ABSTRACT
Mutations in the NSMF gene have been related to Kallmann syndrome. Conflicting results have been reported on the subcellular localization of Jacob/NELF, the protein encoded by the NSMF gene. Some reports indicate an extracellular localization and a function as a guidance molecule for migration of GnRH-positive neurons from the olfactory placode to the hypothalamus. Other studies have shown protein transport of Jacob from synapse-to-nucleus and indicate a role of the protein in neuronal activity-dependent gene expression. A recent publication casts doubts on a major role of Jacob/NELF in Kallmann syndrome and neuronal migration of GnRH-positive neurons during early development. Instead a murine NSMF gene knockout results in hippocampal dysplasia, impaired BDNF-signaling during dendritogenesis, and phenotypes related to the lack of BDNF-induced nuclear import of Jacob in early postnatal development.
Introduction
Kallmann syndrome (KS) is a rare neurodevelopmental disorder considered as a subtype of idiopathic congenital hypogonadotropic hypogonadism (IHH). Symptoms associated with IHH include absence or delay of puberty due to gonadotropin-releasing hormone (GnRH) deficiency, hypogonadism, infertility and, in case of KS, anosmia or hyposmia.Citation1,2 The incidence of IHH is 3–5 times higher in males than in females with a prevalence of 1/5000 to 1/10000, and 50–60% of patients display olfactory dysfunction and KS.
The release of GnRH from a relatively small number of neurons in the hypothalamus (in humans about 7.000-12.000) is a prerequisite for normal hypothalamic-pituitary-gonadal function in puberty and reproduction.Citation3 During embryonic development these GnRH-positive neurons migrate together with olfactory axons from the olfactory placode region into the forebrain and disturbances in migration or proper cellular functions like defective secretion result in KS or IHH, respectively.Citation4,5
In recent years more than 31 different putative candidate loci have been identified and many studies suggested digenic or oligogenic causations for the development of IHH or KS, however, the degree and frequency of oligogenicity is still under debate. Among candidate genes that are mutated in KS and IHH are genes coding for cell adhesion and guidance molecules (KAL1, SEMA3A, SEMA7A), DNA-binding proteins / transcriptional repressors (FEZF1, HESX1, CHD7, SOX10) and molecules involved in placodal development and neurogenesis (FGF8, FGFR1).Citation2,6 Another candidate gene for KS is NSMF (NMDA Receptor Synaptonuclear Signaling And Neuronal Migration Factor). Several publications suggested a link between mutations in the NSMF gene and KS.Citation7-14 So far, 5 different mutations were identified, most of them in a digenic pattern together with mutations in other candidate genes.Citation7,9,13 Only in one case a monogenic causation of Kallmann syndrome was suggested by a point mutation in NSMF.Citation13 All mutations occurred heterozygously, 3 are intronic mutations from which an 8-bp intronic deletion might result in a splicing defect and premature stop codon and 2 missense mutations lead to exchange of a polar to unipolar amino acids or vice versa. Given that a candidate gene was sequenced in numerous patients with KS and in only very few cases a mutation was found, and the lack of any association studies as well as reverse or forward genetics, the evidence for a causal link between KS and a loss-of-function or gain-of-function-mutations in the NSMF gene is less than compelling.
