechanical behaviour of coatings. Continuous load is measured which gives the hardness, Young’s modulus, Yield stress and dynamic mechanical properties through measurements and various calculations [Citation181]. The indentation technique could be tested in two ways: indentation which is perpendicular to the surface and perpendicular to the cross-section [Citation182]. The morphological observations are obtained by SEM, atomic force microscopy, EIS, etc. [Citation183]. Karacan et al. [Citation184] discussed the corrosion occurring over the Ti alloy. They also discuss the nanoindentation tests and the hardness and modulus of elasticity values.
Using the nanoindentation studies, Young’s modulus and nano-hardness were evaluated by Dey et al. [Citation185]. The Weibull distribution statistics is used by most of the authors for theoretical expression since it is the most common and widely used method. The indentation size effects were also evaluated by these studies. The nanoindentation technique makes use of a high-resolution instrument and parameters like load vs. depth of penetration plot along with providing details on other parameters which include contact stiffness. By the Oliver-Pharr model for nanoindentation
(1)
(1)
where Pmax is the maximum applied load and Acr is the real contact area. Nanoindentation studies were done to study the elastic moduli mismatch between HAp and other metal substrates by Samandari et al.. Mechanical compatibility between the implant and coating is highly relevant for long run in implant fixation. Depth serving indentation tests were also carried out and the Oliver-Pharr approach was used for finally determining the properties from the studies. It is revealed through this study that the elastic moduli mismatch is indeed a serious problem to be dealt with when it comes to the mechanical compatibility of coating and implant substitutes. They conclude through the findings that HAp was better coated over commercially pure-Ti rather than Cr Alloy or SS substrates so that there is the least mismatch between them [Citation186].
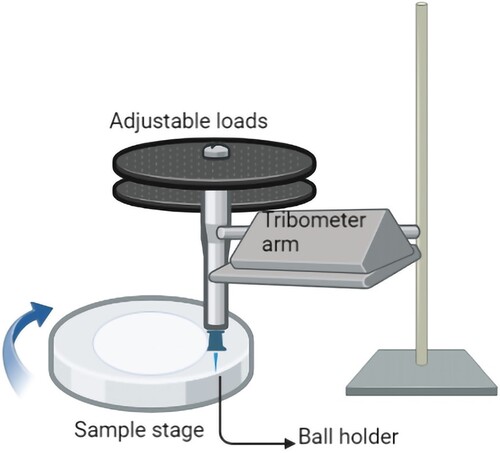
Tribology is the study of wear and tear to estimate the mechanical durability of implants. Wear resistance studies are an important criterion to study when orthopaedic implants and joints are considered. Certain coatings inherently known for wear resistance are ZrO2, Al2O3, etc. Hence, they are combined by researchers along with HAp and other less wear-resistant coatings to improve their characteristics. Rattan et al. [Citation187] reinforced HAp with Al2O3 and observed that the wear resistance has been improved. It is done by evaluating the friction coefficient curves. The coefficient of friction is smaller for pure HAp owing to their surface smoothness and when Al2O3 is added; in addition, surface roughness is observed. Other parameters that determine the wear resistance of a coating are porosity, the thickness of the coating, surface smoothness, etc. along with wear volume loss and wear rate. Baligidad et al. [Citation188] conducted a similar study by adding rGO to HAp and observing the improvement. A tribometer set-up can be used for wear tests. As rGO content is increased, the lubrication between coatings and substrates is improved and further coefficient of friction decreases and hence not normally preferred for orthopaedic joint implants. TiO2 addition on coatings was also studied for wear properties. Tribology studies were carried out by researchers and found that the debris accumulation in the surfaces led to the COF value fluctuations suggesting that abrasive wear is more and depends on the TiO2 concentrations added to the coatings [Citation189]. Surface modifications are also done on the coatings which tend to improve the wear resistance according to certain works of literature. Figure illustrates the wear test apparatus.
6.2. Corrosion studies
The corrosion resistance of the coatings is a vital parameter to keep an eye on for determining the coating effectiveness. Corrosion behaviour can be understood as the effect of the ions in body fluids that causes damage to the coating or the implant, thereby leading to implant failure. Henceforth, corrosion has to be considered ssessentially and superior corrosion-resistant coatings should be preferred. Corrosion studies are normally carried out by electrochemical impedance spectroscopy (EIS) and polarization measurements. Corrosion resistance studies were also carried out for coatings of Mg which detains the capability to degrade in vivo. Chiu et al. [Citation190] studied MgF2 coatings and their corrosion resistance properties by tests such as immersion tests, EIS, polarization tests or electrochemical studies. Electrochemical Impedance Spectroscopy (EIS) was realized to be a strikingly peculiar analyzing technique as observed by Yao et al. [Citation191] while probing into the corrosion studies of ceramic coatings over Ti by the Plasma oxidation technique. EIS technique renders spectra in accordance with the selection of different parameters such as current densities, frequency, etc. The results are correlated to the thickness and strength of the coating. The thicker and denser the coating, the better the corrosion resistance. The immersion test results showed that the untreated MgF2 covered with corrosion products and the treated ones have better resistance show only a slight colour fading and show a milder attack of corrosion [Citation190]. An immersion test in simulating body fluid (SBF) was carried out for the ZrO2/SiO2 composite over the Stainless steel SUS 304. The in vitro test was done for the same to observe the corrosion properties [Citation192]. Raj et al. [Citation183] also discussed α- and γ-alumina and Al2(SiO3)3. They emphasized the evaluation of the corrosion resistance by electrochemical methods and morphology. Thickness was tested by nanoindentation and corrosion resistance by EIS and polarization studies. The polarization tests would be determined by various parameters such as corrosion potential, current density, etc. Figure (a) and (b) represent the schematic representation of the evaluation of corrosion studies by Potentiodynamic polarization and Potentiodynamic polarization curve (Tafel plot). Extrapolation of the Anodic and cathodic regions of Tafel plots gives Corrosion current (Icorr) and corrosion potential (Ecorr) values where they could be correlated with inverse proportionality; that is, with an increase in corrosion current, corrosion potential decreases. Figure (a), (b) and (c) gives the schematic representation of the Electrochemical Impedance spectroscopy method and representation of Nyquist and Bode plots, respectively. A larger semicircle diameter from the Nyquist plots denotes better corrosion resistance. A Bode plot is a graphical representation of frequency vs. impedance.
Figure 16. (a): Schematic representation of potentiodynamic test for corrosion studies. (b) Representation of the potentiodynamic polarization curve (Tafel plot).
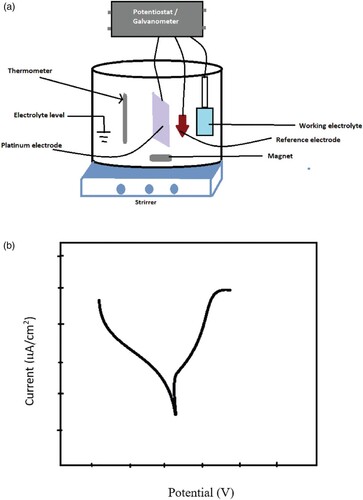
Figure 17. (a): Schematic representation of electrochemical impedance spectroscopy for corrosion studies. (b) Nyquist plot for impedance study. (c) Representation of Bode plot for Impedance study.
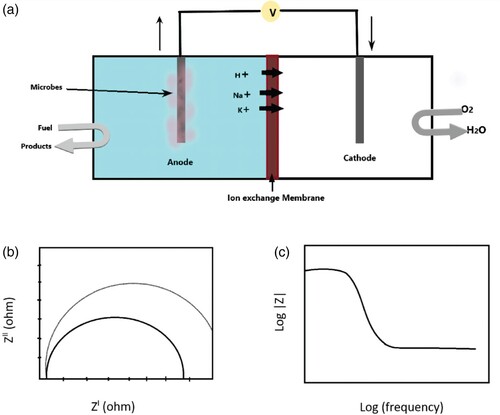
Corrosion resistance was tested on most of the ceramic coatings and only if they provided significant resistance, they were considered a favourable coating. Ceramic coatings on Aluminium were studied by Guan et al. [Citation193] with Plasma Electrolyte coating which possesses sufficient dielectric properties. The Polarization test results demonstrated that the corrosion potential increased in contrast to the Aluminium substrate. The coating at 120 min shows corrosion potential at its peak. Exempting from the corrosion potential the current density of the technique also affects the corrosion resistance. The more the current density, the more the corrosion. Hence, the least current density should be adapted. HAp/TiO2 combined coating over Ti implant produces exceedingly high corrosion resistance and better adhesion strength than pure HAp since they contribute two layers of coating: one of Ti which is densely thin and HAp which is crystalline and thick. The corrosion study using Ringer's SBF revealed highly increased corrosion resistance compared to pure HAp. By furnishing bilayers, they exhibit high resistance to corrosion and the interlayers of the coatings which are anticipated to be dense can adequately prevent the immediate association of body fluid to the metal [Citation194]. Similarly, HAp over the Ti-6Al-4V alloy was fabricated by the sol–gel method to provide desirable corrosion resistance. EIS was carried out for the same coating by Hadad et al. [Citation195] to obtain the results that support improved corrosion resistance. Sidane et al. [Citation196] also considered HAp/Titania coating over stainless steel metal. The outcomes revealed the regulation of corrosion potential and current density over corrosion resistance. The TiO2 coating can diminish these parameters and thereby promote corrosion resistance. Plasma-sprayed Al2O3 was studied for corrosion resistance and showed high corrosion resistance. Lower porosity was exhibited by these coatings. The moderate Calcium Phosphate ceramics exhibited maximum corrosion resistance and moderate hardness [Citation197]. Alumina coatings over Aluminium were fabricated by Atomic Deposition. EIS studies revealed that the thickness of the coating was highly favourable for protecting the coating [Citation198]. Another hybrid coating of ZrO2 and Poly(methyl methacrylate) (PMMA) was studied by Norouzi et al. [Citation199] which made it obvious that it provided good adhesion, low defects and more corrosion protection. The better adhesion was the sole reason behind corrosion resistance and showed better activity than pure ZrO2; because the hybrid coating of ZrO2-PMMA showed the best corrosion by laying in the top layer. YSZ over AISI 316L SS was evaluated for corrosion performance and found that hardly any degradation occurred from the Impedance studies [Citation200]. Alumina Zirconia composites fabricated by PEO gave better coatings with improved mechanical properties and corrosion resistance. Barati et al. [Citation201] studied the corrosion resistance and noticed the improvement due to higher thickness, roughness and porosity. HAp/Graphene oxide composites were studied for the corrosion behaviour by Sebastin et al. [Citation202] using SBF solution in vitro. The EIS studies of the coatings provided data suggesting that the corrosion resistance improved with an increase in Charge Transfer Resistance (Rct) value with the incorporation of graphene into HAp.
