Abstract
Objective: To design an ALS clinical study in which patients are remotely recruited, screened, enrolled and then assessed via daily data collection at home by themselves or caregivers. Methods: This observational, natural-history study included two academic medical centers, one providing overall clinical management and the other overseeing computing and web-services design and management. Both healthy and ALS subjects were recruited on the Internet via advertisement on governmental and foundation websites as well as through Facebook and Google paid advertisements. Individuals underwent screening and enrollment remotely, including signing an electronic informed consent form. Participants were then provided self-measurement equipment and instructed on their use through a series of web-based videos. The equipment included a handgrip dynamometer, spirometer with smartphone connection, electrical impedance myography device, and an activity tracker. ALS Functional Rating Scale-Revised data were also collected. Subjects were asked to collect data daily for three months and twice-weekly for the subsequent six months. Results: One hundred and eleven ALS patients and 30 healthy individuals enrolled in the study from across 41 states (74 men, 62 women). Baseline median ALSFRS-R score was 33. Seventy two percent of the ALS patients sent equipment and 88% of the healthy subjects sent equipment were able to complete a first set of measurements. Expected baseline differences between the ALS patients and healthy participants were identified for all measures. Conclusions: It is possible to design and institute an at-home based study in ALS patients, using a number of state-of-the-art approaches, including web-based consenting and training and Internet-connected measurement devices.
Trial registration: ClinicalTrials.gov identifier: NCT03016897.
Introduction
Over the past several decades, a standard approach to clinical trial design in ALS has emerged (Citation1). In general, patients are screened, enrolled, and studied at a large medical center. A variety of outcome measures or biomarkers are obtained at baseline, the patient is provided active drug or placebo, and then followed over a period of several months to a year. Patient visits to the center occur at bi- or tri-monthly intervals during which outcomes data are gathered as well as safety and adverse event information. At the conclusion of the study, data are analyzed by assessing the rate of change in a given parameter over time, with the goal of determining whether individuals on active treatment show a relative slowing of progression as compared to those on placebo.
A variety of outcome measures have been incorporated into this approach, each of which has some intrinsic variability. These measures have also confirmed that ALS patients progress at very different rates, some declining slowly and others very rapidly. These two factors together mean that late stage trials usually require hundreds of patients per group, studied for six months or more, to have sufficient power to identify a treatment effect. Novel trial designs have concentrated on altering inclusion criteria to enroll a cohort of similarly declining patients. This approach has led to the approval of edaravone in 2017 (Citation2); however, drastic limits on inclusion criteria raise questions about the generalizability of results, and rate of patient accrual is drastically reduced.
In addition to this limitation, the standard clinical trial approach also requires that patients return to a study center for evaluation on a frequent schedule. This limits potential trial participants to those in proximity to such a center. In addition, while travel early in the disease may be easy, with progression, it often becomes difficult for the patient to travel any distance for evaluation. This can put considerable burden on patients and their caregivers and is likely one reason many may discontinue participation at some point during a study (Citation3).
An alternative strategy to help reduce study size requirements and increase convenience would be to have patients obtain quantitative, functional data on themselves at home on a very frequent (e.g., daily) basis (Citation4). By frequent sampling, both variability of measurement and small physiological changes in function would both be eliminated, potentially reducing the variability of the slope of a given measure. Such a measurement strategy should improve the certainty in the estimation of the rate of decline in a given person over time (see ) and thus have the power to reduce to sample size requirements overall. Moreover, such measurements would obviate the need for repeated travel to a distant medical center. Theoretically, essentially all aspects of a clinical trial could be performed remotely, including dispensing drug, collection of blood samples and assessment of adverse events.
Figure 1 Simplified, basic concepts concerning sampling frequency and ability to accurately measure the true slope of disease progression, assuming a linear decline over time. (A) Standard clinical trial design in which only intermittent assessments are obtained in any given patient. There is a great deal of uncertainty in the true rate of progression (see wide 95% confidence intervals). (B) Clinical trial design with frequency measurements. Although there continues to be noise in any individual measurement, by frequent sampling the uncertainty in the estimation of the true slope is much smaller.
