Abstract
Background: The safety of percutaneous endoscopic gastrostomy (PEG) insertion in amyotrophic lateral sclerosis (ALS) patients with significant respiratory compromise has been questioned. Objectives: To review the characteristics of an ALS clinic patient cohort undergoing PEG, and the introduction of a risk stratification tool with procedural adaptations for higher-risk individuals. Methods: Patients undergoing PEG insertion were analysed (n = 107). Cases stratified as higher-risk underwent insertion in a semi-recumbent position, minimising sedation, with the option of nasal non-invasive ventilation. Results: All underwent successful PEG. One-third had pre-procedure FVC ≤50% (mean, 64 ± 22%). Of those who underwent PEG insertion after introduction of risk stratification (n = 58), 39 (67%) met criteria for being higher risk, 16 (41%) of whom had FVC ≤50% (p = 0.005). High-risk patients received lower sedative doses vs. the low-risk group (midazolam 2.1 ± 1.1 vs.2.8 ± 0.95mg, p = 0.021; fentanyl 42 ± 16 vs. 60 ± 21μg, p = 0.015). Four deaths occurred within one month of insertion (attributable to the natural disease course). Conclusions: Risk stratification identified a greater number of patients with evidence of respiratory compromise than using the sole criterion of FVC ≤50%. A modified PEG procedure enabled safe insertion despite respiratory compromise, in those who might not have tolerated attempted insertion by alternative means such as radiologically-inserted gastrostomy.
Key words:
Introduction
Nutrition is an important component of optimal care in patients with amyotrophic lateral sclerosis (ALS). Weight loss at presentation is associated with decreased life span, and recent evidence suggests that higher calorific intake may be associated with improved survival (Citation1,Citation2). The difficulty of maintaining adequate nutrition due to dysphagia is compounded by the increased energy requirements of patients with ALS (Citation3).
Gastrostomy insertion is frequently employed as a means of enteral nutritional supplementation in patients with ALS unable to meet their nutritional requirements orally. It may be associated with modestly prolonged survival (Citation4). Gastrostomy placement is typically performed using endoscopic guidance (percutaneous endoscopic gastrostomy, PEG). Insertion prior to the onset of respiratory dysfunction is not always possible due to multiple factors such as patient preference, delay in diagnosis or presentation after respiratory involvement has already become established. A retrospective study suggested that the PEG procedure may carry increased risk in patients with significant respiratory weakness, as indicated by forced vital capacity (FVC) below 50% predicted (Citation5). This led to recommendations from the American Academy of Neurology and European Federation of Neurological Societies that PEG insertion be performed when FVC is greater than 50% predicted and to otherwise consider alternatives such as radiologically-inserted gastrostomy (RIG) or peroral imaging-guided gastrostomy (PIG) (Citation6,Citation7). However, recent data from the ProGas study, a large prospective cohort study of patients with ALS undergoing gastrostomy insertion via PEG, RIG or PIG, suggests that there is no difference in mortality between PEG and RIG (Citation8).
British Society of Gastroenterology Guidelines also point to sedation risks with patients with neurological ventilatory failure undergoing PEG (Citation9). Aspiration risk with PEG has also been highlighted as a concern (Citation10); however, a recent meta-analysis of low quality studies suggested no absolute mortality difference for PEG vs. radiologically-inserted gastrostomy (2.1%, 95% CI –6.3%–11.2%) (Citation11).
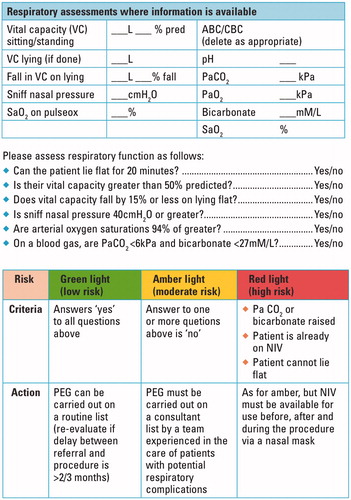
Here we describe experience of a locally developed risk stratification tool to identify ALS patients at potentially increased risk of complications from sedation and analgesia, taking account of factors beyond only the FVC (). Stratification was used as a guide to allow a modified procedure permitting PEG insertion in all ALS patients, including those with established respiratory insufficiency.