6.3. Biological evaluation
6.3.1. In vitro studies
An implant coated with ceramics, if introduced to the human system, may induce rejection by immunological responses by the body. Consequently, in vivo testing is mandatory for any biomedical implant. However, being costlier and requiring animal models, it is preferred to test the bioactivity and the biological responses in vitro. Essentially, two methods are discussed for in vitro testing by Xiao and Wu [Citation203] which are apatite formation evaluation in SBF and osteogenic differentiation by cell experiments. Either one or both are necessary for in vitro testing. Biocompatibility, anti-bacterial and anti-tumour activities of bioactive scaffold fabricated from Arabinoxylan incorporated with Zn and reduced Graphene oxide with hydroxyapatite are studied for bone tissue engineering. Enhanced cell proliferation and viability were studied with preosteoblast cell lines due to their multifunctional properties and the synergistic effect shown by the scaffold. Similar scaffolds were studied for improving their cell adhesion characteristics and cell viability which resulted in the generation of a potent material for bone-tissue engineering [Citation204,Citation205]. Another scaffold comprising acrylic acid, guar gum, hydroxyapatite and titania nanoparticles with the optimum amount of graphene oxide showed improved mechanical properties and compatibility with tissues with better cell proliferation [Citation206]. In addition, Si-substituted HAp fabricated by the ultrasonic spray pyrolysis technique showed improved cell proliferation and osteocalcin production owing to the incorporation of Si ions [Citation207]. Osteoblast-like Human Osteosarcoma cells (HOS) were studied for the composite by Kim et al. [Citation63] who observed the spreading and proliferation of cells and more attachment of cells from alkaline phosphatase activity (ALP). Strontium-doped hydroxyapatite incorporated with chitosan and polypyrrole was studied for its in vitro bioactivity using an MTT Assay and live/dead staining assay for the cell viability and concluded that from the first day of incubation onwards, cells showed adequate proliferation on MG63 cell lines and least toxicity [Citation208]. Ag was coated over Mg alloys to obtain a coating with high anti-bacterial efficiency. This was achieved by combining two methods including micro-arc oxidation and physical vapour deposition. An efficient bi-layer of anti-microbial coating of the porous structure showed its effectiveness against E. coli and S. aureus [Citation209]. HAp/Alumina composites were formerly known to exhibit better mechanical strength with higher concentrations of Alumina. But their bioactivity was discussed in detail by Radha et al. [Citation210] through in vitro studies by immersing the sample in SBF. After 21 days, the conclusion was derived that Alumina affects the crystallinity of HAp. Additionally, the ceramic coatings exhibit a marginal increase in heamolysis, and concurrently, the cell proliferation rate was significantly high. The osteogenic activity was evaluated by the MTT assay for a titania coating and confirmed efficient activity by numerous osteoblast cells on the tissue-coating interface which further concluded that they carry excellent anti-bacterial and osteogenic activities [Citation211]. Zirconia at the same time is a great bioceramic, which shows good mechanical properties as implant coating. Zirconia was coated over the AZ91D Mg alloy, for reducing the bio-corrosion effect of the Mg implant. YSZ is deposited over the implant by the EPD technique and in vitro tests are performed in SBF by immersion. They showed rapid alteration in pH due to the degradation of Mg and 50 min EPD coated ones provided the best corrosion resistance. Also, Zirconia could slightly improve apatite formation on the surface which was substantial in biomedical implants. [Citation212]. Apart from YSZ, monoclinic zirconia coated over Ti alloy by plasma spraying gave bone-like apatite formation in vitro. The Ca and P concentration was lesser with the time of immersion getting higher. The bioactivity also depended on the coating thickness and the nanostructured surface was significant for a coating to show good bioactivity. The monoclinic Zirconia thereby could support cell proliferation as per the study of Wang et al. [Citation213]. Similarly, YZP reinforced with Titania showed apatite formation succeeding three days of immersion in SBF due to the rutile phase of TiO2 that is immensely efficacious in the generation of apatites [Citation214]. Huang et al. [Citation215] studied antibacterial analysers in vitro for Zirconia doped with Ag and Cu. The antibacterial activity of ZrO2-Ag composite gave eminently potent bioactivity in vitro. Robertson et al. [Citation216] gave insights into coatings comprising titania nanotubes/HAp by co-deposition over the Ti alloy using plasma spraying and sol–gel dip coating. These coatings showed a micro-structure that was helping the mechanical behaviour which was apt for orthopaedic applications, besides providing great osteoblastic activity and cell proliferation. Hydroxyapatite with other dopants which was coated over Rex-734 alloy was known to exhibit anti-bacterial activity and biocompatibility. They show fair osteoblastic performance and great cell viability owing to the surface properties that determine the biological performance of the material [Citation217]. Priyadarshini et al. [Citation139] investigated the inhibition performance against certain bacteria by in vitro studies on Ce-doped HAp. Ce-HAp was non-toxic to certain levels. The in vitro studies on MG-63 osteoblast-like cells paved the way for inference regarding the use of the same in orthopaedic implantations due to high biocompatibility and tissue regeneration applications. Studies on haemolytic activity showed that with increased concentration of doping material, the haemolytic ratio is increased. Accordingly, an optimum concentration was confirmed for optimized haemolysis, thereby uncovering applications in tissue engineering. The in vitro studies also confirmed that with higher concentrations, there was visible toxicity to the cells which successively leads to lesser cell viability.
6.3.2. In vivo studies
It is crucial for any biomedical implant to have acquaintance with the immunological response shown by the body to the implant. The coating of the implant also needs to be studied as the coating surface comes into contact with the body fluid, different cells, tissues, etc. On that account, in vivo studies are imperative after the execution of the in vitro studies. It normally concerns implanting the material under study into an animal model like rats or rabbits and sacrificing them for the evaluation of the bioactivity and histocompatibility. Figure represents the image of Wister Albino rats used for in vivo studies. Short-term in vitro test results alone cannot be considered for compromising health. Some of the calcium phosphate ceramics show high in-vivo significance. For new bone formation through in vivo, the composition should contain more CaO, finally leading to the conclusion that CaO containing HAp is more influential for any macrophagic response [Citation218]. Alumina, a known bioinert ceramic, has been incorporated with fluorapatite to display apatite formation and bioactivity with improved cell adhesion, hence a stable implant fixation. The in vivo studies in rabbits indicated strong osseointegration shown by the coating material by evaluating the importance of the bone-implant interface contact ratio [Citation219]. Kim et al. [Citation220] studied hydroxyapatite coated over the Zirconia surface to observe a preferable cell proliferation and attachment from in vivo studies. Profoundly greater osteoconductivity with the least inflammation is observed while coating zirconia which is completely bioinert to become a material with bioactivity. Similarly, Roy et al. [Citation221] studied nano-HAp over Ti by plasma spraying the coating. The rat femur was the medium of in vivo studies which resulted in the formation of osteoid on the surface of the coating which implies bone-tissue integration rather than the uncoated implants. Since HAp is well known for aiding bone mineralization, it helped this coating and the in vivo studies in a possible way. Another study by Jiang et al. [Citation222] was on hydroxyapatite coating over titanium alloy which could also act as a drug carrier for antibiotics. It was confirmed by studies on rabbits that the coating is efficient enough to show great biocompatibility and hence can carry potent drugs with controlled release. Micro-computed tomography imaging confirmed new cancellous bone formation in rabbits after 8-week post-implantation. Alzubaydi et al. [Citation223] carried out in vivo studies in HAp and PSZ-coated Ti implants on rabbits. Having insights into the increased mechanical properties and great bond strength, the in vivo studies were left unexplored. They showed high osseointegration in addition to improved bioactivity. The Hap-coated Titanium enhanced bone healing response required for osseointegration and osteoconductive potential. The PSZ- and Hap-coated implants showed fibroblast tissues and osteoblasts which boosted new bone formation. Apart from new bone formation they also exhibited rapid and superior bone maturation. Cheng et al. [Citation224] studied a silver nanoparticle incorporated with TZP coating which showed extremely high antibacterial and bio-integration properties. Sacrificing thirty rats, which were divided into three groups for convenient studying, revealed that the Ag-coated Ti had extra-ordinary antibacterial activities due to the biofilm formation and prevention of bacterial adherence. The coating surfaces were compatible with osteoblasts and enhanced their proliferation. Comparable antibacterial activity was studied in vivo by Li et al. [Citation225] for Zinc-loaded TiO2 nanotubes. Rats were sacrificed to obtain the conclusion for in vivo studies which comprise enhanced osseointegration and lesser growth of bacteria. The reduction or suppression of bacteria was a boon for an implant that is already effectively showing mechanical properties and bioactivity. Notwithstanding in vivo studies are satisfying supplementary studies are a requisite for clinical testing of all coatings.
6.3.3. Stem cells and tissue regeneration studies
Stem cells are capable of self-renewal and differentiation and vary concerning their sources and potentials and also depending on their interaction with the microenvironment. Among many types of stem cells, adult stem cells are vital for bone, cartilage, etc. Typically bone regeneration is possible inherently by nature, but using bioceramics aids in accelerating bone regeneration. This contributes to the effect on Mesenchymal cells (MSC) which are familiar for improving angiogenesis and possess anti-inflammatory activities including cytokines that can regenerate and hence show high bone-regeneration applications. As they can differentiate into a variety of cells, they are explored for tissue regeneration in connective tissues [Citation226]. A Silica-based bioceramic composed of Calcium Silicates, Calcium Phosphates, ZrO2, etc. promote osteogenic and odontogenic stem cell proliferation according to the study of Chen et al. [Citation227]. They confine beneficial biocompatibility and stimulate cell proliferation, thereby accelerating the healing process. The granular surface of the bioceramic aids the proliferation of cells by attaching to the surface and hastening up spreading. HAp was also studied for its osteogenesis properties which were significantly visible during the study. HAp scaffolds with nanostructured surfaces have the capability of inducing osteogenesis by influencing Mesenchymal stem cells and thereby repair bone damage or injuries.