Nuclear import of Jacob couples the NMDAR-Ca2+-signal to activity-dependent gene expression
The cellular function of Jacob, the protein encoded by the NSMF gene, has been investigated by us in recent years and we could show that Jacob is involved in N-Methyl-D-Aspartate-Receptor (NMDAR) signaling to the nucleus.Citation15-17 NMDAR are ligand- and voltage-gated sodium / calcium channels that play a key role in neuronal signaling. In addition to calcium signals, which represent a major route for communication of NMDAR activity to the nucleus, macromolecules and synapto-nuclear protein messengers have recently appeared to connect synapses and nucleus enabling bidirectional transfer of information.Citation18,19 Several lines of evidence demonstrate that protein transport from synapse-to-nucleus has a role in synaptic function and plasticity.Citation18,19
NMDAR are present at both synaptic and extrasynaptic sites, and the subcellular localization of each receptor profoundly and differentially affects the nuclear response to its activation. Activation of synaptic NMDAR induces the expression of cell survival and plasticity genes, while their extrasynaptic counterparts primarily drive the expression of cell death genes, linking the pathway to disease. Extrasynaptic NMDAR activation induces nuclear translocation of Jacob, which results in sustained dephosphorylation and transcriptional inactivation of the transcription factor CREB, a loss of synaptic contacts, a retraction of dendrites and eventually cell death.Citation15 Moreover, evidence was provided that amyloid-β (Aβ), a causative agent for Alzheimer disease, drives Jacob into the nucleus.Citation20,21 Nuclear import depends upon activation of extrasynaptic NMDAR and is part of pathological Aβ-signaling. However, Jacob also transits to the nucleus of CA1 neurons following induction of Schaffer collateral dependent long-term potentiation (LTP), a form of synaptic plasticity that essentially requires opening and calcium influx through synaptic NMDAR and hence acts as a messenger for both synaptic and extrasynaptic NMDAR pathways.Citation22 In previous work we addressed how the protein gets to the nucleus. Neuronal importins are present in axons, dendrites and synapses and they can associate with a dynein motor for active retrograde transport along microtubuli to the nucleus. Jacob utilizes this transport system after activation of both types of receptors and, in a recent study, we found that Jacob, following its nuclear import, can even encode the synaptic and extrasynaptic origin of NMDAR signals.Citation17 ERK1/2-kinase binding and ERK-dependent phosphorylation of the serine 180 residue in Jacob encodes synaptic but not extrasynaptic NMDAR activation. A stable trimeric complex with proteolytically cleaved fragments of the neurofilament α-internexin is formed which protects Jacob and active ERK against phosphatase activity during retrograde transport. In the nucleus, this signalosome-like complex enhances “plasticity-related” and “CREB-dependent” gene expression as well as synaptic strength. Collectively, the evidence suggests that Jacob operates as a mobile hub that docks NMDA receptor-derived signalosomes to nuclear target sites and thereby plays a role in activity-dependent gene transcription.
Discrepant reports on NELF and Jacob
It has been reported by others that a protein knock-down of the mouse ortholog of Jacob, NELF, results in migration deficits of Gonadotropin-releasing hormone (GnRH) positive neurons from the olfactory bulb to the hypothalamus during early brain development.Citation23,24 In addition, it was claimed that NELF is an extracellular guidance / migration factor for routing of GnRH positive cells along vomeronasal olfactory-derived axons and eventually to the hypothalamus (for overview see ).
Table 1. Overview of different phenotypes observed after Jacob/Nsmf knockdown or knockout approaches with respect to KS.
Thus, the discrepancies in reports on Jacob and NELF concern not only the subcellular localization but also their function.Citation15-17,23-25 This prompted us to delete the gene in vivo and to address the questions of whether inactivation of the Nsmf gene in mice results in phenotypes related to KS and whether a gene knockout supports a role of Jacob for hippocampal circuitry and function.
Jacob/Nsmf ko mice are fertile and do not show clear signs of Kallmann syndrome
In the first set of experiments, we found that mice that are constitutively deficient for the Nsmf gene do not present phenotypic characteristics related to KS.Citation26 Along these lines, we found no indication of hyposmia and hypogonadotropic hypogonadism in neither male nor female knockout mice.Citation26 The mice are viable, fertile and displayed normal life span. The morphology of reproductive organs display no abnormalities in both males and females.Citation26 Sex hormone levels and the estrous cycle are only slightly altered in comparison to wild-type littermates and had no impact on reproduction.Citation26
Collectively, these data are at variance with a recent study of Quaynor et al. (2015) that reported a reduced number of hypothalamic GnRH positive neurons and delayed puberty in female Jacob/Nsmf ko mice.Citation27 Our analysis was mainly focused on male mice but we also found no evidence for subfertility in female mice. Nonetheless, it is possible that sex differences exist and that female knockout mice exhibit subtle alterations toward hypogonadotropic hypogonadism.
The involvement of Jacob/NELF in the pathogenesis of KS was plausible because of previous reports that claimed a role of NELF as a guidance molecule. However, no evidence was presented that the protein can be secreted and, in fact, Jacob/NELF lacks a signal peptide that is mandatory for trafficking through the secretory pathway.Citation15 In addition, we could not support an extracellular localization of Jacob, which would be consistent with a role in axon growth cone guidance.Citation26 Instead, the results show, in accordance to previous results, a nuclear and synaptic localization of Jacob in mouse and rat pyramidal neurons.Citation25 Finally, both Jacob protein and mRNA levels are relatively low during early brain development, when cell migration and axon extension takes place, and increase during dendrito- and synaptogenesis in the second postnatal week in rodents.Citation15,16 Thus, a major functional role of Jacob as a growth cone-associated molecule for the migration of GnRH-positive neurons during development appears unlikely.