Methods
Patients
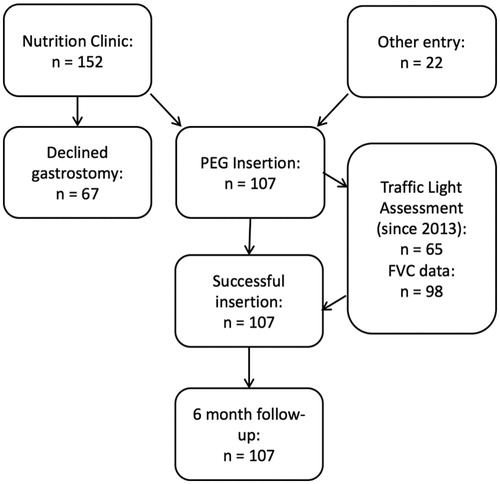
We identified 107 patients admitted for PEG to the John Radcliffe Hospital in Oxford, UK (assessed in the associated tertiary referral clinic by KT and MRT) between February 2011 (when dedicated records began) and October 2015. Approval for data collection through the Oxford Neurodegeneration Database was obtained from the Health and Social Care Northern Ireland Research Ethics Committee B (Ref 15/NI/0096). All patients provided written consent for data collection. Data were prospectively collected on patient age, gender, the site and date of clinical disease onset, disease progression rate (based on ALS functional rating score (ALSFRS)), FVC prior to PEG insertion and a locally-developed risk stratification tool summarised by a ‘traffic light’ score (green: low risk; amber: higher risk; red: highest risk). The main outcome measures considered were successful PEG insertion, and complications within 30 days or 6 months. These data were acquired prospectively at the time of PEG insertion and during subsequent clinic visits. We also studied death at 30 days and at 6 months.
Risk stratification
Patients considering PEG were referred to a dedicated ALS Nutrition Clinic run by a team comprising an ALS Specialist Nurse, Dietician, and Endoscopy Specialist Nurse (Citation12). From February 2013, as part of the counselling process, patients underwent risk stratification according to FVC measurement (% predicted), presence of hypercapnia or raised blood bicarbonate on blood gas sampling, and use of non-invasive ventilation ().
Modified higher risk PEG procedure
Patients were admitted to the regional neurosciences centre for one night prior to and following the procedure. Patients who were stratified as ‘green’ were not considered high risk and therefore underwent routine PEG insertion procedures. Additional precautions were implemented for those in the ‘amber’ and ‘red’ categories. For these patients PEG insertion was performed only by a highly experienced operator (JEE and PJA). The patient was positioned with at least 30° whole body head-up tilt during the procedure to offload the diaphragm, and using a paediatric mouth-guard gently held in place (rather than strapped in place) to minimise tension on the mandible and therefore potential airway compromise. The smaller paediatric mouth guard facilitates use of non-invasive ventilation, which often fails with a full-size adult mouth guard due to lack of pharyngeal seal. Minimal use of sedation was planned with careful titration and pauses for effect. Additional discussion with these patients prior to the procedure was undertaken, explaining the desire for lighter sedation and to counsel on the possibility of a degree of peri-procedural awareness.
For patients who were stratified as ‘red’, nasal non-invasive ventilation was available in the endoscopy room in case it was needed, and could be used during the procedure. Minimal oxygen was used in the procedure room and in recovery, titrated to saturations, particularly in those patients on home non-invasive ventilation or with raised PaCO2.
Statistical analysis
Statistical analysis was performed using SPSS Version 22.0.0.0 (IBM Inc.). Normality of continuous variables was assessed using the Shapiro-Wilk test. Comparisons between groups (FVC >50% and FVC ≤50% and risk assessment strata) were performed using Kruskall-Wallis H test with multiple comparison correction using the Dunn-Bonferroni method for continuous variables. Fisher’s exact test was used for comparison of categorical variables. Patients for whom data for stratification were missing were omitted from the appropriate tests.