Bone morphogenic protein 2 (BMP 2) is a prominent factor in the osteogenic differentiation of stem cells. Among the certain other factors that affect osteogenic differentiation, there is an extracellular matrix (ECM), cytokines and various other growth factors which include the basic Fibroblast Growth Factor (bFGF) from Human umbilical vein endothelial cells [Citation228]. Bioactive ions that are coated over the implants tend to influence the stem cell lines by altering their microenvironment and their surface topography which, in turn, results in the restriction of ECM contact between the material and the cells and consecutively causes osteogenic differentiation. Pang et al. [Citation229] studied HAp and how it affects MC3T3-E1 cell behaviour. The author suggested that the HAp topography influenced osteogenesis by the proliferation of osteoblasts-like cells which includes osteocalcin, osteonectin, etc. and differentiation of certain cells. The rod-like HAp surface behaved more competently with a higher rate of proliferation. Guimarães et al. [Citation230] conducted a similar study, but with HAp and bioactive glass coated over zirconia. The studies on MC3T3-E1 cells showed better cell adhesion with improved surface texture which meant the laser sintering technique used for coatings showed meliorated bioactivity. Tissue regeneration studies are also highly decisive for biological evaluation studies. The topography of bioceramics was an important factor that could enhance tissue regeneration. Stem cells persist in a significant strategy of tissue regeneration till time. Certain authors have noticed that some bioceramics will release bioactive ions so that it would influence the stem cell behaviour, and tissue engineering applications could be met. Hence, bioceramics are an important component to be used as a scaffold [Citation231]. Poinern et al. [Citation232] studied nano-HAp for in vivo behaviour in sheep which offered good regeneration by showing well-grown granulation tissue alongside myofibre regeneration. Some other tissue components were also observed to regenerate including fibrins. A Silicate-based ceramic namely gehlenite showed better adhesion and osteogenesis by the proliferation of osteoblasts. The mechanical properties that were already studied did not give any details on bio-compliance, which made Esfahani et al. [Citation233] execute it and obtain the results which proved bone regeneration application. Similarly, Zhou et al. [Citation234] studied nano-HAp for application in the repair of hard tissues like bone or teeth. This study was a kickstart for future studies on the extensive application of the former as a scaffold in tissue applications. There is clear evidence of bioactive ions influencing the stem cells, thereby causing osteogenic differentiation to stem cells and also inducing osseointegration to the cell-implant interface and providing cell adhesion.
7. Discussions
In this research, we conduct a thorough review of the influence of mechanical behaviour on ceramic coatings achieved through distinct coating techniques. It is essential to comprehend how the coating techniques alter the mechanical behaviour since those assist in determining the method that extends the finest results. The literature review unveils that various factors affect mechanical properties such as microhardness, elastic modulus, adhesion strength, etc. Not only the coating but also the technique is extremely relevant when it comes to the discussion of mechanical properties. The coating techniques diverge in providing different coating characteristics. EPD technique can furnish thicker coatings that are crack-free. As crack-free surfaces can alter the mechanical properties, certain parameters like deposition time could be depleted to minimize the same and improve their properties. The surface cracks reduce the tensile strength of the coatings, also it was admitted that the EPD technique affects the mechanical properties a lot.
Nano-hydroxyapatite/nano-copper-nano titania coating fabricated by the EPD process was studied for its mechanical properties by various tests like nanoindentation. The physical adhesion of the coating was one of the principal aspects which influenced the mechanical behaviour. The process of EPD paved the way for coating complex structures with increased adhesion strength and decreased contact angle. Nanoindentation results suggested that with increased deposition time, an increase in thickness, hardness and adhesion was observed which was further confirmed by a nano-scratch test [Citation235]. Besides, HAp/CNT coatings over the Ti alloy by EPD also showed higher improvement in mechanical properties than pure HAp or pure CNT. Higher adhesion strength was indeed a remarkable property which was due to the EPD method since they provided thicker coatings of around 25–40 µm which are crack-free. Also increasing the deposition time contributed to an increase in the thickness and also caused cracks to form on the surface. Accordingly, minimizing deposition time was essential to minimize crack formation [Citation236]. Plasma spraying also affects the microstructure of the coatings. The process that includes high temperature for fabrication of coatings explains the change in surface microstructure and forms microcracks on the first layer of coating although scale down the same with increased process time, which, in turn, decreases porosity successively increasing the elastic modulus and residual stress of the coatings. Plasma-sprayed coatings form microcracks and pores when YSZ coatings are fabricated. In other words, the increased temperature during the fabricating process decreases the porosity and thereby increases the elastic modulus and residual stress of the coatings. Longer process time leads to denser coatings due to the disappearance of microcracks which further leads to decreased porosity due to the reduction of cracks. Hence deducing the microstructure of coatings attained by coating techniques determines the mechanical properties [Citation237]. Girolamo et al. [Citation238] studied plasma-sprayed Al2O3 coatings which show a porosity of 7.1% with a microhardness of 12.8 GPa. It demonstrated that a higher spraying distance would increase the microhardness as well. Magnetron sputtering technique that can provide smooth film exhibits high mechanical resistance due to increased elastic modulus that is dependent on the sputtering temperature. Magnetron sputtering techniques provide a smooth film that can exhibit increased mechanical resistance, due to higher elastic modulus. The hardness and elastic modulus are highly reliant upon the sputtering temperature. Establishing an association between microstructures and mechanical properties revealed the necessity of optimizing the sputtering parameters. With improved deposition temperature it favours the decline of elastic modulus along with hardness and accepted that mechanical behaviour depended upon surface topography [Citation239,Citation240]. Chernozem [Citation241] studied TiO2 and HAp coating fabricated by Magnetron sputtering. TiO2 nanotubes fabricated by the same method were familiar for their decreased elastic modulus and nano-hardness and TiO2 coating became more densified. Similarly, the sol–gel coating provides greater corrosion resistance instead of providing fair mechanical properties. The presence of cracks formed on sol–gel coated surfaces affects the mechanical behaviour. Sol–gel-coated HAp/TiO2 coatings exhibit prominent mechanical properties. An increase in strength is observed that accounts for an increase in TiO2 concentration and is not attributed to the sol–gel coating. The coating technique offers thin and dense coatings but being a composite, HAP/TiO2 shows advanced properties [Citation63,Citation242]. The PEO technique also aids in increasing the hardness and elastic modulus due to alterations in surface microstructures. Also, they show high surface roughness and porosity. Thus, studies elucidate that there is an evident influence of the coating method on mechanical behaviour. In conclusion, the surface topography and microstructure have an exceptional role in determining mechanical behaviour and they need to be chosen wisely for the best results.
The corrosion behaviour of the coatings should be inferred thoroughly, which may alter the mechanical properties. The studies showed that the corrosion shown by the coatings due to the ions in the body fluid may cause high deterioration in mechanical properties since they affect the tensile strength and fracture toughness of the implant. Also, the corrosion may induce certain structural alterations or ruptures in the coatings which in sequence lead to the direct contact of the implant surface to the body fluid that causes deterioration of all the characteristics of the implants including mechanical properties. The review on biological evaluation unveils that specified coating techniques and their parameters are capable of improving osseointegration. Additionally, each ceramic coating possesses certain biological properties apart from the mechanical properties such as HAp which has high biocompatibility. Hence these coatings fabricated by appropriate coating methods can improve their new-bone-formation capability and osseointegration property.
8. Conclusion
Quite a few surface modification techniques on ceramics such as composite and doping of ions in ceramics and various coating techniques are discussed. Particularly, relevant attributes for exceptional use in biomedical applications and the impact of miscellaneous processes on mechanical behaviour are meticulously reviewed. We pay heed to bioceramics which fall within the category of biomaterials that can assist and aid patients in distress due to underlying disease or injuries. Their advantages could be complemented by reinforcing several dopants to the parent material, each of the elements when doped in optimum concentration renders a specific function that can be tailored in accordance to our inclination and patient’s requirement.
Integration of dopants into parent material results in coatings with enhanced characteristics. It was inferred from different pieces of literature that the microstructure, roughness of the surface, thickness and adhesion of the coating are the rudimentary factors that hinge on the mechanical attributes. The necessity of the implant and the coating analogous to the mechanical behaviour of natural bone compels the optimization of the coating method to procure an efficient coating with appropriate attributes. Corrosion also plays a pivotal role in implant failure that cannot be ignored as it may generate toxicity and produce adverse effects or even damage tissues. Those coatings that successfully showed beneficial biological properties were further explored for in vivo studies which could be further extended to bone-tissue engineering and later on trailed to clinical trials and thereby used effectively in the biomedical domain.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Williams DF. Definitions in biomaterials. Amsterdam: Elsevier; 1987.
- Williams DF. On the nature of biomaterials. Biomaterials. 2009;30(30):5897–5909. doi:10.1016/j.biomaterials.2009.07.027
- Vallet-Regí M. Evolution of bioceramics within the field of biomaterials. C R Chim. 2010;13(1-2):174–185. doi:10.1016/j.crci.2009.03.004
- Navarro M, Michiardi A, Castano O, et al. Biomaterials in orthopaedics. J R Soc Interface. 2008;5(27):1137–1158. doi:10.1098/rsif.2008.0151
- Vert M. Polymeric biomaterials: strategies of the past vs. strategies of the future. Prog Polym Sci. 2007;32(8-9):755–761. doi:10.1016/j.progpolymsci.2007.05.006
- Piconi C. Bioinert ceramics: state-of-the-art. Key Eng Mater. 2017;758:3–13. doi:10.4028/www.scientific.net/KEM.758.3
- Vallet-Regí M, Salinas AJ. Ceramics as bone repair materials. Bone repair biomaterials: regeneration and clinical applications. 2nd ed. Woodhead Publishers; 2019, p. 141–178.
- Juhasz JA, Best SM. Bioactive ceramics: processing, structures and properties. J Mater Sci. 2011;47(2):610–624. doi:10.1007/s10853-011-6063-x
- Hench LL. The future of bioactive ceramics. J Mater Sci: Mater. 2015;26:1–4. doi:10.1007/s10856-015-5425-3
- Tan L, Yu X, Wan P, et al. Biodegradable materials for bone repairs: a review. J Mater Sci Technol. 2013;29(6):503–513. doi:10.1016/j.jmst.2013.03.002
- Godavitarne C, Robertson A, Peters J, et al. Biodegradable materials. J Orthop Trauma. 2017;31(5):316–320. doi:10.1016/j.mporth.2017.07.011
- Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24(13):2161–2175. doi:10.1016/S0142-9612(03)00044-9
- Ratha I, Datta P, Balla VK, et al. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: a review. Ceram Int. 2021;47(4):4426–4445. doi:10.1016/j.ceramint.2020.10.112
- Oluwatosin DA, El Mabrouk K, Bricha M. A review on borate bioactive glasses (BBG): effect of doping elements, degradation, and applications. J Mater Chem B. 2023;11:955–973.
- Albrektsson T, Zarb GA, Worthington P, et al. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11–25.
- Gross KA, Walsh W, Swarts E. Analysis of retrieved hydroxyapatite-coated hip prostheses. J Therm Spray Technol. 2004;13:190–199.
- Lopez-Esteban S, Saiz E, Fujino S, et al. Bioactive glass coatings for orthopedic metallic implants. J Eur Ceram. 2003;23(15):2921–2930. doi:10.1016/S0955-2219(03)00303-0
- Otsuka Y, Kagaya K, Miyashita Y. Effect of delamination of plasma sprayed hydroxyapatite coating on loosening behaviors of acetabular cups subjected to cyclic loading. Eng Failure Analysis. 2021;127:105548.