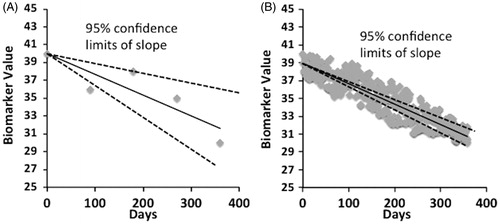
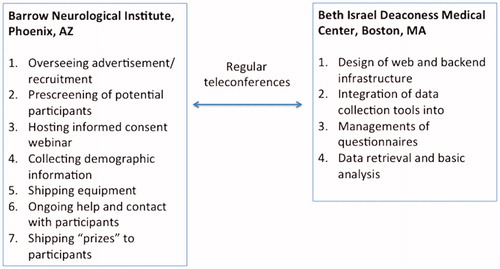
Figure 2 Overall set-up of ALS-at-home study across the 2 sites, Barrow Neurological Institute (BNI) and Beth Israel Deaconess Medical Center (BIDMC). BNI served as the main center for conducting the trial whereas BIDMC oversaw all the electronic-web infrastructure development and application.
In order to better understand the potential of this approach, we designed a study, funded by ALS-Finding-A-Cure, entitled “ALS AT HOME,” specifically focused on assessing possible sample-size improvements, identifying the optimal sampling frequency, and assessing patient acceptance of such an approach. We employed a battery of frequently used outcome measures that were well suited to in-home assessments, as well as several novel endpoints. In this report, we provide an overview of the methods developed, including enrollment and consenting procedures, the set-up of data collection and web design, baseline data, and provide detailed information on the challenges, both expected and unexpected, with such an approach.
Methods
Overall structure ()
Study coordination was carried out at Barrow Neurological Institute (BNI), Phoenix, AZ; the BNI coordination center was also responsible for all clinical aspects of the study, including providing institutional review board oversight, implementing recruitment strategies, enrollment, and conducting all subsequent participant contact, including shipping of study tools and troubleshooting problems as they arose. Beth Israel Deaconess Medical Center, Boston, MA was responsible for all informational technology development and oversight, including design and implementation of the ALS AT HOME web portal, linking the various collection tools with data storage in the REDCap server, and automating the sending of reminders if individuals neglected to collect data on a given day. Weekly to biweekly conference calls were held to ensure that the project was progressing appropriately.
Advertisement and recruitment
Our recruitment efforts were mostly web-based through the Centers for Disease Control (CDC) ALS patient registry (https://www.cdc.gov/als/), and the ALS Association and the Muscular Dystrophy Association websites. We also advertised through Facebook and Google Ads, in addition to maintaining a social media presence on Facebook®, Twitter®, and Reddit®. Some in-person recruitment was completed at ALS clinics. ALS patient inclusion criteria were kept intentionally broad, and included a diagnosis of ALS within five years and no known additional neurological disorder of relevance. Healthy subject criteria included having no history of a generalized neurological condition.
Tools for data collection
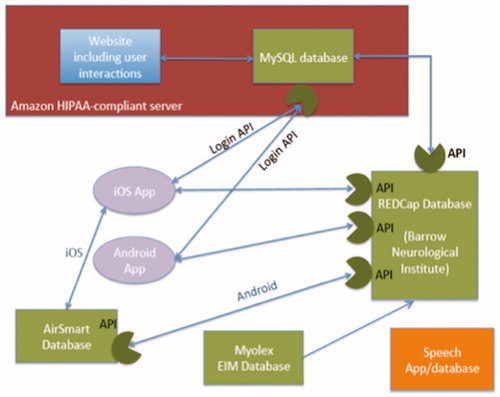
The overall architecture for data collection is summarized in . A single HIPAA-compliant REDCap database was designed for the outcomes data (with the exception of voice data). The REDCap database was populated either from the ALS AT HOME website or from apps on Android or Apple smartphones. A new ALS AT HOME app was also created for both iOS and Android smartphones. A number of data collection tools were utilized, as follows:
Figure 3 Diagram showing the complex interconnection between various components of the data acquisition system.
Hand grip dynamometer
We used the Camry Handgrip Dynamometer (Camry Scale-USA, City Industry, California,). Data was uploaded manually to the Internet via the ALS AT HOME website.