Results
The recruitment pathway is shown in . Patient data are summarised in . All patients underwent successful PEG placement, two on the second attempt. Early procedural complications were rare, with one episode of bowel perforation and one episode of insertion site cutaneous infection. There were no episodes of tube displacement.
Table 1. Study cohort characteristics. Values are mean ± standard deviation, median (IQR) or n/N (%).
Of 98 patients for whom FVC was available, 26 (27%) had FVC ≤50%. Of patients undergoing FVC assessment, death within 30 d occurred in four patients (4%), all of whom had FVC ≤50% (15% (4/26) vs. 0% (0/72), p = 0.003). None was directly attributable to PEG insertion.
Of 58 patients for whom FVC and traffic light stratification data were available, 39 (67%) were stratified ‘amber’ or ‘red’, implying respiratory involvement, and underwent a modified procedure. Of these 39 patients, 23 (59%) had FVC >50%, meaning that over half of these patients would not have been considered high risk using the sole criterion FVC ≤50% (p = 0.005).
The distribution of sedative dose differed between risk strata, with statistically significant differences observed between ‘red’ and ‘green’ strata for both midazolam (mean 2.12 mg, standard deviation 1.12 mg vs. 2.8 ± 0.95 mg, p = 0.021) and fentanyl (41.7 ± 15.9 μg vs. 60.0 ± 20.5 μg, p = 0.015).
Two deaths occurred within 30 days in the group undergoing risk stratification, both of whom had been stratified as ‘red’ (2/22 (9%) vs. 0/38 (0%), p = 0.131). Within six months of insertion there had been 28 deaths overall, 10 in the group FVC ≤50% (10/26 (38%) vs. 19% 14/72 (19%) p = 0.096). Of those undergoing traffic light assessment there were 16 deaths, six of whom were stratified ‘red’, seven ‘amber’ and three ‘green’ (6/25 (24%) vs. 7/20 (35%) vs. 3/20 (15%) (3/20), p = 0.339). Again, no deaths were directly attributable to PEG insertion.
Discussion
Our data suggest that PEG can be carried out safely in ALS patients, including those with significant respiratory involvement. The 30-d mortality in this study of 4% is comparable to previously published mortality for insertion of PEG, RIG or PIG in the ProGas study (overall 4%, CI 2–6%; PEG 3%, CI 1–8%; RIG 3%, CI 1–9%, PIG 7%, CI 2–19%) and a meta-analysis of previously published data (PEG 10%, CI 5–15%; RIG and PIG 6%, CI 3–9%) (Citation8,Citation11). The severity of respiratory involvement is a confounding adverse survival factor, and we conclude that these deaths were related to underlying disease progression rather than a direct consequence of the procedure. The rate of local complications including tube displacement was very low (affecting fewer than 2% of patients), again comparable to the ProGas study and in marked contrast to insertion by RIG, where tube displacement mandates an additional procedure: in our series, PEG is a one-off intervention (Citation8).
The use of a stratification system based on a number of respiratory parameters in this study enables identification of a greater number of patients with respiratory involvement than using FVC alone (including patients who would be considered low risk using FVC ≤50% as the sole criterion) and therefore enabling procedural adaptation in these patients. However, due to the observational nature and small size of this study, no inferences can be drawn about the influence of the stratification tool on mortality. The study design also does not allow for direct comparison of the risks of different insertion methods, did not explore the effect of reduced sedative doses on related outcomes such as procedural awareness or pain, and suffered from incomplete data acquisition for FVC in a small number of subjects.