- Al-Amin M, Abdul Rani AM, Abdu Aliyu AA, et al. Bio-ceramic coatings adhesion and roughness of biomaterials through PM-EDM: a comprehensive review. Mater Manuf. 2020;35(11):1157–1180. doi:10.1080/10426914.2020.1772483
- He LH, Standard OC, Huang TTY, et al. Mechanical behaviour of porous hydroxyapatite. Acta Biomater. 2008;4(3):577–586. doi:10.1016/j.actbio.2007.11.002
- Obada DO, Dauda ET, Abifarin JT, et al. Mechanical properties of natural hydroxyapatite using low cold compaction pressure: effect of sintering temperature. Mater Chem Phys. 2019;239:122099. doi:10.1016/j.matchemphys.2019.122099
- Lacefield WR. Hydroxyapatite coatings. Ann N Y Acad Sci. 1988 Jan 1;523:72–80. doi:10.1111/j.1749-6632.1988.tb38501.x
- Aarthy S, Thenmuhil D, Dharunya G, et al. Exploring the effect of sintering temperature on naturally derived hydroxyapatite for bio-medical applications. J Mater Sci Mater Med. 2019;30:1–1. doi:10.1007/s10856-019-6219-9
- Arcos D, Vallet-Regí M. Substituted hydroxyapatite coatings of bone implants. J Mater Chem B. 2020;8(9):1781–1800. doi:10.1039/C9TB02710F
- Beig B, Liaqat U, Niazi MF, et al. Current challenges and innovative developments in hydroxyapatite-based coatings on metallic materials for bone implantation: a review. Coatings. 2020;10(12):1249. doi:10.3390/coatings10121249
- Zhang AM, Lenin P, Zeng RC, et al. Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys. J Magnes Alloy. 2022;10(5):1154–1170. doi:10.1016/j.jma.2022.01.001
- Lemoine P, Acheson J, McKillop S, et al. Nanoindentation and nano-scratching of hydroxyapatite coatings for magnesium alloy bone implant applications. J Mech Behav Biomed Mater. 2022;133:105306. doi:10.1016/j.jmbbm.2022.105306
- Kim SY, Kim DH, Kim YG, Oh CW, Ihn JC. Early failure of hemispheric Hydroxyapatite-coated acetabular cups. Clin Orthop Relat Res. 2006;446:233–238.
- Tallon C, Chuanuwatanakul C, Dunstan DE, et al. Mechanical strength and damage tolerance of highly porous alumina ceramics produced from sintered particle stabilized foams. Ceram Int. 2016;42(7):8478–8487. doi:10.1016/j.ceramint.2016.02.069
- Yokobori Jr AT, Adachi T, Yokobori T. Time-dependent mechanical behaviour of alumina ceramics. Eng Fract Mech. 1991;40(4-5):863–868. doi:10.1016/0013-7944(91)90245-V
- Hou G, An Y, Zhao X, et al. Improving interfacial, mechanical and tribological properties of alumina coatings on Al alloy by plasma arc heat-treatment of substrate. Appl Surf Sci. 2017;411:53–66. doi:10.1016/j.apsusc.2017.03.170
- Isik M, Avila JD, Bandyopadhyay A. Alumina and tricalcium phosphate added CoCr alloy for load-bearing implants. Addit Manuf. 2020;36:101553.
- Walpole AR, Briggs EP, Karlsson M, et al. Nano-porous alumina coatings for improved bone implant interfaces. Mater Werkst. 2003;34(12):1064–1068.
- Barkallah R, Taktak R, Guermazi N, et al. Mechanical properties and wear behaviour of alumina/tricalcium phosphate/titania ceramics as coating for orthopedic implant. Eng Fract Mech. 2021;241:107399. doi:10.1016/j.engfracmech.2020.107399
- Cheng KY, Gopal V, McNallan M, et al. Enhanced tribocorrosion resistance of hard ceramic coated Ti-6Al-4 V alloy for hip implant application: in-vitro simulation study. ACS Biomater Sci Eng. 2019;5(9):4817–4824. doi:10.1021/acsbiomaterials.9b00609
- Gil J, Pérez R, Herrero-Climent M, et al. Benefits of residual aluminum oxide for sand blasting titanium dental implants: osseointegration and bactericidal effects. Materials. 2022;15(1):178. doi:10.3390/ma15010178
- Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20(1):1–25. doi:10.1016/S0142-9612(98)00010-6
- Thompson I, Rawlings RD. Mechanical behaviour of zirconia and zirconia-toughened alumina in a simulated body environment. Biomaterials. 1990;11(7):505–508. doi:10.1016/0142-9612(90)90066-Y
- Rodríguez-Rojas F, Borrero-López Ó, Sánchez-González E, et al. On the durability of zirconia-reinforced lithium silicate and lithium disilicate dental ceramics under severe contact. Wear. 2022;508:204460. doi:10.1016/j.wear.2022.204460
- Pobloth AM, Mersiowsky MJ, Kliemt L, et al. Bioactive coating of zirconia toughened alumina ceramic implants improves cancellous osseointegration. Sci Rep. 2019;9(1):16692. doi:10.1038/s41598-019-53094-5
- Rajan ST, Terada-Nakaishi M, Chen P, et al. Zirconium-based metallic glass and zirconia coatings to inhibit bone formation on titanium. Biomed Mater. 2020;15(6):065019. doi:10.1088/1748-605X/aba23a
- Luo K, Zhou S, Wu L. High refractive index and good mechanical property UV-cured hybrid films containing zirconia nanoparticles. Thin Solid Films. 2009;517(21):5974–5980. doi:10.1016/j.tsf.2009.03.162
- Li L, Yao L, Wu L, et al. The characteristic and osteogenic effect of a nanoporous coating of zirconia implant. Ceram Int. 2022;48(17):24260–7. doi:10.1016/j.ceramint.2022.06.135
- Huang Z, Wang Z, Li C, et al. Application of plasma-sprayed zirconia coating in dental implants: study in implants. J Oral Implantol. 2018;44(2):102–109. doi:10.1563/aaid-joi-D-17-00020
- Wang G, Meng F, Ding C, et al. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010;6(3):990–1000. doi:10.1016/j.actbio.2009.09.021
- Beger B, Goetz H, Morlock M, et al. In vitro surface characteristics and impurity analysis of five different commercially available dental zirconia implants. Int J Implant Dent. 2018;4(1):1–0. doi:10.1186/s40729-018-0124-8
- Gil J, Delgado-García-Menocal JA, Velasco-Ortega E, et al. Comparison of zirconia degradation in dental implants and femoral balls: an X-ray diffraction and nanoindentation study. Int J Implant Dent. 2021;7(1):1–9. doi:10.1186/s40729-020-00284-w
- Kawanabe K, Yamamuro T, Nakamura T, et al. Effects of injecting massive amounts of bioactive ceramics in mice. J Biomed Mater Res. 1991;25(1):117–128. doi:10.1002/jbm.820250109
- Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74(7):1487–1510. doi:10.1111/j.1151-2916.1991.tb07132.x
- Mohammadi H, Sepantafar M, Ostadrahimi A. The role of bioinorganics in improving the mechanical properties of silicate ceramics as bone regenerative materials. J Ceram Sci Technol. 2015;6(1):1–8.
- Mohammadi H, Muhamad N, Sulong AB, et al. Recent advances on biofunctionalization of metallic substrate using ceramic coating: How far are we from clinically stable implant? J Taiwan Inst Chem Eng. 2021;118:254–270. doi:10.1016/j.jtice.2021.01.013
- Zhou P, Xia D, Ni Z, et al. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact Mater. 2021;6(3):810–822. doi:10.1016/j.bioactmat.2020.09.018
- Brunello G, Elsayed H, Biasetto L. Bioactive glass and silicate-based ceramic coatings on metallic implants: open challenge or outdated topic? Materials. 2019;12(18):2929. doi:10.3390/ma12182929
- Stan GE, Tite T, Popa AC, et al. The beneficial mechanical and biological outcomes of thin copper-gallium doped silica-rich bio-active glass implant-type coatings. Coatings. 2020;10(11):1119. doi:10.3390/coatings10111119
- Vijayalakshmi U, Rajeswari S. Development of silica glass coatings on 316L SS and evaluation of its corrosion resistance behavior in Ringer’s solution. Metall Mater Trans A. 2012;43:4907–4919. doi:10.1007/s11661-012-1283-5
- Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329–1342. doi:10.1016/S0142-9612(97)00217-2
- Smith L. Ceramic-plastic material as a bone substitute. Arch Surg. 1963;87(4):653–661. doi:10.1001/archsurg.1963.01310160115023
- Johnson I, Tian Q, Liu H. Nanostructured ceramic and ceramic-polymer composites as dual functional interface for bioresorbable metallic implants. MRS Online Proc Lib (OPL). 2014;1621:39–45. doi:10.1557/opl.2014.344
- Radtke A, Topolski A, Jędrzejewski T, et al. Bioactivity studies on titania coatings and the estimation of their usefulness in the modification of implant surfaces. Nanomaterials. 2017;7(4):90. doi:10.3390/nano7040090
- Safavi MS, Etminanfar M. A review on the prevalent fabrication methods, microstructural, mechanical properties, and corrosion resistance of nanostructured hydroxyapatite containing bilayer and multilayer coatings used in biomedical applications. J Ultrafine Grained Nanostruct Mater. 2019;52(1):1–7.
- Li J. Behaviour of titanium and titania-based ceramics in vitro and in vivo. Biomaterials. 1993;14(3):229–232. doi:10.1016/0142-9612(93)90028-Z
- Araghi A, Hadianfard MJ. Fabrication and characterization of functionally graded hydroxyapatite/TiO2 multilayer coating on Ti–6Al–4 V titanium alloy for biomedical applications. Ceram Inter. 2015;41(10):12668–12679. doi:10.1016/j.ceramint.2015.06.098
- Kim HW, Kim HE, Salih V, et al. Hydroxyapatite and titania sol–gel composite coatings on titanium for hard tissue implants; mechanical and in vitro biological performance. J Biomed Mater Res B Appl Biomater. 2005;72(1):1–8.
- Lee SH, Kim HE, Kim HW. Nano-sized hydroxyapatite coatings on Ti substrate with TiO2 buffer layer by E-beam deposition. J Am Ceram. 2007;90(1):50–56. doi:10.1111/j.1551-2916.2006.01351.x
- Rath PC, Besra L, Singh BP, et al. Titania/hydroxyapatite bi-layer coating on Ti metal by electrophoretic deposition: characterization and corrosion studies. Ceram Int. 2012;38(4):3209–3216. doi:10.1016/j.ceramint.2011.12.026
- Shojaee P, Afshar A. Effects of zirconia content on characteristics and corrosion behavior of hydroxyapatite/ZrO2 biocomposite coatings codeposited by electrodeposition. Surf Coat Technol. 2015;262:166–172. doi:10.1016/j.surfcoat.2014.12.044
- Prabakaran K, Kannan S, Rajeswari S. Development and characterisation of zirconia and hydroxyapatite composites for orthpaedic applications. Trends Biomater Artif Organs. 2005;18(2):114–116.