Actigraph
The Mi Band® (Xiaomi Corp, Beijing, China) was used for this purpose. It has a direct Bluetooth interface with the Mi Fit app, allowing data to be automatically uploaded to a smartphone. Activity data was populated to REDCap via application program interfaces (APIs), synchronizing with the Apple Health app for iOS and Google Fit for Android. Data automatically transferred to REDCap whenever the participant opened the ALS AT HOME app.
Spirometer
We used the AirSmart Spirometer (Nuvoair AB, Stockholm, Sweden). Respiratory data, including FVC and FEV1, was stored in the Air Smart’s health cloud after measures were obtained. For iOS users, respiratory data was extracted with the Apple Health app via the study app to REDCap. For Android users, a Python code was developed and executed weekly to extract data directly from the Air Smart database before transferring to REDCap.
Electrical impedance myography (EIM) tool
The Skulpt Scanner (Myolex, Inc, Boston, MA) was used for this purpose. EIM data, collected on bilateral biceps, forearms, calves, and quadriceps, were uploaded via Bluetooth to the Skulpt app and transferred to a Myolex, Inc server. A Python code was created to pull data from the Skulpt database and transfer it to REDCap weekly.
Speech app
A separate ALS AT HOME speech app was developed specifically by Aural Analytics (Phoenix, Arizona, USA). Patients downloaded the app to their smartphones and spoke specific phrases into the phone. Speech samples were then automatically uploaded to a separate cloud-based repository for analysis.
Questionnaire data
These data included the ALSFRS-R (Citation5) and patient reported experience measures (PREMs): both surveys were created and built in the REDCap database and appeared at the time of patient log-in at the website on specific days: ALSFRS-R links appeared weekly for ALS patients only while PREM links appeared on day 7, three months, six months, and nine months automatically for all participants. After completion, all answers were stored in REDCap.
The participant study experience
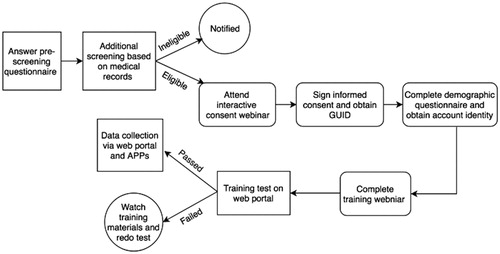
outlines the overall experience of the participant. After learning about the study, the participant would go to the ALS AT HOME website to answer the prescreening questions. These questions were designed to address whether the patient was planning to serve as a healthy control or was an ALS patient, and to make an initial ruling on whether the individual would be eligible for the study. In either case, they would be asked to provide their contact information. They would then be contacted by the study liaison at BNI, provided more details regarding participation, and asked to provide basic medical records (if an ALS patient) to confirm that they had a diagnosis of ALS with time from diagnosis of less than five years. Records were reviewed by Dr. Shefner; subsequent to diagnosis and disease duration confirmation, they were invited to participate in the study. Similarly, for healthy controls, the study would be explained in more detail and questions answered. If the participant was eligible, they were then asked to participate in an informed consent webinar that was held on a regular basis; these were performed in such a manner that all participants were blind to the names of other participants. The study liaison at BNI held these webinars and questions were answered live during the webinar. After participating in the informed consent webinar, the potential participants would be provided a link to an electronic BNI institutional review board-approved ICF that they could complete. They would be able to keep one copy for themselves after signing.
Once formally enrolled, the participant would then be shipped a package containing all the products to be used in the data collection described above. They would then be asked to review a series of instructional videos created at BNI to explain how the devices were used. After reviewing these videos, participants completed an online test to ensure they understood their proper use. Participants received feedback on their performance and could keep taking the test until they demonstrated adequate proficiency. At that point, participants were formally invited to begin collecting data using the various tools.
Data collection schedule
Participants were asked to obtain all outcome measures daily for 90 days, then twice weekly for an additional 180 days. The exception to this was the ALSFRS-R, which ALS participants were asked to fill out online weekly for the duration of the study. Ongoing feedback would be provided to the participants via the study liaison at BNI. All participants had email and phone contact information if they had questions or encountered problems during the data collection procedures. At the 3-month point (the conclusion of daily data collection), participation would be rewarded with an ALS AT HOME t-shirt; at the conclusion of nine months, participants would be sent an ALS AT HOME hat.