The decision to have a PEG is among the most challenging for ALS patients (Citation13), and natural fear and procrastination may result in the procedure having to be considered in late-stage disease. The previous perception of a clear benefit of RIG over PEG in the setting of respiratory failure in ALS, specifically that RIG requires less sedation and has a lower risk of complications, is disputed by the evidence emerging from published clinical practice (Citation8,Citation14). RIG is performed in the supine state and the procedure is typically prolonged compared to PEG, and therefore may preclude ALS patients with an FVC ≤50% predicted, who are likely to suffer significant associated orthopnoea. RIG involves significant discomfort to the patient, so that sedation and high levels of analgesia are routine, with the same concern over exacerbating respiratory failure as with PEG (Citation10,Citation15). Complications such as tube displacement requiring reinsertion or site infection are much more common with RIG (Citation14,Citation16).
Our experience of employing a modified semi-recumbent procedure performed by a team experienced in PEG insertion in patients with respiratory involvement, associated with reduced sedation and with the option of nasal NIV, suggests that PEG can be safely carried out in those ALS patients stratified as higher risk for respiratory complications. We conclude that FVC ≤50% should not preclude successful PEG, indeed we regard it as preferable to RIG for such individuals due to the ability to perform PEG in the semi-recumbent position and the lower risk of complications, in particular tube displacement. More than half of the patients stratified as high-risk by our tool had an FVC >50%. The use of the stratification tool described to identify evidence of respiratory dysfunction not detected by measurement of FVC alone permitted procedural adaptation in patients who might otherwise have been considered low risk.
In conclusion, PEG appears to be a safe and appropriate choice for ALS patients even with respiratory impairment, using a modified technique. Use of a respiratory risk stratification tool may help identify MND patients who would benefit from PEG technique modification.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. The Oxford MND Clinic is funded with a Care Centre Grant from the MND Association.
References
- Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry. 2011;82:628–34.
- Wills AM, Hubbard J, Macklin EA, Glass J, Tandan R, Simpson EP, et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;383:2065–72.
- Bouteloup C, Desport JC, Clavelou P, Guy N, Derumeaux-Burel H, Ferrier A, et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol. 2009;256:1236–42.
- Chio A, Mora G, Leone M, Mazzini L, Cocito D, Giordana MT, et al. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology. 2002;59:99–103.
- Kasarskis EJ, Scarlata D, Hill R, Fuller C, Stambler N, Cedarbaum JM. A retrospective study of percutaneous endoscopic gastrostomy in ALS patients during the BDNF and CNTF trials. J Neurol Sci. 1999;169:118–25.
- Diagnosis ETFo, Management of Amyotrophic Lateral Sclerosis, Andersen PM, Abrahams S, Borasio GD, de Carvalho M, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol. 2012;19:360–75.
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–26.
- ProGas Study Group. Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): a prospective cohort study. Lancet Neurol. 2015;14:702–9.
- Westaby D, Young A, O'Toole P, Smith G, Sanders DS. The provision of a percutaneously placed enteral tube feeding service. Gut. 2010;59:1592–605.
- Allen JA, Chen R, Ajroud-Driss S, Sufit RL, Heller S, Siddique T, et al. Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: a retrospective study of complications and outcome. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:308–14.
- Stavroulakis T, Walsh T, Shaw PJ, McDermott CJ, Progas S. Gastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practice. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:96–104.
- Marsden R, Millard E, Allan PJ, Blackwell V, East J, Lawson C, et al. Nutritional pathway for people with motor neurone disease. Br J Commun Nurs. 2016;21:260–3.
- Oliver DJ, Turner MR. Some difficult decisions in ALS/MND. Amyotroph Lateral Scler 2010;11:339–43.
- Chio A, Galletti R, Finocchiaro C, Righi D, Ruffino MA, Calvo A, et al. Percutaneous radiological gastrostomy: a safe and effective method of nutritional tube placement in advanced ALS. J Neurol Neurosurg Psychiatry. 2004;75:645–7.
- Desport JC, Mabrouk T, Bouillet P, Perna A, Preux PM, Couratier P. Complications and survival following radiologically and endoscopically-guided gastrostomy in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:88–93.
- Rio A, Ampong MA, Turner MR, Shaw AS, Al-Chalabi A, Shaw CE, et al. Comparison of two percutaneous radiological gastrostomy tubes in the nutritional management of ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:177–81.