- Curran DJ, Fleming TJ, Towler MR, et al. Mechanical properties of hydroxyapatite–zirconia compacts sintered by two different sintering methods. J Mater Sci Mater Med. 2010;21:1109–1120. doi:10.1007/s10856-009-3974-z
- Sung YM, Shin YK, Ryu JJ. Preparation of hydroxyapatite/zirconia bioceramic nanocomposites for orthopaedic and dental prosthesis applications. Nanotechnology. 2007;18(6):065602. doi:10.1088/0957-4484/18/6/065602
- Matsuno T, Watanabe K, Ono K, et al. Microstructure and mechanical properties of sintered body of zirconia coated hydroxyapatite particles. J Mater Sci Lett. 2000;19(7):573–576. doi:10.1023/A:1006722110462
- Rapacz-Kmita A, Ślósarczyk A, Paszkiewicz Z. Mechanical properties of HAp–ZrO2 composites. J. Eur. Ceram. 2006;26(8):1481–1488. doi:10.1016/j.jeurceramsoc.2005.01.059
- Jun YK, Kim WH, Kweon OK, et al. The fabrication and biochemical evaluation of alumina reinforced calcium phosphate porous implants. Biomaterials. 2003;24(21):3731–3739. doi:10.1016/S0142-9612(03)00248-5
- An SH, Matsumoto T, Miyajima H, et al. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 2012;28(12):1221–1231. doi:10.1016/j.dental.2012.09.001
- Sanayei M, Nasiri-Tabrizi B, Ebrahimi-Kahrizsangi R, et al. Effect of alumina additives on the crystallite size and lattice strain of nanocrystalline hydroxyapatite obtained by Dry mechanochemical process. J Nano Res. 2010;11:145–149. doi:10.4028/www.scientific.net/JNanoR.11.145. Trans Tech Publications Ltd.
- Raj SV, Rajkumar M, Meenakshi Sundaram N, et al. Synthesis and characterization of hydroxyapatite/alumina ceramic nanocomposites for biomedical applications. Bull Mater Sci. 2018;41:1–8. doi:10.1007/s12034-017-1515-9
- de Lima MG, Leal E, Agnolon Pallone EM, et al. Microstructural and mechanical properties of Al2O3/HAp composites for use as bone substitute material. Mater Sci Forum. 2018;912:153–158. doi:10.4028/www.scientific.net/MSF.912.153. Trans Tech Publications Ltd.
- Juang HY, Hon MH. Fabrication and mechanical properties of hydroxyapatite-alumina composites. Mater Sci Eng C. 1994;2(1–2):77–81. doi:10.1016/0928-4931(94)90033-7
- Shanmugapriya SV, Padma Rao A, Senthil Kumar P, et al. Sol–gel derived Al2O3/Gr/HAP nanocomposite coatings on Ti–6Al–4 V alloy for enhancing tribo-mech properties and antibacterial activity for bone implants. Appl Phys A. 2022;128(8):635. doi:10.1007/s00339-022-05784-7
- Evis Z, Doremus RH. Coatings of hydroxyapatite—nanosize alpha alumina composites on Ti-6Al-4V. Mater Lett. 2005;59(29-30):3824–3827. doi:10.1016/j.matlet.2005.07.020
- Ha P, Kompoziti A, Za N, et al. Porous HA/alumina composites intended for bone-tissue engineering. Mater Tehnol. 2017;51(4):631–636. doi:10.17222/mit.2016.191
- Jia ZQ, Guo ZX, Chen F, et al. Microstructure, phase compositions and in vitro evaluation of freeze casting hydroxyapatite-silica scaffolds. Ceram Int. 2018;44(4):3636–3643. doi:10.1016/j.ceramint.2017.11.114
- Taha MA, Youness RA, Ibrahim M. Biocompatibility, physico-chemical and mechanical properties of hydroxyapatite-based silicon dioxide nanocomposites for biomedical applications. Ceram Int. 2020;46(15):23599–23610. doi:10.1016/j.ceramint.2020.06.132
- Yousefpour M, Taherian Z. The effects of ageing time on the microstructure and properties of mesoporous silica-hydroxyapatite nanocomposite. Superlatt Microstruct. 2013;54:78–86. doi:10.1016/j.spmi.2012.11.002
- Qiu ZY, Noh IS, Zhang SM. Silicate-doped hydroxyapatite and its promotive effect on bone mineralization. Front Mater Sci. 2013;7:40–50. doi:10.1007/s11706-013-0193-9
- Chellappa M, Vijayalakshmi U. Electrophoretic deposition of silica and its composite coatings on Ti-6Al-4V, and its in vitro corrosion behaviour for biomedical applications. Mater Sci Eng C. 2017;71:879–890. doi:10.1016/j.msec.2016.10.075
- Chellappa M, Vijayalakshmi U. Improved corrosion resistant and mechanical behavior of distinct composite coatings (silica/titania/zirconia) on Ti–6Al–4 V deposited by EPD. J Asian Ceram Soc. 2017;5(3):326–333. doi:10.1016/j.jascer.2017.06.005
- Ballarre J, Aydemir T, Liverani L, et al. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: improving early stage performance of titanium implants. Surf Coat Technol. 2020;381:125138. doi:10.1016/j.surfcoat.2019.125138
- Khan MU, Abd Razak SI, Rehman S, et al. Bioactive scaffold (sodium alginate)-g-(nHAp@ SiO2@ GO) for bone tissue engineering. Int J Biol Macromol. 2022;222:462–472. doi:10.1016/j.ijbiomac.2022.09.153
- White AA, Best SM, Kinloch IA. Hydroxyapatite–carbon nanotube composites for biomedical applications: a review. Int J Appl Ceram. 2007;4(1):1–3. doi:10.1111/j.1744-7402.2007.02113.x
- Ajayan PM. Nanotubes from carbon. Chem Rev. 1999;99(7):1787–1800. doi:10.1021/cr970102g
- Pei X, Zeng Y, He R, et al. Single-walled carbon nanotubes/hydroxyapatite coatings on titanium obtained by electrochemical deposition. Appl Surf Sci. 2014;295:71–80. doi:10.1016/j.apsusc.2014.01.009
- Xu JL, Khor KA, Sui JJ, et al. Preparation and characterization of a novel hydroxyapatite/carbon nanotubes composite and its interaction with osteoblast-like cells. Mater Sci Eng C. 2009;29(1):44–49. doi:10.1016/j.msec.2008.05.009
- Lahiri D, Ghosh S, Agarwal A. Carbon nanotube reinforced hydroxyapatite composite for orthopedic application: a review. Mater Sci Eng C. 2012;32(7):1727–1758. doi:10.1016/j.msec.2012.05.010
- Thom NT, Nam PT, Phuong NT, et al. Electrodeposition of hydroxyapatite/functionalized carbon nanotubes (HAp/fCNTs) coatings on the surface of 316L stainless steel. Vietnam J Sci Technol. 2017;55(6):706–715. doi:10.15625/2525-2518/55/6/9153
- Stango SA, Vijayalakshmi U. Synthesis and characterization of hydroxyapatite/carboxylic acid functionalized MWCNTS composites and its triple layer coatings for biomedical applications. Ceram Int. 2019;45(1):69–81. doi:10.1016/j.ceramint.2018.09.135
- Sivaraj D, Vijayalakshmi K, Ganeshkumar A, et al. Tailoring Cu substituted hydroxyapatite/functionalized multiwalled carbon nanotube composite coating on 316L SS implant for enhanced corrosion resistance, antibacterial and bioactive properties. Int J Pharm. 2020;590:119946. doi:10.1016/j.ijpharm.2020.119946
- Xavier SA, Vijayalakshmi U. Electrochemically grown functionalized multi-walled carbon nanotubes/hydroxyapatite hybrids on surgical grade 316L SS with enhanced corrosion resistance and bioactivity. Colloids Surf. B. 2018;171:186–196. doi:10.1016/j.colsurfb.2018.06.058
- Zhang Y, Nayak TR, Hong H, et al. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4(13):3833–3842. doi:10.1039/c2nr31040f
- Papageorgiou DG, Kinloch IA, Young RJ. Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci. 2017;90:75–127. doi:10.1016/j.pmatsci.2017.07.004
- Xie H, Cao T, Rodríguez-Lozano FJ, et al. Graphene for the development of the next-generation of biocomposites for dental and medical applications. Dent Mater. 2017;33(7):765–774. doi:10.1016/j.dental.2017.04.008
- Lee WC, Lim CH, Shi H, et al. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–7341. doi:10.1021/nn202190c
- Li M, Xiong P, Yan F, et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact Mater. 2018;3(1):1–8. doi:10.1016/j.bioactmat.2018.01.001
- Baudín C, Benet T, Pena P. Effect of graphene on setting and mechanical behaviour of tricalcium phosphate bioactive cements. J Mech Behav Biomed Mater. 2019;89:33–47. doi:10.1016/j.jmbbm.2018.09.002
- Baradaran S, Moghaddam E, Basirun WJ, et al. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon. 2014;69:32–45. doi:10.1016/j.carbon.2013.11.054
- Sebastin AX, Uthirapathy V. In vitro electrochemical behavior of sol-gel derived hydroxyapatite/graphene oxide composite coatings on 316L SS for biomedical applications. ChemistrySelect. 2020;5(39):12140–7. doi:10.1002/slct.202003368
- Li M, Liu Q, Jia Z, et al. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon. 2014;67:185–197. doi:10.1016/j.carbon.2013.09.080
- Aslam Khan MU, Haider A, Abd Razak SI, et al. Arabinoxylan/graphene-oxide/nHAp-NPs/PVA bionano composite scaffolds for fractured bone healing. J Tissue Eng Regen. 2021;15(4):322–335. doi:10.1002/term.3168
- Al-Arjan WS, Aslam Khan MU, Nazir S, et al. Development of arabinoxylan-reinforced apple pectin/graphene oxide/nano-hydroxyapatite based nanocomposite scaffolds with controlled release of drug for bone tissue engineering: In-vitro evaluation of biocompatibility and cytotoxicity against MC3T3-E1. Coatings. 2020;10(11):1120. doi:10.3390/coatings10111120
- Arul Xavier Stango S, Vijayalakshmi U. Electrolytic deposition of composite coatings on 316L SS and its in vitro corrosion resistive behavior in simulated body fluid solution. Chem Pap. 2021;75(9):4779–4791. doi:10.1007/s11696-021-01657-0
- Wu H, Zhang R, Li X, et al. Doping inorganic ions to regulate bioactivity of Ca–P coating on bioabsorbable high purity magnesium. Prog Nat Sci. 2014;24(5):479–485. doi:10.1016/j.pnsc.2014.08.004
- Nguyen TD, Jang YS, Lee MH, et al. Effect of strontium doping on the biocompatibility of calcium phosphate-coated titanium substrates. J Appl Biomater Funct Mater. 2019;17(1):2280800019826517.