Results
Enrollees/drop-outs to date
Five hundred and fifty three potentially eligible individuals completed the eligibility questionnaire on the ALS AT Home website over the 14-month period of recruitment. Of these, 101 were healthy subjects of whom 32 attended an informed consent webinar, 30 signed the informed consent and received equipment, and 25 began contributing data. Similarly, 452 potentially eligible participants with ALS completed the eligibility questionnaire and were asked to submit medical records, and we received records from 175 individuals. Of this group, 157 individuals met the inclusion criteria (diagnosis of ALS less than five years from date of records receipt), and were invited to participate in an informed consent webinar. One hundred and eleven individuals were consented, 106 received equipment, and 75 began contributing data.
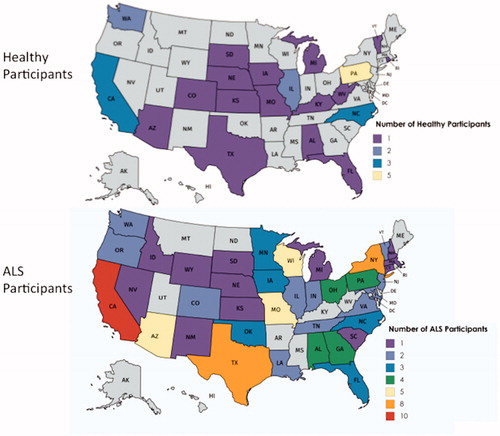
Geographic distribution of subjects
Subjects were recruited from 41 states, with the highest total number of participants in California, and multiple states with only one participant. The number of ALS and healthy participants in each state are depicted in and .
Baseline values
provides baseline data for the entire group of individuals enrolled. Most notably, most ALS patients and healthy volunteers were able to successfully collect initial data. Reduced mean values in most parameters were observed in the ALS patients compared to the healthy controls, although the range of values for both groups across parameters was quite large.
Table 1 Baseline data.
Discussion
This is a novel study in many respects. First, participants were enrolled, consented, and trained online, without ever going to a study site. Thus, we have shown that it is possible to solicit, prescreen, enroll, screen, and successfully begin collecting data in a completely remote study. Perhaps the overall most important finding of the study to date is that it is feasible to institute an almost entirely remote study with individuals collecting data on themselves or with the help of a caregiver. At the outset of this effort, we had concerns that people might be suspicious of this study or simply unwilling to enroll given their lack of human connection with the study organizers, as well as there being no therapeutic intervention. However, we have found that having a dedicated study liaison who is available to answer queries either by voice phone call or email is sufficient to engage subjects through the enrollment process to the point of initial data collection.
Another aspect that was novel included enrollment entirely based on patient’s medical records without our physically evaluating the potential participant. This was generally not challenging, although we inevitably lost some potential participants who had trouble accessing or delivering the needed documentation. Similarly, the consenting webinar was easily arranged and applied using standard Internet tools. Inasmuch as possible, these were organized so as to present the information to as many individuals as possible at a time; approximately one dozen webinars were provided during the course of the enrollment period.
We also had concerns that the computer and smartphone applications might prove difficult for participants to navigate. However, the participants who reached the point of entering data were sufficiently familiar with digital devices so as to be able to manage the various apps and over-the-Internet communications. Another concern we had from the outset was that the study might be too tech-heavy, making it excessively onerous and complex to obtain data. However, it appears that the majority of individuals are sufficiently comfortable to collect and transmit the data.
We have also demonstrated that online recruitment tools can be used successfully in this patient population. Rather than recruiting through clinics via face-to-face interactions, almost all of our advertising was through the CDC ALS patient registry (https://www.cdc.gov/als/) and foundation websites. In addition, we used standard online recruitment tools including Facebook and Google paid ads. All of these were successful in bringing people to our webpage.