Nuclear import of Jacob is important for dendrite development in the hippocampus
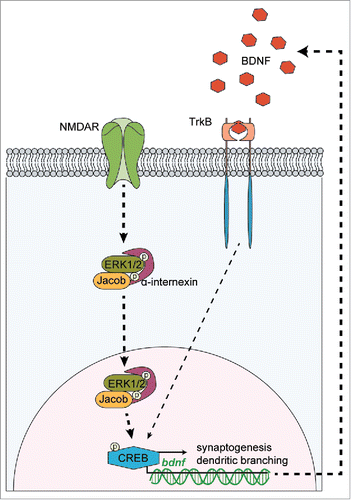
Our further analysis then revealed that these mice exhibit hippocampal dysplasia with a reduced number of synapses and simplification of dendrites, reduced hippocampal long-term potentiation (LTP) at CA1 synapses and deficits in hippocampus dependent learning.Citation26 Further structural anomalies include an altered catecholaminergic innervation and mossy fiber projection.Citation26 Brain-derived neurotrophic factor (BDNF) activation of CREB-activated gene expression plays a documented role in hippocampal CA1 synapse and dendrite formation. We found that BDNF induces the nuclear translocation of Jacob in an NMDAR-dependent manner in early development, which results in increased phosphorylation of CREB and enhanced CREB-dependent Bdnf gene transcription.Citation26 Most strikingly, the BDNF-induced nuclear import of pERK, which likely acts upstream of CREB, was clearly reduced in Jacob-deficient neurons. In consequence, Nsmf knockout mice show reduced hippocampal Bdnf mRNA and protein levels as well as reduced pCREB levels during dendritogenesis.Citation26 Moreover, BDNF reexpression can rescue the morphological deficits in hippocampal pyramidal neurons devoid of Jacob. Taken together, the data suggest that the absence of Jacob in early development interrupts a positive feedback loop between BDNF signaling, subsequent nuclear import of Jacob, activation of CREB, and enhanced Bdnf gene transcription, ultimately leading to hippocampal dysplasia (see ).Citation26
Figure 1. Translocation of Jacob/Nsmf to the nucleus is a key factor for a positive feedback loop involved in BDNF synthesis. BDNF induces the NMDAR-dependent translocation of phosphorylated Jacob to the nucleus in a trimeric complex with pERK1/2 and α-internexin. Higher levels of nuclear pJacob and pERK1/2 substantially contribute to expression of CREB-dependent genes including bdnf. BDNF synthesis enhances dendritic and synaptic development, necessary for unaltered synapto-nuclear communication, cell survival and expression of plasticity-related genes.
Conclusions
We think it is unlikely that Jacob/NELF plays a major role in early development as an extracellular guidance molecule for the migration of GnRH-positive neurons from the olfactory bulb to the hypothalamus. In consequence, a monogenic causation of Kallmann syndrome by mutations in the Jacob/Nsmf gene that lead to a loss of function is unlikely, although the previous work that reported migration deficits was based on acute antisense-mediated protein knockdown (see ) and compensatory mechanisms of a constitutive gene knock-out cannot be completely excluded.
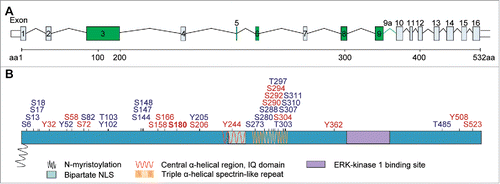
On the other hand, we found several indications for a role of the protein in hippocampal dendrito- and synaptogenesis. It will be interesting to see whether this role is specific for certain brain regions and whether it might play a role in human disease. The human, mouse, and rat genes are highly conserved with 96% identity across species at the amino acid level and an identical exon/intron organization in all mammals. Of note, Jacob undergoes extensive splicing and more than 20 splice isoforms can arise from transcription of the gene (see ).Citation16 This high degree of conservation in amino acid sequence and gene organization is surprising since Jacob harbors no clear-cut domains. In silico analysis predicts a protein with long disordered stretches and it is likely that the protein will only acquire a defined structure when bound to a target. Jacob is most likely a phosphoprotein that contains several motifs for protein interactions () and we are currently underway to establish a larger Jacob-interactome (). Database and literature searches have so far revealed very few mutations and polymorphisms in the human NSMF gene and it will be interesting to test whether mutations, including those already identified, might interfere with protein function within this interactome. Finally, we speculate that a role in human disease might be related to transcriptional regulation of the gene, aberrant splicing and pathological signaling similarly to what has been reported for Aβ-signaling.