- Singh SS, Roy A, Lee BE, et al. A study of strontium doped calcium phosphate coatings on AZ31. Mater Sci Eng C. 2014;40:357–365. doi:10.1016/j.msec.2014.03.062
- Curran DJ, Fleming TJ, Towler MR, et al. Mechanical parameters of strontium doped hydroxyapatite sintered using microwave and conventional methods. J Mech Behav Biomed Mater. 2011;4(8):2063–2073. doi:10.1016/j.jmbbm.2011.07.005
- Pal A, Nasker P, Paul S, et al. Strontium doped hydroxyapatite from Mercenaria clam shells: synthesis, mechanical and bioactivity study. J Mech Behav Biomed Mater. 2019;90:328–336. doi:10.1016/j.jmbbm.2018.10.027
- Chetan VU. Structural phase formation and in vitro bioactivity evaluations of strontium phosphosilicate for orthopedic applications. J Biomed Mater Res Part B Appl Biomater. 2020;108(8):3286–3301. doi:10.1002/jbm.b.34665
- Pietak AM, Reid JW, Stott MJ, et al. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28(28):4023–4032. doi:10.1016/j.biomaterials.2007.05.003
- Fu Q, Liu Q, Li L, et al. Effect of doping different Si source on Ca-P bioceramic coating fabricated by laser cladding. J Appl Biomater Funct Mater. 2020;18:2280800020917322.
- Vladescu A, Birlik I, Braic V, et al. Enhancement of the mechanical properties of hydroxyapatite by SiC addition. J Mech Behav Biomed Mater. 2014;40:362–368. doi:10.1016/j.jmbbm.2014.08.025
- Yong TC, Aw KL, Yeo WH, et al. Influence of magnesium doping in hydroxyapatite ceramics. In: 4th Kuala Lumpur International Conference on Biomedical Engineering 2008: BIOMED 2008 25–28 June 2008 Kuala Lumpur, Malaysia; 2008. p. 326–329. Springer Berlin Heidelberg.
- Shahrezaee M, Raz M, Shishehbor S, et al. Synthesis of magnesium doped amorphous calcium phosphate as a bioceramic for biomedical application: in vitro study. Silicon. 2018;10:1171–1179. doi:10.1007/s12633-017-9589-y
- Xu Z, Li M, Li X, et al. Antibacterial activity of silver doped titanate nanowires on Ti implants. ACS Appl Mater Interfaces. 2016;8(26):16584–16594. doi:10.1021/acsami.6b04161
- Dubnika A, Loca D, Rudovica V, et al. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram Int. 2017 Mar 1;43(4):3698–3705. doi:10.1016/j.ceramint.2016.11.214
- Mazare A, Anghel A, Surdu-Bob C, et al. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Films. 2018;657:16–23. doi:10.1016/j.tsf.2018.04.036
- Yanovska AA, Stanislavov AS, Sukhodub LB, et al. Silver-doped hydroxyapatite coatings formed on Ti–6Al–4 V substrates and their characterization. Mater Sci Eng C. 2014;36:215–220. doi:10.1016/j.msec.2013.12.011
- Thukkaram M, Cools P, Nikiforov A, et al. Antibacterial activity of a porous silver doped TiO2 coating on titanium substrates synthesized by plasma electrolytic oxidation. Appl Surf Sci. 2020;500:144235. doi:10.1016/j.apsusc.2019.144235
- Huang HL, Chang YY, Weng JC, et al. Anti-bacterial performance of zirconia coatings on titanium implants. Thin Solid Films. 2013;528:151–156. doi:10.1016/j.tsf.2012.07.143
- Anjaneyulu U, Priyadarshini B, Nirmala Grace A, et al. Fabrication and characterization of Ag doped hydroxyapatite-polyvinyl alcohol composite nanofibers and its in vitro biological evaluations for bone tissue engineering applications. J Solgel Sci Technol. 2017;81:750–761. doi:10.1007/s10971-016-4243-5
- Refaat A, Youness RA, Taha MA, et al. Effect of zinc oxide on the electronic properties of carbonated hydroxyapatite. J Mol Struct. 2017;1147:148–154. doi:10.1016/j.molstruc.2017.06.091
- Robinson L, Salma-Ancane K, Stipniece L, et al. The deposition of strontium and zinc Co-substituted hydroxyapatite coatings. J Mater Sci Mater Med. 2017;28:1–4. doi:10.1007/s10856-017-5846-2
- Vijayalakshmi U, Chellappa M, Anjaneyulu U, et al. Influence of coating parameter and sintering atmosphere on the corrosion resistance behavior of electrophoretically deposited composite coatings. Mater Manuf. 2016;31(1):95–106. doi:10.1080/10426914.2015.1070424
- Zhang C, Uchikoshi T, Liu L, et al. Synthesis of Eu-doped hydroxyapatite whiskers and fabrication of phosphor layer via electrophoretic deposition process. J Am Ceram. 2020;103(12):6780–6792. doi:10.1111/jace.17147
- Chen F, Huang P, Zhu YJ, et al. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials. 2011;32(34):9031–9039. doi:10.1016/j.biomaterials.2011.08.032
- Stojadinović S, Vasilić R. Formation and photoluminescence of Eu3 doped zirconia coatings formed by plasma electrolytic oxidation. J Lumin. 2016;176:25–31. doi:10.1016/j.jlumin.2016.03.012
- Xiaohong W, Wei Q, Xianbo D, et al. Photocatalytic activity of Eu-doped TiO2 ceramic films prepared by microplasma oxidation method. J Phys Chem Solids. 2007;68(12):2387–2393. doi:10.1016/j.jpcs.2007.07.073
- Kumar A, Babu S, Karakoti AS, et al. Luminescence properties of europium-doped cerium oxide nanoparticles: role of vacancy and oxidation states. Langmuir. 2009;25(18):10998–11007. doi:10.1021/la901298q
- Zhang K, Holloway T, Bahoura M, et al. Europium doped Gd2O3 and FeCo nanoparticles for biomedical application. Nanosens Biosens Info-Tech Sens Syst. 2009;7291:37–46. SPIE.
- Oesterle A, Boehm AV, Müller FA. Photoluminescent Eu3+-doped calcium phosphate bone cement and its mechanical properties. Materials. 2018;11(9):1610. doi:10.3390/ma11091610
- Ciobanu G, Harja M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram Int. 2019;45(2):2852–2857. doi:10.1016/j.ceramint.2018.07.290
- Priyadarshini B, Anjaneyulu U, Vijayalakshmi U. Preparation and characterization of sol-gel derived Ce4 + doped hydroxyapatite and its in vitro biological evaluations for orthopedic applications. Mater Des. 2017;119:446–455. doi:10.1016/j.matdes.2017.01.095
- Montazerian M, Hosseinzadeh F, Migneco C, et al. Bioceramic coatings on metallic implants: an overview. Ceram Int. 2022;48(7):8987–9005. doi:10.1016/j.ceramint.2022.02.055
- Lugscheider E, Weber T, Knepper M, et al. Production of biocompatible coatings by atmospheric plasma spraying. Mater Sci Eng A. 1991;139:45–48. doi:10.1016/0921-5093(91)90594-D
- Cheang P, Khor KA. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials. 1996;17(5):537–544. doi:10.1016/0142-9612(96)82729-3
- Kuo TY, Chin WH, Chien CS, et al. Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf Coat Technol. 2019;372:399–409. doi:10.1016/j.surfcoat.2019.05.003
- Yang CY, Wang BC, Chang E, et al. The influences of plasma spraying parameters on the characteristics of hydroxyapatite coatings: a quantitative study. J Mater Sci Mater Med. 1995;6:249–257. doi:10.1007/BF00120267
- Bansal P, Singh G, Sidhu HS. Investigation of surface properties and corrosion behavior of plasma sprayed HA/ZnO coatings prepared on AZ31 Mg alloy. Surf Coat Technol. 2020;401:126241. doi:10.1016/j.surfcoat.2020.126241
- Singh S, Prakash C, Singh H. Deposition of HA-TiO2 by plasma spray on β-phase Ti-35Nb-7Ta-5Zr alloy for hip stem: characterization, mechanical properties, corrosion, and in-vitro bioactivity. Surf Coat Technol. 2020;398:126072. doi:10.1016/j.surfcoat.2020.126072
- Singh H, Kumar R, Prakash C, et al. HA-based coating by plasma spray techniques on titanium alloy for orthopedic applications. Mater Today: Proc. 2022;50:612–628. doi:10.1016/j.matpr.2021.03.165
- Bargavi P, Chitra S, Durgalakshmi D, et al. Zirconia reinforced bio-active glass coating by spray pyrolysis: structure, surface topography, in-vitro biological evaluation and antibacterial activities. Mater Today Commun. 2020;25:101253. doi:10.1016/j.mtcomm.2020.101253
- Chou YJ, Ningsih HS, Shih SJ. Preparation, characterization and investigation of antibacterial silver-zinc co-doped β-tricalcium phosphate by spray pyrolysis. Ceram Int. 2020;46(10):16708–16715. doi:10.1016/j.ceramint.2020.03.245
- Jokanovic V, Uskokovic D. Calcium hydroxyapatite thin films on titanium substrates prepared by ultrasonic spray pyrolysis. Mater Trans. 2005;46(2):228–235. doi:10.2320/matertrans.46.228
- Sivaraj D, Vijayalakshmi K. Enhanced corrosion resistance and antibacterial activity of Zn-HA decorated MWCNTs film coated on medical grade 316L SS implant by novel spray pyrolysis technique. J Anal Appl Pyrolysis. 2018;134:176–182. doi:10.1016/j.jaap.2018.06.006
- Cho JS, Jung DS, Han JM, et al. Nano-sized α and β-TCP powders prepared by high temperature flame spray pyrolysis. Mater Sci Eng C. 2009;29(4):1288–1292. doi:10.1016/j.msec.2008.10.020
- Visentin F, El Habra N, Fabrizio M, et al. TiO2-HA bi-layer coatings for improving the bioactivity and service-life of Ti dental implants. Surf Coat Technol. 2019;378:125049. doi:10.1016/j.surfcoat.2019.125049
- Ghanbari A, Khiabani AB, Zamanian A, et al. The competitive mechanism of plasma electrolyte oxidation for the formation of magnesium oxide bioceramic coatings. Mater Today Proc. 2018;5(7):15677–15685. doi:10.1016/j.matpr.2018.04.178
- Hryniewicz T. Plasma electrolytic oxidation of metals and alloys. Metals. 2018;8(12):1058. doi:10.3390/met8121058
- Kazek-Kęsik A, Leśniak K, Zhidkov IS, et al. Influence of alkali treatment on anodized titanium alloys in wollastonite suspension. Metals. 2017;7(9):322. doi:10.3390/met7090322
- Rokosz K, Hryniewicz T, Gaiaschi S, et al. Characterization of porous phosphate coatings enriched with magnesium or zinc on cp titanium grade 2 under DC plasma electrolytic oxidation. Metals. 2018;8(2):112. doi:10.3390/met8020112
- Sieber M, Simchen F, Morgenstern R, et al. Plasma electrolytic oxidation of high-strength aluminium alloys—substrate effect on wear and corrosion performance. Metals. 2018;8(5):356. doi:10.3390/met8050356
- Sampatirao H, Radhakrishnapillai S, Dondapati S, et al. Developments in plasma electrolytic oxidation (PEO) coatings for biodegradable magnesium alloys. Mater Today: Proc. 2021;46:1407–1415. doi:10.1016/j.matpr.2021.02.650
- Molaei M, Fattah-alhosseini A, Nouri M, et al. Systematic optimization of corrosion, bioactivity, and biocompatibility behaviors of calcium-phosphate plasma electrolytic oxidation (PEO) coatings on titanium substrates. Ceram Int. 2022;48(5):6322–6337. doi:10.1016/j.ceramint.2021.11.175
- Fattah-alhosseini A, Chaharmahali R, Keshavarz MK, et al. Surface characterization of bioceramic coatings on Zr and its alloys using plasma electrolytic oxidation (PEO): a review. Surf. Interf. 2021;25:101283.