There were several challenges that we encountered in the implementation and performance of this study. First, given that many of the Internet and device tools used in this study were proprietary, intermittent but frequent updates to the various software components are needed, even during the enrollment period. This included regular updates to smartphone operating systems, to the specific smart phone apps, and to the firmware embedded in any Bluetooth enabled Internet-connected devices. While most of these did not interfere with our ability to collect data, some required urgent modifications of our software in order to maintain continued error-free operation. Second, none of the tools have been problem-free, and even the relatively simple digital grip dynamometer presented challenges. It can give data in pounds/kilograms, and we need to ensure they are providing us the accurate data. Similarly the air smart spirometer, the Mi-Band, and Skulpt Scanner have had technical challenges. Some people were unable to interface the device with the Internet effectively while others simply could get the device to work properly at first. Whether this is a fault with the device itself or simply inexperience on the user’s part was often unclear. Third, participants often complained of difficulties with one more aspects of data collection or device function. While most of these were relatively straightforward to address, some problems remained entirely unresolved despite a dedicated effort by our study to team to identify the problem.
Another challenge was that the online nature of this study limited our ability to effectively understand challenges to participant attrition. Unlike most studies in which the study team develops a close rapport with the participants, the lack of any meaningful connection with participants meant that when people went “radio silent” during the enrollment and training process we often lacked the ability to determine the reason for the lack of contact.
Many of the challenges discussed above were expected from the outset, although we did not have clear expectations of which would be most serious. In addition, a number of unexpected challenges were noted. For example, shortly after funding, two of the companies whose activity trackers we were going to utilize could no longer supply the tracker, either because the company went out of business or it stopped producing the product. Accordingly, we found ourselves needing to stock up on products to ensure availability to participants throughout the study. Another unexpected issue was that Nuvoair changed its policy regarding data and required us to pay an extra fee to have access to all the AirSmart data that was to be collected.
The online nature of the study also led to some unique concerns. For example, several providers reached out to us suspicious that the study was not legitimate and was an Internet gimmick of some sort. These concerns were fairly easy to overcome, but underscores perceptions about the Internet and the solicitation of participants sight-unseen.
For convenience and simplicity, this study was restricted to the United States with only one set of governing laws overseeing clinical studies. Nevertheless, it remains possible to perform such a study across international borders. There would be some additional challenges associated with this beyond requiring additional approvals in different countries. For example, shipping devices to different countries may be problematical in certain situations, and it is conceivable that Internet censoring in some countries could hamper recruitment or other aspects of study data collection.
Regardless of the challenges associated with this approach, we suggest that it may be possible to start moving clinical trials out of the clinic and, inasmuch as possible, into the home. Once this study draws to conclusion in the coming months, we will provide a full data set that will likely reveal additional challenges and successes to undertaking this new approach to ALS data collection. The potential improvements in sample size requirements, the added convenience to participants, and the new insights obtained into the short-term behavior of the disease could make such an approach the preferred method for most future ALS therapeutic trials.
Disclosure statement
Dr. Rutkove has equity in, and serves a consultant and scientific advisor to, Myolex, Inc. a company that designs impedance devices for clinical and research use; he is also a member of the company’s Board of Directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named as an inventor. Drs. Berisha and Liss serve as Chief Science Officer and Chief Clinical Officer at Aural Analytics, Inc, a company that develops objective measures of neurological health. Both Dr. Berisha and Dr. Liss have equity in the company and serve on the Board of Directors.
Additional information
Funding
References
- Petrov D, Mansfield C, Moussy A, Hermine O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Vol. 9, Frontiers in Aging Neuroscience. 2017.
- Abe K, Aoki M, Tsuji S, Itoyama Y, Sobue G, Togo M, et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol [Internet]. 2017;[cited 2017 Jun 18]16:505–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28522181
- Atassi N, Yerramilli-Rao P, Szymonifka J, Yu H, Kearney M, Grasso D, et al. Analysis of start-up, retention, and adherence in ALS clinical trials. Neurology. 2013;81:1350–5.
- Dodge HH, Zhu J, Mattek NC, Austin D, Kornfeld J, Kaye JA. Use of high-frequency in-home monitoring data may reduce sample sizes needed in clinical trials. PLoS One. 2015;10:e0138095.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.