Figure 2. Genomic structure of the mouse Jacob/Nsmf gene, key motifs and phosphorylation sites of the Jacob protein. (A) The Nsmf mouse gene consists of 16 exons. Exons 3, 5, 6, 8, 9 (marked in green) can be alternatively spliced. In addition, intron 9 has been predicted to constitute for one further isoform (denoted 9a) (B). The Jacob/Nsmf protein is largely unstructured but contains several motifs like a N-myristoylation site, a bipartite NLS, an IQ domain, central α-helical region, ERK-1 kinase binding site, and Triple α-helical spectrin-like repeats described before. Disorder Enhanced Phosphorylation Predictor (DEEP) revealed numerous phosphorylation sites (blue; only phosphorylation sites with DEEP score above 0.7 were included). Analysis of Jacob mouse protein with PhosphoSitePlus tool (Cell Signaling) revealed numerous phosphorylation sites reported by more than one Mass Spectrometry analysis studies (red). In bold, a S180 phosphorylation site confirmed by site-specific method, i.e. site-directed mutagenesis, mass-spectrometry and specific antibodies.Citation25-27
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG Kr1879 / 5–1, 6–1; SFB779 TPB8), WGL Pakt f. Forschung, JPND (STAD), BMBF EnergI and People Program (Marie Curie Actions) of the European Union's Seventh Framework Program FP7/2007-2013/ under REA grant agreement n [289581] NPlast to MRK.
References
- Valdes-Socin H, Rubio Almanza M, Tomé Fernández-Ladreda M, Debray FG, Bours V, Beckers A. Reproduction, smell, and neurodevelopmental disorders: genetic defects in different hypogonadotropic hypogonadal syndromes. Front Endocrinol 2014; 5:109; http://dx.doi.org/10.3389/fendo.2014.00109
- Kim SH. Congenital Hypogonadotropic Hypogonadism and Kallmann Syndrome: Past, Present, and Future. Endocrinol Metab 2015; 30:456-66; http://dx.doi.org/10.3803/EnM.2015.30.4.456
- Rance NE, Young WS, 3rd, McMullen NT. Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol 1994; 339:573-86; PMID:8144747; http://dx.doi.org/10.1002/cne.903390408
- Cariboni A, Maggi R, Parnavelas JG. From nose to fertility: the long migratory journey of gonadotropin-releasing hormone neurons. Trends Neurosci 2007; 30:638-44; PMID:17981344; http://dx.doi.org/10.1016/j.tins.2007.09.002
- Wierman ME, Kiseljak-Vassiliades K, Tobet S. Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol 2011; 32(1):43-52; PMID:20650288; http://dx.doi.org/10.1016/j.yfrne.2010.07.005
- Forni PE, Wray S. GnRH, anosmia and hypogonadotropic hypogonadism–where are we? Front Neuroendocrinol 2015; 36:165-77; PMID:25306902; http://dx.doi.org/10.1016/j.yfrne.2014.09.004
- Miura K, Acierno JS, Jr, Seminara SB. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J Hum Genet 2004; 49:265-8; PMID:15362570; http://dx.doi.org/10.1007/s10038-004-0137-4
- Trarbach EB, Baptista MT, Garmes HM, Hackel C. Molecular analysis of KAL-1, GnRH-R, NELF and EBF2 genes in a series of Kallmann syndrome and normosmic hypogonadotropic hypogonadism patients. J Endocrinol 2005; 187:361-8; PMID:16423815; http://dx.doi.org/10.1677/joe.1.06103
- Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 2007; 117:457-63; PMID:17235395; http://dx.doi.org/10.1172/JCI29884
- Pedersen-White JR, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of intragenic deletions in patients with idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Mol Hum Reprod 2008; 14:367-70; PMID:18463157; http://dx.doi.org/10.1093/molehr/gan027
- Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A 2010; 107:15140-4; PMID:20696889; http://dx.doi.org/10.1073/pnas.1009622107
- Quaynor SD, Kim HG, Cappello EM, Williams T, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of digenic mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil Steril 2011; 96:1424-30; PMID:22035731; http://dx.doi.org/10.1016/j.fertnstert.2011.09.046
- Xu N, Kim HG, Bhagavath B, Cho SG, Lee JH, Ha K, Meliciani I, Wenzel W, Podolsky RH, Chorich LP, et al. Nasal embryonic LHRH factor (NELF) mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil Steril 2011; 95:1613-20.e1-7; PMID:21300340; http://dx.doi.org/10.1016/j.fertnstert.2011.01.010