- Boccaccini AR, Zhitomirsky I. Application of electrophoretic and electrolytic deposition techniques in ceramics processing. Curr Opin Solid State Mater Sci. 2002;6(3):251–260. doi:10.1016/S1359-0286(02)00080-3
- Corni I, Ryan MP, Boccaccini AR. Electrophoretic deposition: from traditional ceramics to nanotechnology. J Eur Ceram. 2008;28(7):1353–1367. doi:10.1016/j.jeurceramsoc.2007.12.011
- Boccaccini AR, Cho J, Subhani T, et al. Electrophoretic deposition of carbon nanotube–ceramic nanocomposites. J Eur Ceram. 2010;30(5):1115–1129. doi:10.1016/j.jeurceramsoc.2009.03.016
- Kwok CT, Wong PK, Cheng FT, et al. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Appl Surf Sci. 2009;255(13–14):6736–6744. doi:10.1016/j.apsusc.2009.02.086
- Aksoy ME, Aksakal B, Aslan N, et al. Enhanced adhesion and corrosion properties of boron doped bioceramic coated 316L implants. Prot Met Phys Chem. 2021;57:1040–1050. doi:10.1134/S2070205121050026
- Drevet R, Jaber NB, Fauré J, et al. Electrophoretic deposition (EPD) of nano-hydroxyapatite coatings with improved mechanical properties on prosthetic Ti6Al4V substrates. Surf Coat Technol. 2016;301:94–99. doi:10.1016/j.surfcoat.2015.12.058
- Nawaz Q, Fastner S, Rehman MA, et al. Multifunctional stratified composite coatings by electrophoretic deposition and RF co-sputtering for orthopaedic implants. J Mater Sci. 2021;56:7920–7935. doi:10.1007/s10853-020-05725-w
- Barrow DA, Petroff TE, Sayer MB. Thick ceramic coatings using a sol gel based ceramic-ceramic 0–3 composite. Surf Coat Technol. 1995;76:113–118. doi:10.1016/0257-8972(95)02562-6
- Guglielmi M. Sol-gel coatings on metals. J Solgel Sci Technol. 1997;8:443–449.
- Tranquillo E, Bollino F. Surface modifications for implants lifetime extension: an overview of sol-gel coatings. Coatings. 2020;10(6):589. doi:10.3390/coatings10060589
- Advincula MC, Petersen D, Rahemtulla F, et al. Surface analysis and biocorrosion properties of nanostructured surface sol–gel coatings on Ti6Al4V titanium alloy implants. J Biomed Mater Res Part B Appl Biomater. 2007;80(1):107–120.
- Vijayalakshmi U, Rajeswari S. Influence of process parameters on the sol–gel synthesis of nano hydroxyapatite using various phosphorus precursors. J Solgel Sci Technol. 2012;63:45–55. doi:10.1007/s10971-012-2762-2
- Priyadarshini B, Ramya S, Shinyjoy E, et al. Structural, morphological and biological evaluations of cerium incorporated hydroxyapatite sol–gel coatings on Ti–6Al–4 V for orthopaedic applications. J Mater Res Technol. 2021;12:1319–1338. doi:10.1016/j.jmrt.2021.03.009
- Nichol T, Callaghan J, Townsend R, et al. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Joint J. 2021;103(3):522–529. doi:10.1302/0301-620X.103B3.BJJ-2020-0347.R1
- Valli J, Mäkelä U. Applications of the scratch test method for coating adhesion assessment. Wear. 1987;115(1-2):215–221. doi:10.1016/0043-1648(87)90211-0
- Schwarzer N, Duong QH, Bierwisch N, et al. Optimization of the scratch test for specific coating designs. Surf Coat Technol. 2011;206(6):1327–1335. doi:10.1016/j.surfcoat.2011.08.051
- Bull SJ, Berasetegui EG. An overview of the potential of quantitative coating adhesion measurement by scratch testing. Tribol Int. 2006;39(2):99–114. doi:10.1016/j.triboint.2005.04.013
- Drevet R, Jaber NB, Fauré J, et al. Electrophoretic deposition (EPD) of nano-hydroxyapatite coatings with improved mechanical properties on prosthetic Ti6Al4V substrates. Surf Coat Technol. 2016;301:94–99. doi:10.1016/j.surfcoat.2015.12.058
- Wei M, Ruys AJ, Swain MV, et al. Interfacial bond strength of electrophoretically deposited hydroxyapatite coatings on metals. J Mater Sci Mater Med. 1999;10:401–409. doi:10.1023/A:1008923029945
- Bull SJ. Nanoindentation of coatings. J Phys D. 2005;38(24):R393. doi:10.1088/0022-3727/38/24/R01
- Chen Z, Zhou K, Lu X, et al. A review on the mechanical methods for evaluating coating adhesion. Acta Mech. 2014;225:431–452. doi:10.1007/s00707-013-0979-y
- Raj V, Ali MM. Formation of ceramic alumina nanocomposite coatings on aluminium for enhanced corrosion resistance. J Mater Process Technol. 2009;209(12-13):5341–5352. doi:10.1016/j.jmatprotec.2009.04.004
- Karacan I, Ben-Nissan B, Wang HA, et al. Mechanical testing of antimicrobial biocomposite coating on metallic medical implants as drug delivery system. Mater Sci Eng C. 2019;104:109757. doi:10.1016/j.msec.2019.109757
- Dey A, Mukhopadhyay AK, Gangadharan S, et al. Nanoindentation study of microplasma sprayed hydroxyapatite coating. Ceram Int. 2009;35(6):2295–2304. doi:10.1016/j.ceramint.2009.01.002
- Saber-Samandari S, Berndt CC, Gross KA. Selection of the implant and coating materials for optimized performance by means of nanoindentation. Acta Biomater. 2011;7(2):874–881. doi:10.1016/j.actbio.2010.09.023
- Rattan V, Sidhu TS, Mittal M. Wear studies on plasma-sprayed pure and reinforced hydroxyapatite coatings. Mater Today Proc. 2022;60:1731–1735. doi:10.1016/j.matpr.2021.12.306
- Baligidad SM, Arunkumar T, Thodda G, et al. Fabrication of HAp/rGO nanocomposite coating on PEEK: tribological performance study. Surf Interfaces. 2023;38:102865. doi:10.1016/j.surfin.2023.102865
- Molaei M, Fattah-Alhosseini A, Nouri M, et al. Role of TiO2 nanoparticles in wet friction and wear properties of PEO coatings developed on pure titanium. Metals. 2023;13(4):821. doi:10.3390/met13040821
- Chiu KY, Wong MH, Cheng FT, et al. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf Coat Technol. 2007;202(3):590–598. doi:10.1016/j.surfcoat.2007.06.035
- Yao Z, Jiang Z, Wang F. Study on corrosion resistance and roughness of micro-plasma oxidation ceramic coatings on Ti alloy by EIS technique. Electrochim Acta. 2007;52(13):4539–4546. doi:10.1016/j.electacta.2006.12.052
- Morks MF, Kobayashi A. Development of ZrO2/SiO2 bioinert ceramic coatings for biomedical application. J Mech Behav Biomed Mater. 2008;1(2):165–171. doi:10.1016/j.jmbbm.2007.09.002
- Guan Y, Xia Y, Li G. Growth mechanism and corrosion behavior of ceramic coatings on aluminum produced by autocontrol AC pulse PEO. Surf Coat Technol. 2008;202(19):4602–4612. doi:10.1016/j.surfcoat.2008.03.031
- Rath PC, Besra L, Singh BP, et al. Titania/hydroxyapatite bi-layer coating on Ti metal by electrophoretic deposition: characterization and corrosion studies. Ceram Int. 2012;38(4):3209–3216. doi:10.1016/j.ceramint.2011.12.026
- Hadad AAE, Peón E, García-Galván FR, et al. Biocompatibility and corrosion protection behaviour of hydroxyapatite sol-gel-derived coatings on Ti6Al4V alloy. Materials. 2017;10(2):94. doi:10.3390/ma10020094
- Sidane D, Chicot D, Yala S, et al. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol–gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films. 2015;593:71–80. doi:10.1016/j.tsf.2015.09.037
- Aruna ST, Balaji N, Shedthi J, et al. Effect of critical plasma spray parameters on the microstructure, microhardness and wear and corrosion resistance of plasma sprayed alumina coatings. Surf Coat Technol. 2012;208:92–100. doi:10.1016/j.surfcoat.2012.08.016
- Mirhashemihaghighi S, Światowska J, Maurice V, et al. Corrosion protection of aluminium by ultra-thin atomic layer deposited alumina coatings. Corros Sci. 2016;106:16–24. doi:10.1016/j.corsci.2016.01.021
- Norouzi M, Garekani AA. Corrosion protection by zirconia-based thin films deposited by a sol–gel spin coating method. Ceram Int. 2014;40(2):2857–2861. doi:10.1016/j.ceramint.2013.10.027
- Tiwari SK, Adhikary J, Singh TB, et al. Preparation and characterization of sol–gel derived yttria doped zirconia coatings on AISI 316L. Thin Solid Films. 2009;517(16):4502–4508. doi:10.1016/j.tsf.2008.12.025
- Barati N, Yerokhin A, Golestanifard F, et al. Alumina-zirconia coatings produced by plasma electrolytic oxidation on Al alloy for corrosion resistance improvement. J Alloys Compd. 2017;724:435–442. doi:10.1016/j.jallcom.2017.07.058
- Sebastin AX, Uthirapathy V. In vitro electrochemical behavior of sol-gel derived hydroxyapatite/graphene oxide composite coatings on 316L SS for biomedical applications. ChemistrySelect. 2020;5(39):12140–7. doi:10.1002/slct.202003368
- Wu C, Xiao Y. Evaluation of the in vitro bioactivity of bioceramics. Bone Tissue Regen. Insights. 2009;2:25–29.
- Khan MU, Al-Arjan WS, Ashammakhi N, et al. Multifunctional bioactive scaffolds from ARX-g-(Zn@ rGO)-HAp for bone tissue engineering: In vitro antibacterial, antitumor, and biocompatibility evaluations. ACS Appl Bio Mater. 2022;5(11):5445–5456. doi:10.1021/acsabm.2c00777
- Khan MU, Razak SI, Ansari MN, et al. Development of biodegradable bio-based composite for bone tissue engineering: synthesis, characterization and in vitro biocompatible evaluation. Polymers. 2021;13(21):3611. doi:10.3390/polym13213611
- Khan MUA, Al-Arjan WS, Binkadem MS, et al. Development of biopolymeric hybrid scaffold-based on AAc/GO/nHAp/TiO2 nanocomposite for bone tissue engineering: In-vitro analysis. Nanomaterials. 2021;11(5):1319. doi:10.3390/nano11051319
- Honda M, Kikushima K, Kawanobe Y, et al. Enhanced early osteogenic differentiation by silicon-substituted hydroxyapatite ceramics fabricated via ultrasonic spray pyrolysis route. J Mater Sci Mater Med. 2012;23:2923–2932. doi:10.1007/s10856-012-4744-x
- Sasireka A, Renji R, Raj RM, et al. Exploration on in vitro bioactivity, antibacterial activity and corrosion behavior of strontium doped hydroxyapatite reinforced chitosan-polypyrrole/TNT for bone regeneration. Inorg Chem Commun. 2022;142:109621. doi:10.1016/j.inoche.2022.109621
- Aktug SL, Durdu S, Aktas S, et al. Surface and in vitro properties of Ag-deposited antibacterial and bioactive coatings on AZ31 Mg alloy. Surf Coat Technol. 2019;375:46–53. doi:10.1016/j.surfcoat.2019.07.013
- Radha G, Balakumar S, Venkatesan B, et al. Evaluation of hemocompatibility and in vitro immersion on microwave-assisted hydroxyapatite–alumina nanocomposites. Mater Sci Eng C. 2015;50:143–150. doi:10.1016/j.msec.2015.01.054
- Lv Y, Zhang T, Zhang X, et al. The synergistic effect of Ag and ZnO on the microstructure, corrosion resistance and in vitro biological performance of titania coating. Surf Coat Technol. 2021;426:127798. doi:10.1016/j.surfcoat.2021.127798
- Amiri H, Mohammadi I, Afshar A. Electrophoretic deposition of nano-zirconia coating on AZ91D magnesium alloy for bio-corrosion control purposes. Surf Coat Technol. 2017;311:182–190. doi:10.1016/j.surfcoat.2016.12.103
- Wang G, Meng F, Ding C, et al. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010;6(3):990–1000. doi:10.1016/j.actbio.2009.09.021
- Jemat A, Ghazali MJ, Razali M, et al. Effects of TiO2 on microstructural, mechanical properties and in-vitro bioactivity of plasma sprayed yttria stabilised zirconia coatings for dental application. Ceram Int. 2018;44(4):4271–4281. doi:10.1016/j.ceramint.2017.12.008
- Huang HL, Chang YY, Weng JC, et al. Anti-bacterial performance of zirconia coatings on titanium implants. Thin Solid Films. 2013;528:151–156. doi:10.1016/j.tsf.2012.07.143
- Robertson SF, Bandyopadhyay A, Bose S. Titania nanotube interface to increase adhesion strength of hydroxyapatite sol-gel coatings on Ti-6Al-4 V for orthopedic applications. Surf Coat Technol. 2019;372:140–147. doi:10.1016/j.surfcoat.2019.04.071
- Aksakal B, Say Y, Sinirlioglu ZA. Effects of silver/selenium/chitosan doped hydroxyapatite coatings on REX-734 alloy: morphology, antibacterial activity, and cell viability. Mater Today Commun. 2022;33:104246. doi:10.1016/j.mtcomm.2022.104246
- Thrivikraman G, Madras G, Basu B. In vitro/in vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates. RSC Adv. 2014;4(25):12763–12781. doi:10.1039/c3ra44483j
- Ghorbel H, Guidara A, Guidara R, et al. Assessment of the addition of fluorapatite–alumina coating for a durable adhesion of the interface prosthesis/bone cells: implementation in vivo. J Med Biol Eng. 2020;40:158–168. doi:10.1007/s40846-019-00498-3
- Kim J, Kang IG, Cheon KH, et al. Stable sol–gel hydroxyapatite coating on zirconia dental implant for improved osseointegration. J Mater Sci: Mater. 2021;32:1–0.
- Roy M, Bandyopadhyay A, Bose S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf Coat Technol. 2011;205(8-9):2785–2792. doi:10.1016/j.surfcoat.2010.10.042
- Jiang J, Han G, Zheng X, et al. Characterization and biocompatibility study of hydroxyapatite coating on the surface of titanium alloy. Surf Coat Technol. 2019;375:645–651. doi:10.1016/j.surfcoat.2019.07.067
- Alzubaydi TL, AlAmeer SS, Ismaeel T, et al. In vivo studies of the ceramic coated titanium alloy for enhanced osseointegration in dental applications. J Mater Sci Mater Med. 2009;20:35–42. doi:10.1007/s10856-008-3479-1
- Cheng H, Li Y, Huo K, et al. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J Biomed Mater Res A. 2014;102(10):3488–3499. doi:10.1002/jbm.a.35019
- Li Y, Xiong W, Zhang C, et al. Enhanced osseointegration and antibacterial action of zinc-loaded titania-nanotube-coated titanium substrates: In vitro and in vivo studies. J Biomed Mater Res A. 2014;102(11):3939–3950. doi:10.1002/jbm.a.35060
- Köse S, Kankilic B, Gizer M, et al. Stem cell and advanced nano bioceramic interactions. Novel Biomater Regen Med. 2018: 317–342. doi:10.1007/978-981-13-0947-2_17
- Chen I, Salhab I, Setzer FC, et al. A new calcium silicate–based bioceramic material promotes human osteo-and odontogenic stem cell proliferation and survival via the extracellular signal-regulated kinase signaling pathway. J Endod. 2016;42(3):480–486. doi:10.1016/j.joen.2015.11.013
- Zhou Y, Wu C, Chang J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater Today. 2019;24:41–56. doi:10.1016/j.mattod.2018.07.016
- Pang S, He Y, He P, et al. Fabrication of two distinct hydroxyapatite coatings and their effects on MC3T3-E1 cell behavior. Colloids Surf B. 2018;171:40–48. doi:10.1016/j.colsurfb.2018.06.046
- Mesquita-Guimarães J, Detsch R, Souza AC, et al. Cell adhesion evaluation of laser-sintered HAp and 45S5 bioactive glass coatings on micro-textured zirconia surfaces using MC3T3-E1 osteoblast-like cells. Mater Sci Eng C. 2020;109:110492. doi:10.1016/j.msec.2019.110492
- Zhou Y, Wu C, Chang J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater Today. 2019;24:41–56. doi:10.1016/j.mattod.2018.07.016
- Poinern GE, Brundavanam RK, Thi Le X, et al. The synthesis, characterisation and in vivo study of a bioceramic for potential tissue regeneration applications. Sci Rep. 2014;4(1):6235. doi:10.1038/srep06235
- Roohani-Esfahani SI, No YJ, Lu Z, et al. A bioceramic with enhanced osteogenic properties to regulate the function of osteoblastic and osteocalastic cells for bone tissue regeneration. Biomed Mater. 2016;11(3):035018. doi:10.1088/1748-6041/11/3/035018
- Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7(7):2769–2781. doi:10.1016/j.actbio.2011.03.019
- Bartmanski M, Zielinski A, Jazdzewska M, et al. Effects of electrophoretic deposition times and nanotubular oxide surfaces on properties of the nanohydroxyapatite/nanocopper coating on the Ti13Zr13Nb alloy. Ceram Int. 2019;45(16):20002–20010. doi:10.1016/j.ceramint.2019.06.258
- Kaya C. Electrophoretic deposition of carbon nanotube-reinforced hydroxyapatite bioactive layers on Ti–6Al–4 V alloys for biomedical applications. Ceram Int. 2008;34(8):1843–1847. doi:10.1016/j.ceramint.2007.06.007
- Qiao X, Wang YM, Weng WX, et al. Influence of pores on mechanical properties of plasma sprayed coatings: case study of YSZ thermal barrier coatings. Ceram Int. 2018;44(17):21564–21577. doi:10.1016/j.ceramint.2018.08.220
- Di Girolamo G, Brentari A, Blasi C, et al. Microstructure and mechanical properties of plasma sprayed alumina-based coatings. Ceram Int. 2014;40(8):12861–7. doi:10.1016/j.ceramint.2014.04.143
- Nelea V, Morosanu C, Iliescu M, et al. Microstructure and mechanical properties of hydroxyapatite thin films grown by RF magnetron sputtering. Surf Coat Technol. 2003;173(2-3):315–322. doi:10.1016/S0257-8972(03)00729-1
- Bramowicz M, Braic L, Azem FA, et al. Mechanical properties and fractal analysis of the surface texture of sputtered hydroxyapatite coatings. Appl Surf Sci. 2016;379:338–346. doi:10.1016/j.apsusc.2016.04.077
- Chernozem RV, Surmeneva MA, Krause B, et al. Hybrid biocomposites based on titania nanotubes and a hydroxyapatite coating deposited by RF-magnetron sputtering: surface topography, structure, and mechanical properties. Appl Surf Sci. 2017;426:229–237. doi:10.1016/j.apsusc.2017.07.199
- Catauro M, Bollino F, Papale F, et al. Corrosion behavior and mechanical properties of bioactive sol-gel coatings on titanium implants. Mater Sci Eng C. 2014;43:375–382. doi:10.1016/j.msec.2014.07.044