- Costa-Barbosa FA, Balasubramanian R, Keefe KW, Shaw ND, Al-Tassan N, Plummer L, Dwyer AA, Buck CL, Choi JH, Seminara SB, Quinton R, Monies D, et al. Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J Clin Endocrinol Metab 2013; 98:E943-953; PMID:23533228; http://dx.doi.org/10.1210/jc.2012-4116
- Dieterich DC, Karpova A, Mikhaylova M, Zdobnova I, König I, Landwehr M, Kreutz M, Smalla KH, Richter K, Landgraf P, et al. Caldendrin-Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol 2008; 6:e34; PMID:18303947; http://dx.doi.org/10.1371/journal.pbio.0060034
- Kindler S, Dieterich DC, Schutt J, Sahin J, Karpova A, Mikhaylova M, Schob C, Gundelfinger ED, Kreienkamp HJ, Kreutz MR. Dendritic mRNA targeting of Jacob and N-methyl-d-aspartate-induced nuclear translocation after calpain-mediated proteolysis. J Biol Chem 2009; 284:2543140; PMID:19608740; http://dx.doi.org/10.1074/jbc.M109.022137
- Karpova A, Mikhaylova M, Bera S, Bär J, Reddy PP, Behnisch T, Rankovic V, Spilker C, Bethge P, Sahin J, et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell 2013; 152:1119-33; PMID:23452857; http://dx.doi.org/10.1016/j.cell.2013.02.002
- Kaushik R, Grochowska KM, Butnaru I, Kreutz MR. Protein trafficking from synapse to nucleus in control of activity-dependent gene expression. Neuroscience 2014; 280:340-50; PMID:25230285; http://dx.doi.org/10.1016/j.neuroscience.2014.09.011
- Panayotis N, Karpova A, Kreutz MR, Fainzilber M. Macromolecular transport in synapse to nucleus communication. Trends Neurosci 2015; 38:108-16; PMID:25534890; http://dx.doi.org/10.1016/j.tins.2014.12.001
- Rönicke R, Mikhaylova M, Rönicke S, Meinhardt J, Schröder UH, Fändrich M, Reiser G, Kreutz MR, Reymann KG. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging 2011; 32:2219-28; PMID:20133015; http://dx.doi.org/10.1016/j.neurobiolaging.2010.01.011
- Gomes GM, Dalmolin GD, Bär J, Karpova A, Mello CF, Kreutz MR, Rubin MA. Inhibition of the polyamine system counteracts β-amyloid peptide-induced memory impairment in mice: involvement of extrasynaptic NMDA receptors. PLoS One 2014; 9:e99184; PMID:24921942; http://dx.doi.org/10.1371/journal.pone.0099184
- Behnisch T, Yuanxiang P, Bethge P, Parvez S, Chen Y, Yu J, Karpova A, Frey JU, Mikhaylova M, Kreutz MR. Nuclear translocation of Jacob in hippocampal neurons after stimuli inducing long-term potentiation but not long-term depression. PLoS One 2011; 6:e17276; PMID:21364755; http://dx.doi.org/10.1371/journal.pone.0017276
- Kramer PR, Wray S. Novel gene expressed in nasal region influences outgrowth of olfactory axons and migration of luteinizing hormone-releasing hormone (LHRH) neurons. Genes Dev 2000; 14:1824-34; PMID:10898796
- Palevitch O, Abraham E, Borodovsky N, Levkowitz G, Zohar Y, Gothilf Y. Nasal embryonic LHRH factor plays a role in the developmental migration and projection of gonadotropin-releasing hormone 3 neurons in zebrafish. Dev Dyn 2009; 238:66-75; PMID:19097186; http://dx.doi.org/10.1002/dvdy.21823
- Mikhaylova M, Karpova A, Bär J, Bethge P, YuanXiang P, Chen Y, Zuschratter W, Behnisch T, Kreutz MR. Cellular distribution of the NMDA-receptor activated synapto-nuclear messenger Jacob in the rat brain. Brain Struct Funct 2014; 219:843-60; PMID:23539133; http://dx.doi.org/10.1007/s00429-013-0539-1
- Spilker C, Nullmeier S, Grochowska KM, Schumacher A, Butnaru I, Macharadze T, Gomes GM, Yuanxiang P, Bayraktar G, Rodenstein C, et al. A Jacob/Nsmf gene knockout results in hippocampal dysplasia and impaired BDNF signaling in dendritogenesis. PLoS Genet 2016; 12(3):e1005907; PMID:26977770; http://dx.doi.org/10.1371/journal.pgen.1005907
- Quaynor SD, Ko EK, Chorich LP, Sullivan ME, Demir D, Waller JL, Kim HG, Cameron RS, Layman LC. NELF knockout is associated with impaired pubertal development and subfertility. Mol Cell Endocrinol 2015; 407:26-36; PMID:25731822; http://dx.doi.org/10.1016/j.mce.2015.02.015
